Abstract
Chronic kidney disease is common after hematopoietic cell transplant. We prospectively measured urinary albumin to creatinine ratios (ACR) in 142 patients. Total (intact) monomeric albumin was determined by liquid chromatography of untreated urine samples collected weekly to day 100 after transplant. Albuminuria was defined as ACR (mg/g creatinine) >30 and proteinuria as ACR >300. Cox and logistic regressions analyses evaluated ACR as a risk factor for clinical events.
The prevalence of albuminuria at baseline, day 100 and 1 year was 37%, 64% and 50%, respectively. Proteinuria occurred in 4% of patients at baseline, 15% at day 100 and 4% at 1 year. Characteristics associated with albuminuria include age, gender, donor type, hypertension and sinusoidal obstruction syndrome. Albuminuria was associated with an increased risk of acute GVHD and bacteremia, but not acute kidney injury. Albuminuria at day 100 was associated with chronic kidney disease at 1 year (OR= 4.0; 95%CI 1.1–14.6). Non-relapse mortality risk was elevated (HR=6.8; 95%CI 1.1–41.5) among patients with overt proteinuria at day 100.
Albuminuria occurs frequently after HCT and correlates with acute GVHD, bacteremia, hypertension and progression of renal disease. Proteinuria at day 100 is associated with an 6-fold increased risk of non-relapse mortality by one year post transplant.
INTRODUCTION
Albuminuria, defined as a urine albumin to urine creatinine ratio (ACR) of 30 to 300 mg/g creatinine, is thought to be a marker of endothelial dysfunction and inflammation, reflecting a systemic endothelial injury that affects multiple organs including the kidney. Newer work postulates that albuminuria results from tubular dysfunction in the trafficking and degradation of albumin 1,2. In both the general population and in cohorts of patients with specific diseases (hypertension, diabetes, inflammatory bowel disease and critically ill patients), albuminuria is a marker for adverse events and poor outcomes. For example, in patients with hypertension and diabetes, albuminuria is a risk factor for cardiovascular morbidity and mortality 3,4. In the general population, the presence of albuminuria predicts the later development of cardiovascular disease and the development of de novo chronic kidney disease 5. Albuminuria can be detected in patients with active inflammatory bowel disease and improves when the disease is quiescent 6. In the ICU setting, albuminuria is associated with multi-organ failure and an increased mortality 7. Both diabetic and non-diabetic patients with albuminuria are at increased risk of developing overt proteinuria and chronic kidney disease 3,8–10.
To better understand the pathophysiology of CKD in patients who have received hematopoietic cell transplants, we prospectively measured urine albumin:creatinine ratios in patients undergoing their first transplant. The process of hematopoietic cell transplant and its complications frequently affect tubular and glomerular function leading to both acute and chronic kidney disease. Epidemiologic studies have identified risk factors for kidney disease in HCT patients; however, little is known about mechanisms of injury, early markers of renal injury, or factors that lead to progression of CKD in transplant patients. In the data reported here, we determined the prevalence of albuminuria and its clinical correlates, including outcomes related to development of CKD.
PATIENTS AND METHODS
Patient Selection
Patients over the age of 2 years undergoing their first hematopoietic cell transplant (HCT) during 2003–2006 participated in this study if they met the following eligibility criteria: a) a baseline creatinine at screening within the limits of normal for age in children and <1.3 mg/dL in women and < 1.5 mg/dL in men, b) not currently taking angiotensin receptor blockers or angiotensin converting enzyme inhibitors, and c) no history of diabetes mellitus; d) signed consent forms approved by our Institutional Review Board.
Technique of HCT
All patients undergoing HCT received a preparative regimen followed by infusion of donor hematopoietic cells. The day of stem cell infusion is termed “day zero,” by convention. Myeloablative regimens were typically cyclophosphamide-based (with either total body irradiation (TBI) or targeted busulfan) for allogeneic transplants; autologous graft recipients received a combination regimens of busulfan or cyclophosphamide with other agents. Non-myeloablative preparative regimens consisted of fludarabine and low-dose TBI 11. The kidneys are not shielded during TBI. Allogeneic graft recipients received prophylaxis against acute GVHD with immunosuppressive drugs, usually cyclosporine or tacrolimus plus methotrexate 12. Prophylaxis for infections included acyclovir for patients seropositive for herpes simplex virus, trimethoprim/sulfamethoxazole to prevent Pneumocystic jivecii infection, oral fluconazole or itraconazole for prophylaxis of candidal infection, and pre-emptive ganciclovir for cytomegalovirus disease among viremic patients 13–15.
Specimen Collection and Analytical Methods
Urine samples were collected from patients at baseline, (prior to any conditioning therapy), weekly through day 100, and monthly through the first year after transplant. Urine was collected between the hours of 8–10 a.m., immediately placed on ice, separated into 2 mL aliquots and frozen at −80 degrees Celsius until time of analysis. Total (intact) monomeric albumin (immuno-reactive plus immuno-unreactive) was measured in aliquots of untreated urine samples with a Hewlett Packard Agilent 1100 high performance liquid chromatography (HPLC) system (Santa Clara, CA). The detection limit of the HPLC assay is <2 mg/dL and coefficient of variation at 100 mg/dL is 0.6%, and at 20 mg/dL, 0.3%. Albuminuria was defined as an ACR ≥30 mg/g creatinine in a urine sample and overt proteinuria as an ACR ≥300 mg/g creatinine.
Definition of Clinical Variables
Baseline characteristics analyzed included age, gender, diagnosis, type of transplant, conditioning therapy, total body irradiation (TBI) yes vs. no. The patient’s weight, blood pressure, medications, and temperature were recorded in the morning on the day samples were collected. The occurrence of aGVHD, grades 0–1 and grades 2–4; sepsis; sinusoidal obstruction syndrome (SOS); acute kidney injury (AKI); and hypertension in the first 100 days after transplant were also noted. AKI was defined as a doubling of baseline serum creatinine within the first 100 days after transplant 16. Hypertension was defined as two blood pressure readings >130/80 mm Hg or the need for anti-hypertensive medications. Bacteremia was defined as the presence of one positive blood culture. The diagnosis of SOS was based on previously published criteria, as applied to data obtained during days 0 through 20 (patient weights, total serum bilirubin, imaging studies, patient symptoms and concomitant medical events) 17. One of us (GBM) reviewed this material blinded to albuminuria results. Chronic kidney disease (CKD) was defined as a GFR <60 mL/min/1.73 m2 at 1 year post-transplant. Baseline clinical and demographic data of the 142 patients included in this analysis are presented in Table 1.
Table 1.
Patient demographic and transplant characteristics (N=142)
| Patient Characteristic | Frequency (%) | |
|---|---|---|
| Age at transplantation | <20 | 13 (9%) |
| (years) | 20–39 | 28 (20%) |
| 40–59 | 78 (55%) | |
| ≥60 | 23 (16%) | |
| Median age = 47 | ||
| Gender: | Female | 52 (37%) |
| Male | 90 (63%) | |
| Race: | African American | 6 (4%) |
| Caucasian | 114 (80%) | |
| Hispanic | 7 (5%) | |
| Other | 13 (9%) | |
| Not available | 2 (1%) | |
| Diagnosis | Acute myeloid leukemia | 52 (37%) |
| Myelodysplastic syndrome | 29 (20%) | |
| Chronic myeloid leukemia | 19 (13%) | |
| Non-Hodgkins lymphoma | 13 (9%) | |
| Acute lymphocytic leukemia | 8 (6%) | |
| Multiple Myeloma | 6 (4%) | |
| Chronic lymphocytic leukemia | 3 (2%) | |
| Aplastic anemia | 5 (4%) | |
| Other | 7 (5%) | |
| Donor type | Allogeneic | 59 (42%) |
| Autologous | 17 (12%) | |
| Unrelated donor | 66 (46%) | |
| Conditioning regimen | Reduced intensity regimens (200cGy*) | 37 (26%) |
| Myeloablative: CY‡/TBI† 12–13.5 Gy | 25 (18%) | |
| Myeloablative: BU⋄, CY only | 61 (43%) | |
| Other myeloablative regimens | 19 (13%) | |
| Baseline serum creatinine (mg/dL) | Median (5th to 95th percentile) | 0.9 (0.5–1.3) |
cGy: centigray;
CY: cyclophosphamide;
BU: busulfan;
TBI: total body irradiation
Statistical Methods
Albumin:creatinine ratios (ACR, mg/g creatinine) were summarized overall and then by patient demographic variables (age, gender) and clinical characteristics (TBI, donor type, hypertension) at baseline, day 35 posttransplant and day 100 posttransplant. Wilcoxon rank sum tests were used to compare ACR values between patients with different demographic and clinical characteristics at each of those time points. Kruskal-Wallis testing was used for the comparisons by donor type, because there are more than two donor categories. The day 35 value was the ACR measurement obtained closest to day 35 in a window of ±10 days. The day 100 value was the ACR measurement obtained closest to day 100 in the window from day 70 to day 100.
Cox regression analysis was used to evaluate ACR as a risk factor for later development of HCT complications including aGVHD II–IV, bacteremia and acute kidney injury. Both baseline ACR, as a fixed covariate, and ACR as a time-dependent covariate were evaluated as risk factors. For the time-dependent model, subjects lacking pretransplant ACR measurements enter the “at risk” cohort at the time of their first post-transplant ACR observation. A unique model was created for each of the clinical outcomes, aGVHD, AKI and sepsis. For the outcome aGVHD, variables adjusted for in the multivariable model were age (<40 and ≥ 40 years), donor type (unrelated vs. allogeneic; autologous transplants excluded because they are not at risk for aGVHD) and intensity of conditioning therapy (myeloablative vs. reduced intensity). For the outcome AKI, the multivariable model was adjusted for age, acute GVHD grade II-IV, SOS and amphotericin use. In this analysis, acute GVHD and amphotericin use were modeled as time dependent covariates. For the outcome bacteremia, the model included adjustments for age and intensity of the conditioning regimen. The adjustment variables included in each model were chosen a priori based on previous studies and knowledge of risk factors related to the clinical events. Although SOS is considered a transplant complication, it begins during administration of the conditioning regimen prior to transplant, and thus could not be analyzed via the same Cox modeling approach. Instead, ACR summaries for subjects with and without SOS are provided in Table 3.
Table 3.
Albumin to Creatinine Ratios (ACR) at Selected Time Points by Clinical Characteristics
| Patient/transplant characteristic | baseline ACR: median (range) | day 35 ACR: median (range) | day 100ACR: median (range) |
|---|---|---|---|
| Age <40 | 14 (2, 424) | 41 (5, 5644) | 29 (1, 1490) |
| Age ≥40 | 24 (2, 1346) | 80 (10, 16948) | 70 (9, 3367) |
| p=0.05 | p=0.11 | p=0.04 | |
| Female | 20 (2, 1346) | 92 (10, 2863) | 91 (7, 3367) |
| Male | 20 (2, 517) | 59 (5, 16948) | 45 (1,1490) |
| p=0.54 | p=0.32 | p=0.04 | |
| No TBI | 19 (2, 517) | 63 (7, 16948) | 59 (3, 3367) |
| TBI | 27 (3, 1346) | 98 (5, 2863) | 53 (1, 1490) |
| p=0.19 | p=0.31 | p=0.98 | |
| Autologous donor | 32 (8, 291) | 41 (13, 106) | 25 (9, 88) |
| Allogeneic (related) donor | 15 (2, 1346) | 59 (5, 5644) | 45 (1, 3367) |
| Allogeneic HLA-matched unrelated donor | 23 (2, 517) | 96 (8, 16948) | 90 (3, 1490) |
| p=0.12 | p=0.04 | p=0.006 | |
| No SOS* | 19 (2, 1346) | 60 (5, 16948) | 45 (1, 3367) |
| SOS | 40 (8, 319) | 231 (23, 5644) | 129 (21, 822) |
| p=0.02 | p=0.0006 | p=0.01 | |
| No hypertension | 20 (2, 517) | 52 (5, 5644) | 30 (1, 3367) |
| Hypertension | 22 (3, 1346)‡ | 121 (8, 16948)** | 100 (3, 1490) |
| p=0.67 | p=0.0001 | p=0.0005 |
Defined as having an elevated BP measurement recorded between study enrollment and the start of conditioning regiment (day minus 7).
Defined as having an elevated BP measurement on at least two consecutive measurements taken between transplant and the timepoint specified in the respective column.
SOS: Sinusoidal obstruction syndrome
Among subjects who survived to day 100, Kaplan-Meier curves were used to illustrate the differences in subsequent survival through 1 year post-transplant based on the day 100 ACR value. For non-relapse mortality, cumulative incidence curves stratified by day 100 ACR level were provided instead. Cumulative incidence methodology was needed because of the presence of the competing risk of relapse for this endpoint. Cox regression analysis was used to estimate the relative risk associated with day 100 ACR category and subsequent non-relapse mortality and survival. Models were adjusted for acute GVHD grade (0/I vs. grade II vs. grade III/IV), bacteremia before day 100 and chronic GVHD. Logistic regression was also used to evaluate the association between albuminuria at day 100 and chronic kidney disease status at 1 year post-transplant adjusted for age >40 years, chronic GVHD, hypertension, and diabetes. Chronic GVHD was included in this model as an indicator of cyclosporine use.
RESULTS
Study Demographics
One hundred forty-two subjects supplied urine samples for ACR measurement between baseline and day 100. The median number of samples provided was 10 (range 1–15). The median age at transplant was 47 years (Table 1). Sixty-three percent of patients were male and 80% were Caucasian. The two most common reasons for transplant were AML (acute myeloid leukemia) (37%) and MDS (myelodysplastic syndrome) (20%). Fifty-nine (42%) patients received an allogeneic transplant from a related donor and 66 (46%) were from an HLA-matched unrelated donor. The median serum creatinine at baseline was 0.9 mg/dL and 90% of baseline observations fell between 0.5–1.3 mg/dL. After HCT, 42 patients developed AKI, 48 patients developed bacteremia within the first 100 days, and 20 patients developed SOS. Eighty-two people were diagnosed with grade II–IV acute GVHD prior to day 100. Twenty-seven patients developed CKD at 1 year post-transplant. There were 5 deaths on or before d100 and 28 deaths from day 100 to 1 year post-transplant.
Prevalence of Albuminuria
The prevalence of albuminuria was 37% at baseline and 64% by day 100 post-transplant (Table 2). Overt proteinuria was seen in 4% of patients at baseline and in 15% of patients by day 100 post-transplant. Among 46 patients who were evaluated at 1 year, 50% had albuminuria and 4% had overt proteinuria.
Table 2.
Descriptive summaries of albumin to creatinine (ACR) levels at key time points after HCT
| Time point | # subjects with data available at these times | ACR Median (Range) | % subjects with ACR ≥30 | % subjects with ACR ≥300 |
|---|---|---|---|---|
| Pre HCT | 94 | 20 (1.5, 1346) | 37% | 4% |
| Day 35* | 133 | 68 (4.7, 16948) | 76% | 17% |
| Day 100‡ | 121 | 57 (1.4, 3367) | 64% | 15% |
| 1 year † | 46 | 31 (4.4, 494) | 50% | 4% |
Observation closest to day 35 within a window of +/− 10 days.
Observation closest to day 100 within the window d70-d100.
Observation closest to day 365 within a window of +/− 90 days.
Clinical Associations with Albuminuria from Day 0 to Day 100
ACR levels were generally higher in patients over 40 years of age. Men and women had similar ACR levels at baseline, but at day 100 more women had albuminuria (Table 3). With regard to donor type, data for post-transplant time points clearly showed increasing ACR levels across time with increasing HLA disparity between donor and recipient. There was no association between ACR level and TBI as part of the conditioning regimen. Patients with sinusoidal obstruction syndrome (SOS) exhibited dramatically higher ACR levels post-transplant, rising within the first week after transplant. To a lesser degree, the difference in ACR in patients who later developed SOS and those who did not was apparent even at baseline, before starting the conditioning regimen and before SOS developed. Forty percent of patients who developed SOS had been transplanted for MDS compared to 17% of patients who did not develop SOS (p=0.02). There was no difference in age, gender and baseline hypertension between the SOS and non-SOS groups. Post-transplant hypertension was associated with higher ACR at both the day 35 and day 100 time points. At 1 year post-transplant, 57% of patients with albuminuria had hypertension compared to 33% of patients without albuminuria (p=0.12).
Albuminuria as a Predictor of Clinical Events Before Day 100
In this analysis, albuminuria at baseline and prior to the diagnosis of clinical events was used. Albuminuria was associated with an increased risk of developing acute GVHD and bacteremia, but not AKI (Table 4). To further explore the potential clinical applications of this finding, the risk differential estimated using the baseline ACR observation alone was contrasted with a predictive model that incorporated the serial ACR observations as a time-dependent variable in the Cox regression. The presence of albuminuria at baseline was associated with a 2-fold increased risk of bacteremia during the first 100 days post-transplant. Of the 43 subjects who were diagnosed with bacteremia prior to day 100 and who had sufficient ACR data to be included in the Cox regression analyses, 24 subjects had already evinced an ACR measurement >30 prior to transplant, either at baseline or during conditioning. For the remaining bacteremia cases without elevated ACR prior to transplant, 18 developed ACR>30 a median of 19 days prior to their bacteremia diagnosis (interquartile range 14 – 33 days) and one subject did not develop ACR>30 before being diagnosed with bacteremia.
Table 4.
Cox regression analysis of albuminuria as a predictor of clinical events in the first 100 days after transplant: relative risk and 95% confidence intervals
| Outcome event | Albuminuria term incorporated as: | Relative risk (95% CI) | Adjusted Relative Risk (95% CI) |
|---|---|---|---|
| aGVHD* grade II–IV | Time-varying | 1.9 (1.1–3.3) | 1.8 (1.0–3.2) |
| Baseline only | 1.7 (1.0–3.0) | 1.6 (0.9–2.8) | |
| AKI† (2X baseline creatinine) | Time-varying | 1.0 (0.5–1.9) | 0.7 (0.3–1.8) |
| Baseline only | 1.4 (0.6–3.3) | 1.1 (0.4–3.2) | |
| Bacteremia | Time-varying | 9.3 (2.2–38.5) | 10.0 (2.4–42.0) |
| Baseline only | 2.4 (1.1–5.2) | 2.3 (1.0–5.1) |
aGVHD: acute graft vs. host disease;
AKI: acute kidney injury
Albuminuria at baseline was not significantly associated with the development of acute GVHD, after adjusting for other factors, but including albuminuria as a time-varying term yielded a statistically significant relative risk of 1.8 (95% CI 1.0–3.2). Of 78 subjects who were diagnosed with aGVHD grade II–IV prior to day 100 and who had sufficient ACR data to be included in the Cox regression analyses, 35 subjects already had showed an ACR measurement >30 prior to transplant, either at baseline or during conditioning. For the remaining aGVHD cases without elevated ACR prior to transplant, 34 developed ACR>30 a median of 18 days prior to their aGVHD grade II–IV diagnosis (interquartile range 10 – 33 days) and nine subjects did not develop ACR>30 before being diagnosed with aGVHD.
Albuminuria was not associated with AKI, regardless of whether it was modeled as a fixed baseline term or time-varying term.
Albuminuria and 1-Year Outcomes
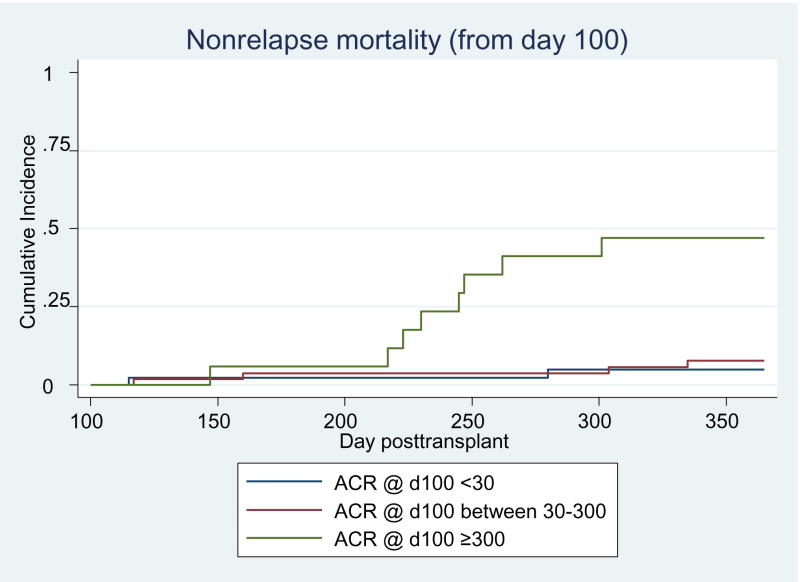
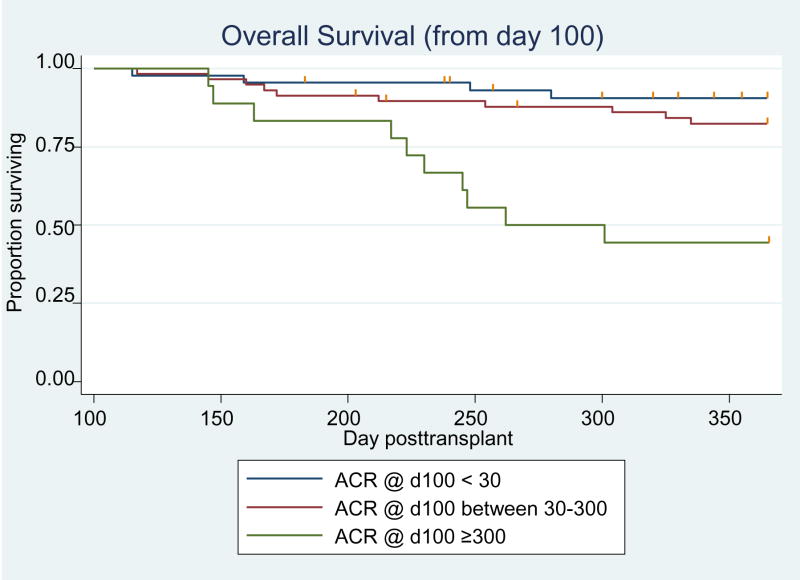
We evaluated how ACR information from the acute phase of transplant care relates to the risk for adverse clinical outcomes from the time of discharge from our transplant center to 1 year post transplant. In univariable analysis, overt proteinuria at day 100 was strongly associated with increased risk of non-relapse mortality (HR=12.8; 95% CI 2.7–60.6) and overall mortality (HR=7.7; 95%CI 2.4–24.7) (Table 5). In multivariable analyses, subjects with overt proteinuria at day 100 were at an almost 7-fold increased risk of non-relapse mortality (HR=6.8; 95%CI 1.1–41.5) through 1 year post-HCT after adjustment for acute GVHD grade, bacteremia before day 100 and chronic GVHD. The elevation in overall mortality risk with over proteinuria at day 100 was less pronounced (HR=2.4; 95%CI 0.6–0.7) after adjustment for the same set of factors. Subjects with day 100 ACR between 30 and 300 mg/g creatinine, did not exhibit increased risk for non-relapse or overall mortality (Figures 1 and 2). Among surviving patients, 17% (5/29) of those with day 100 ACR <30, had CKD at 1 year compared to 48% (21/44) of patients with an ACR ≥ 30 who had CKD at 1 year post-transplant (p<0.01). An ACR ≥30 mg/g creatinine at day 100 was associated with a four-fold increased risk of CKD (OR= 4.0; 95%CI 1.1–14.6) at 1 year post-transplant after adjusting for chronic GVHD, hypertension, diabetes and age.
Table 5.
Cox regression analysis of nonrelapse mortality from d100 through 1year posttransplant: relative risk and 95% confidenc intervals
| Day 100 ACR* Category | Relative risk (95% CI) (unadjusted) | Relative risk (95% CI) (adjusted)+ |
|---|---|---|
| d100 ACR below 30 | n/a (baseline category) | |
| d100 ACR between 30–300 | 1.7 (0.3, 9.3) | 1.4 (0.2, 7.9) |
| d100 ACR above 300 | 12.8 (2.7, 60.6) | 6.8 (1.1, 41.5) |
ACR: albumin to creatinine ratio
Adjusted for acute GVHD grade II, grade III/IV, bacteremia and chronic GVHD
Figure 1.
Cumulative incidence curves of non-relapse mortality from day 100 to 1 year post-HCT displayed by degree of albuminuria. N=43 for ACR <30; N=54 for ACR 30–300; N=17 for ACR ≥300.
Figure 2.
Kaplan-Meier curves of overall survival from day 100 to 1 year post-HCT by degree of albuminuria. N=44 for ACR<30; N=58 for ACR 30–300; N=18 for ACR ≥ 300.
DISCUSSION
This is the first study to describe the relationship of albuminuria to clinical outcomes in the HCT population. We found that albuminuria occurs commonly after HCT, with 94% of patients developing it at some point within the first 100 days post-transplant. Though some patients have resolution of their albuminuria, in the majority of patients it persists, with 64% of patients having albuminuria at day 100 post-transplant and 50% of patients having albuminuria at 1 year. Among patients alive at 1 year after HCT, 4% had overt proteinuria. Post-transplant ACR elevations are more pronounced among patients undergoing an allogeneic transplant, especially those with an unrelated donor, compared to autologous HCT. Post-transplant complications associated with higher ACR include elevated blood pressure, liver disease with portal hypertension and bacteremia. TBI, as part of the conditioning therapy, was not associated with ACR.
Compared to other populations where albuminuria can take years to develop, albuminuria occurs rapidly after HCT, being present before day 100 in the majority of patients. In a study of pediatric patients, 15% of children developed albuminuria after conditioning therapy and prior to infusion of their cells 18. On a follow-up study of these patients 1–2 years later, the albuminuria had normalized 19. The presence of albuminuria in the diabetic population is suggestive of glomerular pathology and is associated with progressive loss of kidney function over time. Recent research has focused on the direct role of albuminuria and proteinuria on progression of CKD [reviewed in 20]. It is thought that albuminuria triggers the local release of pro-inflammatory cytokines and chemokines that recruit macrophages and other inflammatory cells into the interstitium causing fibrosis and progression of CKD. Similar findings are present in the HCT population as patients with albuminuria at day 100 are more likely to progress to CKD Stage 3 by 1 year post-transplant.
The association between albuminuria and clinical events post-transplant and the presence of albuminuria prior to the development of acute GVHD and bacteremia suggests that it is a marker of systemic as well as local (within the kidney) inflammation and vascular injury. Our finding of increased ACR levels in allogeneic transplant recipients (who are more likely to develop GVHD) compared to autologous transplant recipients further supports the association of albuminuria and GVHD. GVHD can cause direct endothelial injury via cytotoxic T-lymphocytes in addition to a pro-inflammatory cytokine profile 21. Tissue destruction by acute GVHD does not require alloantigen expression on target epithelium for cellular cytotoxicity; injury can be mediated by inflammatory cytokines 22. Both minimal change nephrotic syndrome and membranous nephropathy post-HCT are thought to be manifestations of GVHD in the kidney 23,24. In minimal change nephrotic syndrome following HCT, increased production of TNF-α and IFN-gamma by donor T-cells was related to the development of nephrotic syndrome; the lack of cellular infiltrates on biopsy suggested that the glomerular injury was secondary to cytokine production stimulated by alloantigens at extrarenal sites25. In membranous nephropathy, subepithelial immune-complex deposition is present along the glomerular basement membrane. There is also some evidence suggesting that albuminuria is caused by defective tubular trafficking and degradation of albumin 1,2. It may be that in HCT patients, inflammatory damage to the tubules from GVHD leads to albuminuria and the latter is a manifestation of renal GVHD. These data support the hypothesis that albuminuria is a sub-clinical marker of GVHD that can be detected before the disease becomes clinically manifest in the gut, skin and liver. These data also suggest that the renal vasculature, glomerulus and perhaps the proximal tubular cells are affected by the GVHD process making the kidney another target organ in acute GVHD.
Among diabetics, hypertensive patients, and the general population, the presence of albuminuria is associated with an increased risk of cardiovascular morbidity and mortality even after adjustment for other known risk factors 3,4,26,27. We found an increased risk of non-relapse mortality and decrease in overall survival in patients with proteinuria at day 100 post-transplant independent of acute GVHD grade, chronic GVHD and bacteremia. Though the cause of death is often multifactorial in the HCT population (many patients die from infections, complications of GVHD and/or relapse of their primary disease), our data does not address the mechanisms by which the presence of proteinuria confers an additional risk in this patient population.
The findings in this study have clinical implications. First, the incidence of renal injury as detected by the presence of albuminuria is higher than previous studies have found using serum creatinine and estimated GFR. The presence of albuminuria may be a marker of both systemic and renal inflammation and vascular injury perpetuated by bacteremia and/or acute GVHD. These data suggest that both inflammation and vascular injury may play a role in the development of renal disease in this patient population. Second, albuminuria may serve as a useful clinical marker of patients at increased risk for late complications post-transplant, specifically the development of CKD at 1 year post-transplant. We recommend that patients have routine urinalyses done at baseline and day 100 to identify patients at high risk for these adverse, long-term outcomes. Though we have shown an increased risk of CKD in patients with albuminuria at day 100 and an increased risk of non-relapse mortality in patients with overt proteinuria, it is not known whether intervention to reduce the albuminuria or proteinuria will prove beneficial in this patient population. There is evidence in patients with diabetes and albuminuria that treatment with an angiotensin converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB) slows the progression of CKD 28,29. Extrapolating from the studies in the diabetic population, we speculate that ACEI and ARB would be useful in patients with albuminuria after HCT. However, clinical trials using an ACEI or ARB to treat HCT patients with albuminuria at day 100 are needed to correlate albuminuria with progression to end-stage renal disease and to determine whether or not interventions to reduce albuminuria and proteinuria will impact outcomes in the HCT population. A recent study using captopril after engraftment in 55 HCT patients, demonstrated a trend towards improvement in 1 year GFR and serum creatinine compared to untreated patients 30.
In summary, albuminuria occurs frequently in the HCT population and is associated with subsequent acute GVHD, bacteremia and progression of renal disease. Overt proteinuria at day 100 is associated with a 6-fold increased risk of non-relapse mortality and a decrease in overall survival in patients 1 year after HCT. In the HCT population, renal injury exists early after transplant, is not always reflected by changes in serum creatinine, and impacts long-term outcomes.
Acknowledgments
We thank Dr. Wayne Comper and AusAm Biotech for their help with the analysis of the urine samples for ACR measurements. This research was supported by the following: National Institutes of Health (NIDDK) K23 DK63038, American Society of Nephrology/Renal Physicians Association Health Scholars Grant and the National Kidney Foundation Young Investigators Grant (SH), by National Institutes of Health Grant M01-RR-00037 of the University of Washington General Clinical Research Center and CA18029 (GBM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 2.Hilliard L, Russo L, Comper W. Insights into the Relationaship Between Hypertension and Albuminuria. Current Hypertension Reviews. 2007;3:29–37. [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 4.Segura J, Campo C, Ruilope LM. Effect of proteinuria and glomerular filtration rate on cardiovascular risk in essential hypertension. Kidney Int Suppl. 2004:S45–49. doi: 10.1111/j.1523-1755.2004.09212.x. [DOI] [PubMed] [Google Scholar]
- 5.Verhave JC, Hillege HL, Burgerhof JG, Navis G, de Zeeuw D, de Jong PE. Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol. 2003;14:1330–1335. doi: 10.1097/01.asn.0000060573.77611.73. [DOI] [PubMed] [Google Scholar]
- 6.Mahmud N, Stinson J, O’Connell MA, et al. Microalbuminuria in inflammatory bowel disease. Gut. 1994;35:1599–1604. doi: 10.1136/gut.35.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abid O, Sun Q, Sugimoto K, Mercan D, Vincent JL. Predictive value of microalbuminuria in medical ICU patients: results of a pilot study. Chest. 2001;120:1984–1988. doi: 10.1378/chest.120.6.1984. [DOI] [PubMed] [Google Scholar]
- 8.Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 9.Mann JF, Gerstein HC, Yi QL, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. J Am Soc Nephrol. 2003;14:641–647. doi: 10.1097/01.asn.0000051594.21922.99. [DOI] [PubMed] [Google Scholar]
- 10.Pinto-Sietsma SJ, Janssen WM, Hillege HL, Navis G, De Zeeuw D, De Jong PE. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000;11:1882–1888. doi: 10.1681/ASN.V11101882. [DOI] [PubMed] [Google Scholar]
- 11.Carella AM, Champlin R, Slavin S, McSweeney P, Storb R. Mini-allografts: ongoing trials in humans. Bone Marrow Transplant. 2000;25:345–350. doi: 10.1038/sj.bmt.1702204. [DOI] [PubMed] [Google Scholar]
- 12.Chao NJ. Pharmacology and Use of Immunosuppressive Agents After Hematopoietic Cell Transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2. Malden: Blackwell Science; 1999. pp. 176–185. [Google Scholar]
- 13.Brown JM. Fungal Infections After Hematopoietic Cell Transplant. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplant. 3. Malden: Blackwell Science; 2004. pp. 683–700. [Google Scholar]
- 14.Wingard JR. Bacterial Infections. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplant. 3. Malden: Blackwell Science; 2004. pp. 665–682. [Google Scholar]
- 15.Zaia JA. Cytomegalovirus Infections. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 3. Malden: Blackwell Science; 2004. pp. 701–726. [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Patzer L, Hempel L, Ringelmann F, et al. Renal function after conditioning therapy for bone marrow transplantation in childhood. Med Pediatr Oncol. 1997;28:274–283. doi: 10.1002/(sici)1096-911x(199704)28:4<274::aid-mpo6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Patzer L, Ringelmann F, Kentouche K, et al. Renal function in long-term survivors of stem cell transplantation in childhood. A prospective trial. Bone Marrow Transplant. 2001;27:319–327. doi: 10.1038/sj.bmt.1702763. [DOI] [PubMed] [Google Scholar]
- 20.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 21.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 22.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 23.Silva S, Maximino J, Henrique R, et al. Minimal change nephrotic syndrome after stem cell transplantation: a case report and literature review. J Med Case Reports. 2007;1:121. doi: 10.1186/1752-1947-1-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006;1:685–694. doi: 10.2215/CJN.00380705. [DOI] [PubMed] [Google Scholar]
- 25.Seconi J, Watt V, Ritchie DS. Nephrotic syndrome following allogeneic stem cell transplantation associated with increased production of TNF-alpha and interferon-gamma by donor T cells. Bone Marrow Transplant. 2003;32:447–450. doi: 10.1038/sj.bmt.1704151. [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 27.Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: An angiographic study. Am J Kidney Dis. 1999;34:918–925. doi: 10.1016/S0272-6386(99)70051-X. [DOI] [PubMed] [Google Scholar]
- 28.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 29.Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. Bmj. 2004;329:828. doi: 10.1136/bmj.38237.585000.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen EP, Irving AA, Drobyski WR, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]