Abstract
Purpose
To apply an intensity-based nonrigid registration algorithm to MRI-guided prostate brachytherapy clinical data and to assess its accuracy.
Materials and Methods
A nonrigid registration of preoperative MRI to intraoperative MRI images was carried out in 16 cases using a Basis-Spline algorithm in a retrospective manner. The registration was assessed qualitatively by experts’ visual inspection and quantitatively by measuring the Dice similarity coefficient (DSC) for total gland (TG), central gland (CG), and peripheral zone (PZ), the mutual information (MI) metric, and the fiducial registration error (FRE) between corresponding anatomical landmarks for both the nonrigid and a rigid registration method.
Results
All 16 cases were successfully registered in less than 5 min. After the nonrigid registration, DSC values for TG, CG, PZ were 0.91, 0.89, 0.79, respectively, the MI metric was −0.19 ± 0.07 and FRE presented a value of 2.3 ± 1.8 mm. All the metrics were significantly better than in the case of rigid registration, as determined by one-sided t-tests.
Conclusion
The intensity-based nonrigid registration method using clinical data was demonstrated to be feasible and showed statistically improved metrics when compare to only rigid registration. The method is a valuable tool to integrate pre- and intraoperative images for brachytherapy.
Keywords: prostate brachytherapy, signal intensity-based nonrigid registration, B-Spline
MRI-GUIDED BRACHYTHERAPY is a method to treat localized prostate cancer (1–3). It enables treatment planning on site through its delineation of prostate substructures and surrounding tissue, especially when using an endorectal (ER) coil. D’Amico et al and Landis et al demonstrated the feasibility of this approach by performing 248 cases of MRI-guided prostate brachytherapy using a 0.5 Tesla (T) open-configuration MRI scanner, and comparing the clinical outcomes with other forms of brachytherapy (4,5). Their study reported that MR-guided prostate brachy-therapy presented fewer complications and a better quality of life than when using ultrasound for image guidance. One of the drawbacks of the MR-guided procedure, however, is that the open-configuration 0.5T scanner had significantly lower field strength and less gradient performance than standard high-field scanner cylindrical magnets, resulting in a lower signal-to-noise ratio and low contrast and resolution. Image registration between preoperative MRI obtained in a high-field scanner and intraoperative images used for guidance in the open magnet can help contribute information from the diagnostic scan into the procedure (6).
Once registration is performed at the beginning of the procedure, the fused image is useful to better comprehend the prostate substructures and suspicious foci that would otherwise be more difficult to detect using intraoperative MRI alone. Hirose et al found that a significant prostate shape change occurred between preoperative 1.5T ER coil images and intraoperative 0.5T imaging due to postural change from a supine to lithotomy position and the absence of an ER coil at 0.5T. In particular, in the preoperative images, the prostate gland appeared to have a smaller anterior–posterior dimension and wider transverse dimension than in the intraoperative images. Furthermore, the shape change of the peripheral zone was greater than that of the central gland between pre- and intraoperative images (7).
Nonrigid registration, as opposed to rigid registration is, therefore, necessary to compensate for the deformation of the prostate between pre- and intra-operative imaging. Bharatha et al reported nonrigid registration of ER coil-based 1.5T preoperative MRI with 0.5T intraoperative images using segmented data sets. The results from this study indicated that the method was feasible and significantly improved the accuracy of registration when compared to its rigid counterpart (8,9). The method, however, requires manual segmentation of the prostate as a preprocessing step, which can introduce intra- and interobserver variability. Furthermore, the manual segmentation step considerably prolongs the registration process, which impacts the procedure time of the brachytherapy intervention. Intensity-based nonrigid registration which does not require manual segmentation would allow the mapping of high-resolution images to the intraoperative ones in less time. In response to this need, Wang et al used such an approach to effectively track anatomical variations within CT images (10). While intensity-based registration was documented in this study, the method was tested in phantom trials and only a few clinical data sets, and was not tested for MR images.
Our objective is to test and validate the feasibility of intensity-based nonrigid registration between preoperative MRI and intraoperative MRI obtained during MRI-guided brachytherapy of the prostate. Specifically, we tested the accuracy of the intensity-based registration method using a 1.5T preoperative MRI taken with an ER coil and a 0.5T intraoperative MRI taken without the ER coil.
MATERIALS AND METHODS
Patient Selection and Imaging Protocols
This study included 16 men (mean age, 63.4 years; range, 54 to 72 years) who were randomly selected from MRI-guided prostate brachytherapy procedures performed at our institution between December 1998 and August 2002. Informed consent was obtained in each case after description of the nature of the procedures. The entry criteria for patients into the MRI-guided brachytherapy program at the institution has been described elsewhere (1). All patients underwent preoperative 1.5T MRI using an ER coil with integrated pelvic phased multi-coil array (Signa LX, GE Healthcare, Milwaukee, WI). The ER coil was a receive-only coil mounted inside a latex balloon with a diameter of 4–6 cm when inflated inside the patient’s rectum. The patients were placed in the supine position in the closed-bore magnet for the imaging examination that included T2-weighted fast spin echo (FSE) images (repetition time/echo time [TR/TE] of 4050/135 ms; field of view of 12 cm, section thickness of 0.3 cm, section gap of 0 cm, matrix of 256 × 256, 3 signal averages). Typical acquisition times were 5–6 min.
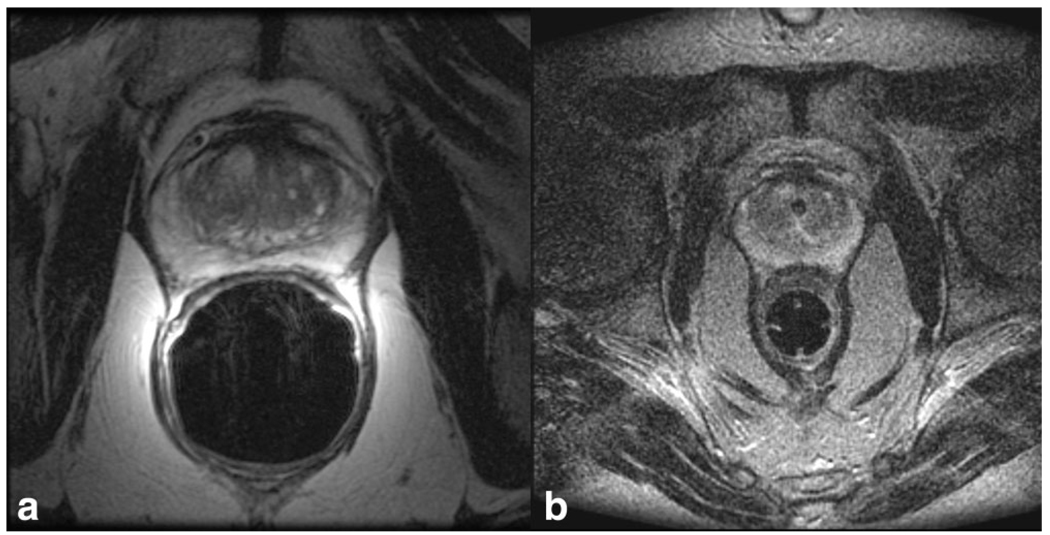
Intraoperative imaging was performed in the open-configuration 0.5T MR scanner (Signa SP, GE Healthcare, Milwaukee, WI) (11). Each patient was placed in the lithotomy position to facilitate prostate brachy-therapy by means of a perineal template. The template was fixed in place by a rectal obturator (2-cm diameter). T2-weighted FSE images (TR/TE 6400/100 ms, field of view of 24 cm, section thickness of 0.5 cm, section gap of 0 cm, matrix 256 × 256, 2 signal averages) were acquired in the scanner using a flexible external pelvic wrap-round coil, with typical acquisition times of 6 min. An example of pre- and intra-operative images is presented in Figure 1.
Figure 1.
a,b: An example of preoperative axial T2-weighted image using an endorectal coil in a 1.5T closed bore scanner (a), and intraoperative axial T2-weighted image in a low-field 0.5T interventional scanner (b).
Image Registration
We used the 3D Slicer (http://www.slicer.org/) and ImageJ (http://rsbweb.nih.gov/ij/) software packages for image registration. Both are free open source software for visualization and image-based computing. A specific module for 3D Slicer has been developed to automate the process of rigid and then nonrigid registration of image data sets. A rigid registration step was necessary before applying nonrigid registration, to center the pre- and intraoperative image series around the prostate.
The nonrigid registration was done using a B-Spline method, a well-known nonrigid registration method (12). This method works by placing a uniformly spaced three dimensional (3D) grid over the volume to be registered, the lattice points of which act as control points for the displacement of tissue. Displacement of each control point results in a deformation of the region surrounding the point, in a way that makes the overall deformation as smooth as possible. The displacement is easily calculated using cubic polynomials. For our application, we used a lattice with 5 × 5 × 5 control points. For our 3D application, this allowed for 125 × 3 = 375 degrees of freedom in the determination of the B-Spline registration. This choice in the number of control points was made based on experimenting with trial cases; we found that a 5 × 5 × 5 grid provided a reasonable amount of control and acceptable processing time. To guide the registration, a measure of image similarity based on mutual information (MI) was used (13). Informally, this similarity measure is maximized when the information in one image volume “best explains” the information content of the other. This measure has a precise meaning within the field of information theory. The B-Spline registration algorithm thus adjusts the control points until the resulting deformation aligns the image volumes in a way that maximizes the information–theoretic similarity between them.
The registration method involved the following steps: (i) both images were cropped for size using the ImageJ software to make the field of view the same on each image, (ii) the 1.5T preoperative images were rigidly registered with the 0.5T intraoperative images using the corresponding function in 3D Slicer, and (iii) the 1.5T images were nonrigidly registered with the 0.5T images using the nonrigid B-Spline registration module in 3D Slicer. We then measured the required processing time and core computation time for the registration procedure.
Accuracy Assessment
The volume of the prostate gland in the preoperative MRI was measured using 3D Slicer and the distribution of the prostate volume was compared with similar studies previously reported in the literature (14–16).
Evaluating the accuracy of a registration method is difficult because there is no gold standard with which to compare it. Therefore, accuracy or validation of the nonrigid registration method was evaluated both qualitatively and quantitatively in several different ways to include both local and global measures of registration success. A qualitative measure included a checker-board comparison between the registered image datasets. Quantitative methods involved a 3D registration error map based on contour alignment between datasets using Hausdorff distance (HD). We also obtained the Dice similarity coefficient (DSC) between the segmented registered prostate images, measured the MI value of the images before and after registration, and finally calculated fiducial registration error (FRE) among common anatomical landmarks.
The registered images from the two data sets were merged for comparison in a checkerboard pattern, to qualitatively evaluate the difference between the pre-and intraoperative images (17,18). The contour of the total gland (TG), central gland (CG), and peripheral zone (PZ) of the prostate were drawn by an experienced radiologist (S.O.) under the supervision of a senior radiologist (C.T.). The radiologists then assessed the continuity of the contours of the different regions of the prostate gland in the checkerboard images.
A 3D distance map indicating the HD were created to assess the accuracy of registration across the prostate gland surface, and investigate if the registered images correctly separate the prostate gland from the surrounding critical structure so that the radiation dose to the gland does not damage those surrounding structures. A similar assessment were performed in the related study (19). The HD between two images were measured by extracting the edges of the segmented prostate from the intraoperative MR images and from the registered preoperative MR images and calculating the distance from each point on the contour to the nearest point on the contour of the registered prostate (20). Perfect alignment between contours will give a HD of zero at every point.
The DSC was calculated to evaluate the coincidence between the segmented registered preoperative prostate volume and the segmented intraoperative one (8,21,22). The DSC is clinically relevant to MRI-guided brachytherapy because the standard radiation plan in the brachytherapy suggests higher dose on the peripheral zone to avoid overdose to urethra causing urinary complications (23). Thus, having a correct volumetric map of peripheral zone in the fused (registered) images, i.e. higher value in DSC, enables more accurate and safer dose planning in the brachytherapy. For two images with segmented volumes (voxels) S1 and S2, the DSC is defined as the ratio of the volume of their intersection to their total volume as,
where Volume (S1) and Volume (S2) represent the volumes of the segmented tissue region in each image and Volume (S1∩S2) is the volume of the intersection between the two. The DSC has a value of 1 for perfect agreement of S1 and S2, and 0 when there is no overlap. A DSC value greater than 0.7 has been reported to indicate good match between two regions in assessing image segmentation (24). In our study, the DSC values were calculated between different substructures of the prostate in the preoperative images and those same structures in the intraoperative images, first using only rigid registration and then using both rigid followed by nonrigid registration. The DSC values calculated in both cases were quantified and compared to measure any improvement. To this end, the radiologists segmented TG, CG, and PZ in all images for each data set.
Calculating the MI value of the images before and after registration gives another measure of image alignment. In this study, we used the implementation by Mattes et al to calculate the MI values to evaluate the similarity between the registered datasets (25,26). The MI value is negative and a smaller value indicates better similarity.
The validation using FRE consists of identifying well-defined corresponding anatomical features on registered images and measuring the 3D distance between them. The FRE is more directly linked to the accuracy of dose planning in the substructure of the prostate. Based on a related study, it was decided to verify the registration accuracy of the urethra (27). It is internal to the prostate, and passes through the prostate from base to apex. We selected the urethra at the level of the verumontanum, where the ejaculatory duct opens into the urethra (28,29). Perfect agreement corresponds to an FRE of 0 mm. Unlike DSC, FRE tells the level of accuracy in a more intuitive manner using the metric system, in millimeters.
Summary statistics include the mean and standard deviation of the DSC values for TG, CG, PZ, MI, and FRE with and without nonrigid image registration were applied. A one-sided paired t-test was then performed to analyze the DSC values MI and FRE to see if the proposed nonrigid registration method significantly improved the level of image matching between pre- and intraoperative images compared with rigid registration.
In addition, a grid motion model was made to estimate the local deformation of the prostatic tissue during registration. A volume made of 3D grid lines was constructed, and then the same deformable transform calculated in step 3 of the nonrigid registration method was applied to the grid lines. Finally, the deformed grid lines were superimposed on the deformed preoperative image, and the anteroposterior (AP) distances of the deformed grid lines were assessed providing a 3D deformation vector field of the registered prostate image.
RESULTS
Processing time for the registration, including preprocessing, was less than 5 min. The mean core computation time for nonrigid registration in 3D Slicer was 24.3 s (range, 15–51 s) on a mid-end scientific computer (CPU, XEON 4 × 2.8 GHz; RAM memory, 8 GB). The mean prostatic volume measured in preoperative MRI was 40.0 mL (range, 21.0–107.5 mL), which is similar to that found in other studies (14–16). Figure 2 illustrates the checkerboard images and the prostate images before and after nonrigid registration was performed. The checkerboard display shows improved matching of TG, CG, and PZ structures. Figure 3 shows a 3D distance map indicating the HD at each contour point that is a measure of the magnitude of misalignment of the contours of the registered prostates. Larger errors were shown at the anterior part of the base and bilateral side of the apex (Figure 3).
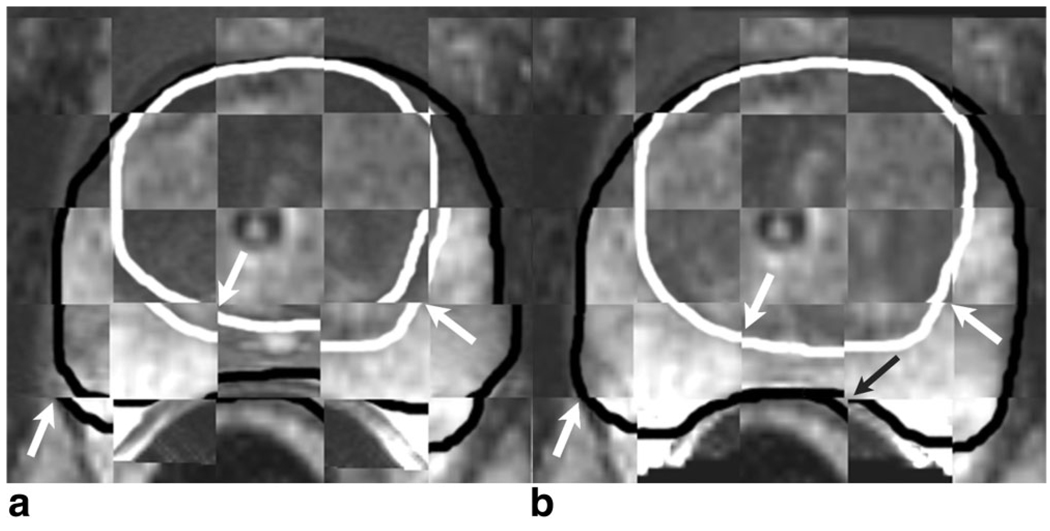
Figure 2.
a,b: In the checkerboard display between the preoperative image (before deformation) and intraoperative image, a black line indicates a contour of the total gland, whereas a white line indicates a contour of the central gland. a: The contours showed discrepancy at white arrows. b: In a checkerboard display between deformed preoperative and intraoperative images, the contours match closely after registration has been performed. A slight discrepancy remained at the posterior part of the prostate (black arrow).
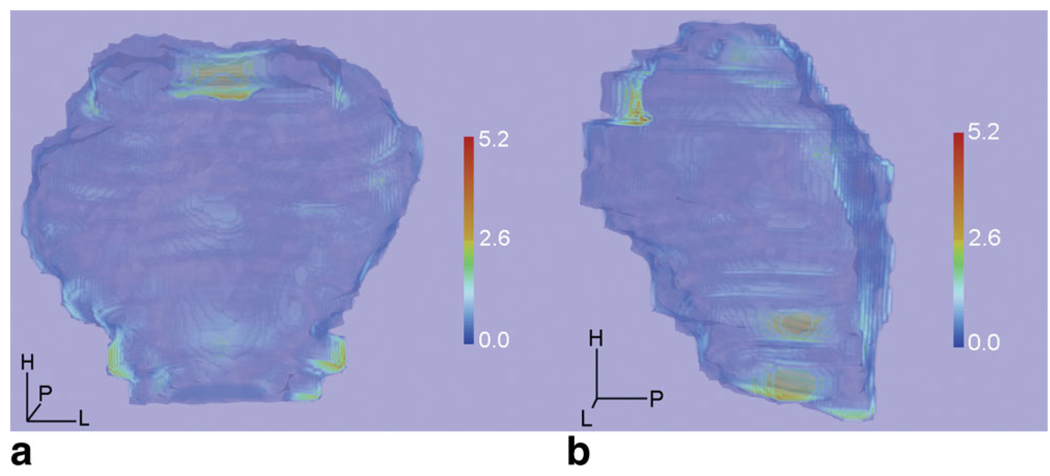
Figure 3.
a,b: An example of a 3D distance map obtained from measured Hausdorff distance (HD) at each contour point of the registered prostates. The colored distance map indicating the HD was superimposed on the prostate model based on intraoperative MR image (blue). Red indicates a larger distance between intraoperative and deformed preoperative MR images, and dark blue means smaller distances. Discrepancy was less than 2.6 mm in almost all surface of the prostate. Larger errors up to 5.2 mm were shown at the anterior part of the base and bilateral side of the apex. The 3D distance map was obtained using the ParaView (http://www.paraview.org/) software package, which is a free open source software for visualization and image-based computing.
Mean DSC values and standard deviations for both rigid and nonrigid registration are presented in Table 1, which shows a significant improvement of the coefficient with nonrigid registration (P < 0.05). Mean MI value was −0.19 ± 0.07. For the FRE, the verumontanum at the urethra was observed in only 10 of the cases. We therefore computed the metric on only these 10 cases, leading us to conclude that mean FRE at urethra was 2.3 ± 1.8 mm. A box-plot of MI and FRE are shown in Figure 4, in which nonrigid registration showed significant improvement of matching accuracy compared with rigid registration (P < 0.05).
Table 1.
Mean DSC Values and Standard Deviations for Both Rigid and Nonrigid Registration*
| DSC Rigid |
DSC Nonrigid |
|||
|---|---|---|---|---|
| Region | Mean | SD | Mean | SD |
| TG | 0.82 | ±0.07 | 0.91 | ±0.02 |
| CG | 0.78 | ±0.08 | 0.89 | ±0.03 |
| PZ | 0.64 | ±0.13 | 0.79 | ±0.07 |
When comparing the underlying mean DSC values for rigid and nonrigid registration for all areas of the prostate, one-sided t-tests showed that the DSC values improved with statistical significance (P < 0.05) in the total prostate gland and its substructures.
DSC = Dice similarity coefficient; TG = total gland; CG = central gland; PZ = peripheral zone; SD = standard deviation.
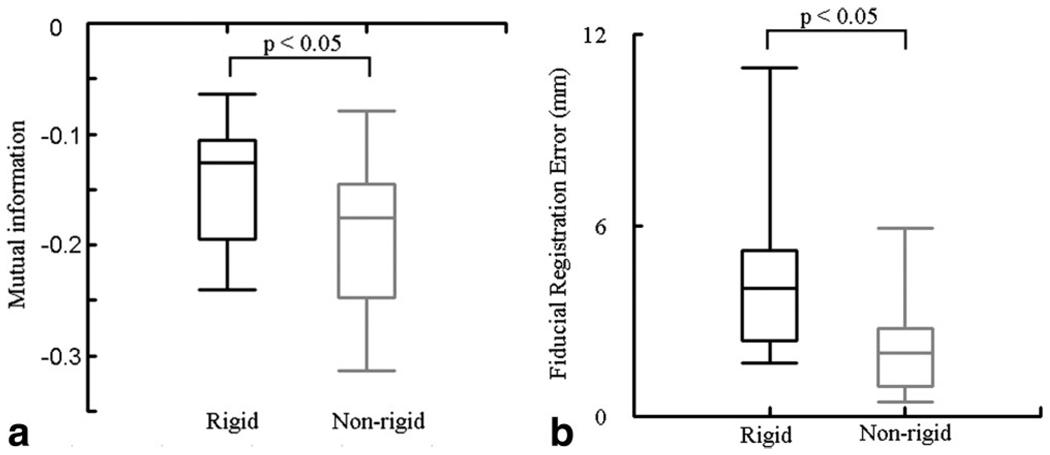
Figure 4.
a,b: Boxplot of the mutual information (MI) metric and the fiducial registration error (FRE) for rigid and nonrigid registrations. Median values with lower and upper quartile (box) and 1.5 times the interquartile range (whiskers) were shown in each figure. The mean MI value for nonrigid registration (−0.19) was significantly better than rigid registration (−0.14) (P < 0.05), and the mean FRE (2.3 mm) was significantly better than for rigid registration (4.5 mm) (P < 0.05).
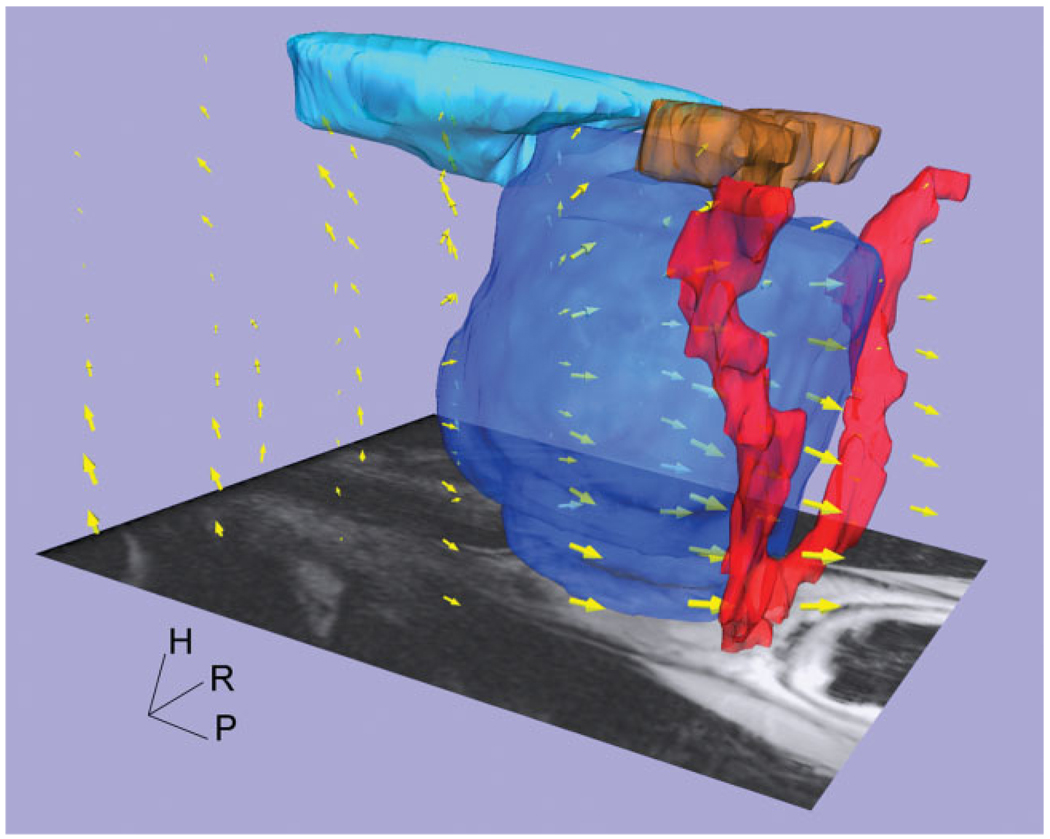
Figure 5 shows the deformed preoperative image superimposed with grid lines indicating the magnitude of the deformation in the different areas of the gland. The AP distance of the grid lines at PZ was larger than at CG at mid-gland and apex levels of the prostate, indicating a larger deformation at PZ. Furthermore, in the 3D deformation vector field, increased deformation was observed at the posterior part of the prostate (Figure 6).
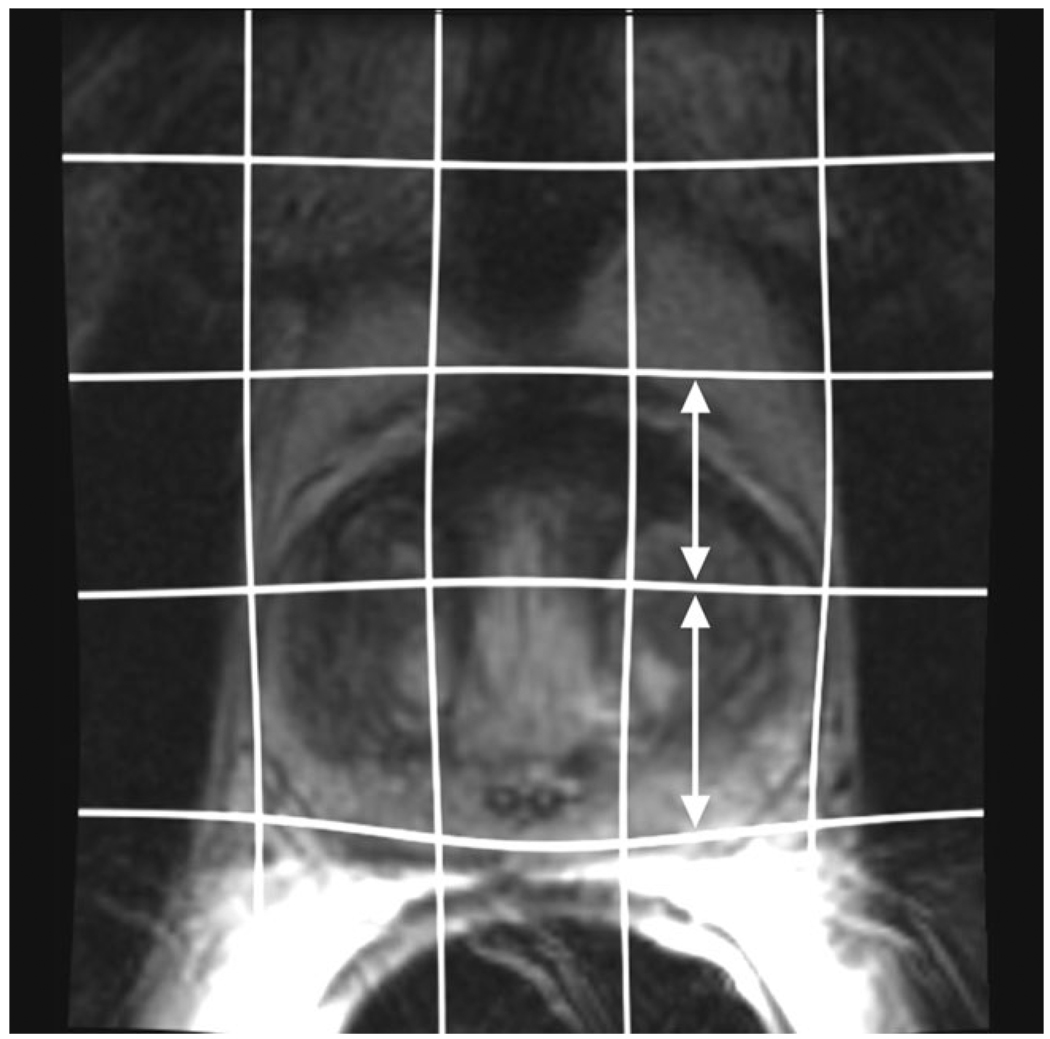
Figure 5.
Preoperative axial image after nonrigid registration has been applied. The grid lines indicating the image deformation are superimposed. Anteroposterior distance of the grid line near the peripheral zone was larger than near the central gland (white arrows), showing increased deformation in the area.
Figure 6.
Three-dimensional representation of deformation vector field, shown with normal pelvic anatomical models (prostate [dark blue], bladder [light blue], seminal vesicle [brown], and neurovascular bundle [red]). Yellow arrows’ size describes magnitude of deformation. Larger deformations are present at the posterior part of the prostate. The 3D deformation vector map was obtained using the ParaView software package.
DISCUSSION
We tested and validated the feasibility of intensity-based nonrigid registration between 1.5T preoperative MRI acquired with an ER coil and 0.5T intraoperative MRI acquired without the ER coil during MRI-guided brachytherapy of the prostate. Using B-Spline registration available in the open source software package 3D Slicer, these data series were successfully registered in clinically acceptable time frames. In the qualitative evaluation, the checkerboard display showed improved matching between images. In quantitative evaluation, DSC values were improved when nonrigid registration was applied in the TG, CG, and PZ, as were FRE and MI metrics.
Our experiments suggest that MI, the similarity measure used in the B-Spline registration algorithm, is likely to be maximized when the DSC value of the segmented total prostate gland is at or near its maximum theoretical value. In this sense, it seems the two measures are correlated. In fact, the mean DSC values obtained in this study are comparable to those obtained in Bharatha’s segmentation-based nonrigid registration method (0.94, 0.86, and 0.76 for TG, CG, and PZ, respectively) (8). This demonstrates that equally good results can be obtained using an intensity-based method, which requires only a fraction of the time.
The deformed grid lines and 3D deformation vector field showed increased deformation in the peripheral zone of the prostate compared with the central zone, due to the presence of the ER coil in the preoperative MRI of the prostate, which was then removed during the intervention (7). The calculated DSC values in the CG and PZ regions are higher than in Bharatha’s segmentation-based method. This is because our B-Spline technique is combined with optimization of likelihood functions that measure the similarity between the intensities within the two images, providing a better coincidence in regions with large deformation for the CG and PZ.
To show clinical relevancy of the registration, we measured the HD at each point of the registered prostate’s surface, and created a 3D color map indicating the HD, which showed only small errors, quantified at less than 2.6 mm in almost every part of the prostate except at the anterior part of the base and bilateral side of the apex with a maximum distance of 5.2 mm. The largest error present in the base is indicative of the large amount of deformation, the prostate undergoes due to the presence of a urethral balloon catheter during the intervention. The registration method, based on B-Spline deformation, and thus presenting limited deformation, struggles to deform the preoperative MR image of the prostate the required amount in this area. The large error at the bilateral side of the apex also could be found in the FRE measurement in our study. This finding also coincides with other studies investigating deformable registration of the prostate (30–32). In those studies, maximum registration errors were from 4.4 mm to 7 mm at the apex. We give rise to the view, as did the study by Reynier et al (30), that an accuracy of up to 3 mm at mid-gland is clinically acceptable in providing preoperative MRI for treatment planning in brachytherapy. There remains an argument whether current accuracy of deformable registration near the apex and base is acceptable in brachytherapy planning.
The processing time of our method took less than 5 min, including preprocessing. By not requiring manual segmentation of the prostate gland, our processing time is considerably shorter than in other studies (8,10,33–35). We believe that 5 min is short enough to make registration feasible during a clinical intervention.
Several limitations are evident in this study. First, it involved analyzing only 16 cases in a retrospective manner. Further validation is needed to demonstrate that this method can be applied during clinical interventions in a robust manner and without considerable interruption to the workflow. Second, a DSC value was calculated to evaluate the coincidence between the deformed preoperative image and the intraoperative one, which requires manual segmentation of specific regions of the prostate. The segmentation process is subject to potential error from interobserver variability (36). A method that does not require segmentation would be less susceptible to observer error.
In this report, we retrospectively tested the feasibility of our intensity-based method on patient data. Future work involves applying registration during the actual clinical brachytherapy procedure and to quantify the impact this information has on clinical outcomes. The ultimate goal of such methodologies is to identify the target in a highly accurate manner using registered preoperative images and to deliver highly localized therapy under real-time guidance.
In conclusion, this study provides new and important information on the intensity-based nonrigid registration between preoperative high-field MR image of the prostate and intraoperative MR images obtained during brachytherapy. The registration method significantly improved the matching between images and could be performed within a clinically acceptable time frame.
ACKNOWLEDGMENTS
The authors acknowledge advisory comments from Dr. Sachio Kuribayashi, Department of Radiology, Keio University School of Medicine. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Contract grant sponsor: NIH; Contract grant numbers: 5U41RR019703, 5P01CA067165, 1R01CA111288, 5U54EB005149, 5R01CA109246, 3P41RR013218. Part of this study was funded by Intelligent Surgical Instruments Project of METI, Japan.
REFERENCES
- 1.D’Amico AV, Cormack R, Tempany CM, et al. Real-time magnetic resonance image-guided interstitial brachytherapy in the treatment of select patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:507–515. doi: 10.1016/s0360-3016(98)00271-5. [DOI] [PubMed] [Google Scholar]
- 2.Van Gellekom MPR, Moerland MA, Battermann JJ, Lagendijk JJW. MRI-guided prostate brachytherapy with single needle method - a planning study. Radiother Oncol. 2004;71:327–332. doi: 10.1016/j.radonc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Zangos S, Eichler K, Thalhammer A, et al. MR-guided interventions of the prostate gland. Minim Invasive Ther Allied Technol. 2007;16:222–229. doi: 10.1080/13645700701520669. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Cormack R, Kumar S, Tempany CM. Real-time magnetic resonance imaging-guided brachytherapy in the treatment of selected patients with clinically localized prostate cancer. J Endourol. 2000;14:367–370. doi: 10.1089/end.2000.14.367. [DOI] [PubMed] [Google Scholar]
- 5.Landis DM, Schultz D, Cormack R, et al. Acute urinary retention after magnetic resonance image-guided prostate brachytherapy with and without neoadjuvant external beam radiotherapy. Urology. 2005;65:750–754. doi: 10.1016/j.urology.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Tempany C, Straus S, Hata N, Haker S. MR-guided prostate interventions. J Magn Reson Imaging. 2008;27:356–367. doi: 10.1002/jmri.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose M, Bharatha A, Hata N, et al. Quantitative MR imaging assessment of prostate gland deformation before and during MR imaging-guided brachytherapy. Acad Radiol. 2002;9:906–912. doi: 10.1016/s1076-6332(03)80460-9. [DOI] [PubMed] [Google Scholar]
- 8.Bharatha A, Hirose M, Hata N, et al. Evaluation of three-dimensional finite element-based deformable registration of pre- and intraoperative prostate imaging. Med Phys. 2001;28:2551–2560. doi: 10.1118/1.1414009. [DOI] [PubMed] [Google Scholar]
- 9.d’Aische AD, De Craene M, Haker S, et al. Improved non-rigid registration of prostate MRI. Med Image Comput Comput Assist Interv Miccai. 2004;7(Pt 1):845–852. [Google Scholar]
- 10.Wang H, Dong L, Lii MF, et al. Implementation and validation of a three-dimensional deformable registration algorithm for targeted rostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:725–735. doi: 10.1016/j.ijrobp.2004.07.677. [DOI] [PubMed] [Google Scholar]
- 11.Schenck JF, Jolesz FA, Roemer PB, et al. Superconducting open-configuration MR imaging system for image-guided therapy. Radiology. 1995;195:805–814. doi: 10.1148/radiology.195.3.7754014. [DOI] [PubMed] [Google Scholar]
- 12.Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 13.Wells WM, III, Viola P, Atsumi H, Nakajima S, Kikinis R. Multimodal volume registration by maximization of mutual information. Med Image Anal. 1996;1:35–51. doi: 10.1016/s1361-8415(01)80004-9. [DOI] [PubMed] [Google Scholar]
- 14.Jeong CW, Park HK, Hong SK, Byun SS, Lee HJ, Lee SE. Comparison of prostate volume measured by transrectal ultrasonography and MRI with the actual prostate volume measured after radical prostatectomy. Urol Int. 2008;81:179–185. doi: 10.1159/000144057. [DOI] [PubMed] [Google Scholar]
- 15.Mir MC, Planas J, Raventos CX, et al. Is there a relationship between prostate volume and Gleason score? BJU Int. 2008;102:563–565. doi: 10.1111/j.1464-410X.2008.07696.x. [DOI] [PubMed] [Google Scholar]
- 16.Brock KK, Sharpe MB, Dawson LA, Kim SM, Jaffray DA. Accuracy of finite element model-based multi-organ deformable image registration. Med Phys. 2005;32:1647–1659. doi: 10.1118/1.1915012. [DOI] [PubMed] [Google Scholar]
- 17.El Naqa I, Yang D, Apte A, et al. Concurrent multimodality image segmentation by active contours for radiotherapy treatment planning. Med Phys. 2007;34:4738–4749. doi: 10.1118/1.2799886. [DOI] [PubMed] [Google Scholar]
- 18.Schreibmann E, Xing L. Narrow band deformable registration of prostate magnetic resonance imaging, magnetic resonance spectroscopic imaging, and computed tomography studies. Int J Radiat Oncol Biol Phys. 2005;62:595–605. doi: 10.1016/j.ijrobp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Smith WL, Lewis C, Bauman G, et al. Prostate volume contouring: a 3D analysis of segmentation using 3DTRUS, CT, and MR. Int J Radiat Oncol Biol Phys. 2007;67:1238–1247. doi: 10.1016/j.ijrobp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Mount DM, Netanyahu NS, Le Moigne J. Efficient algorithms for robust feature matching. Pattern Recognit. 1999;32:17–38. [Google Scholar]
- 21.Dice LR. Measures of the amount of ecologic association between Species. Ecology. 1945;26:297–302. [Google Scholar]
- 22.Alterovitz R, Goldberg K, Pouliot J, et al. Registration of MR prostate images with biomechanical modeling and nonlinear parameter estimation. Med Phys. 2006;33:446–454. doi: 10.1118/1.2163391. [DOI] [PubMed] [Google Scholar]
- 23.Nag S, Beyer D, Friedland J, Grimm P, Nath R. American Brachy-therapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44:789–799. doi: 10.1016/s0360-3016(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 24.Zou KH, Warfield SK, Bharatha A, et al. Statistical validation of image segmentation quality based on a spatial overlap index - Scientific reports. Acad Radiol. 2004;11:178–189. doi: 10.1016/S1076-6332(03)00671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao M, Li T, Schreibmann E, Koong A, Xing L. Automated contour mapping with a regional deformable model. Int J Radiat Oncol Biol Phys. 2008;70:599–608. doi: 10.1016/j.ijrobp.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Mattes D, Haynor D, Vesselle H, Lewellyn TK, Eubank W. Nonrigid multimodality image registration. Proc SPIE. 2001;4322:1609–1620. [Google Scholar]
- 27.Fuller DB, Jin H, Koziol JA, Feng AC. CT-ultrasound fusion prostate brachytherapy: a dynamic dosimetry feedback and improvement method. A report of 54 consecutive cases. Brachytherapy. 2005;4:207–216. doi: 10.1016/j.brachy.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Hricak H, Dooms GC, McNeal JE, et al. MR imaging of the prostate gland: normal anatomy. AJR Am J Roentgenol. 1987;148:51–58. doi: 10.2214/ajr.148.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Curran S, Akin O, Agildere AM, Zhang J, Hricak H, Rademaker J. Endorectal MRI of prostatic and periprostatic cystic lesions and their mimics. AJR Am J Roentgenol. 2007;188:1373–1379. doi: 10.2214/AJR.06.0759. [DOI] [PubMed] [Google Scholar]
- 30.Reynier C, Troccaz J, Fourneret P, et al. MRI/TRUS data fusion for prostate brachytherapy. Preliminary results. Med Phys. 2004;31:1568–1575. doi: 10.1118/1.1739003. [DOI] [PubMed] [Google Scholar]
- 31.Susil RC, Menard C, Krieger A, et al. Transrectal prostate biopsy and fiducial marker placement in a standard 1.5T magnetic resonance imaging scanner. J Urol. 2006;175:113–120. doi: 10.1016/S0022-5347(05)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller DB, Jin H. Computed tomography-ultrasound fusion brachytherapy: description and evolution of the technique. Brachytherapy. 2007;6:272–279. doi: 10.1016/j.brachy.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Foskey M, Davis B, Goyal L, et al. Large deformation three-dimensional image registration in image-guided radiation therapy. Phys Med Biol. 2005;50:5869–5892. doi: 10.1088/0031-9155/50/24/008. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Dibiase SJ, Gullapalli R, Yu CX. Deformable image registration for the use of magnetic resonance spectroscopy in prostate treatment planning. Int J Radiat Oncol Biol Phys. 2004;58:1577–1583. doi: 10.1016/j.ijrobp.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 35.Fei B, Kemper C, Wilson DL. A comparative study of warping and rigid body registration for the prostate and pelvic MR volumes. Comput Med Imaging Graph. 2003;27:267–281. doi: 10.1016/s0895-6111(02)00093-9. [DOI] [PubMed] [Google Scholar]
- 36.Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;47:285–292. doi: 10.1016/s0167-8140(98)00021-8. [DOI] [PubMed] [Google Scholar]