Abstract
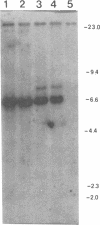
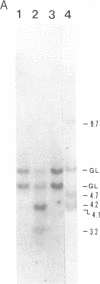
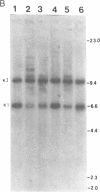
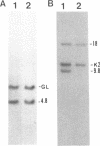
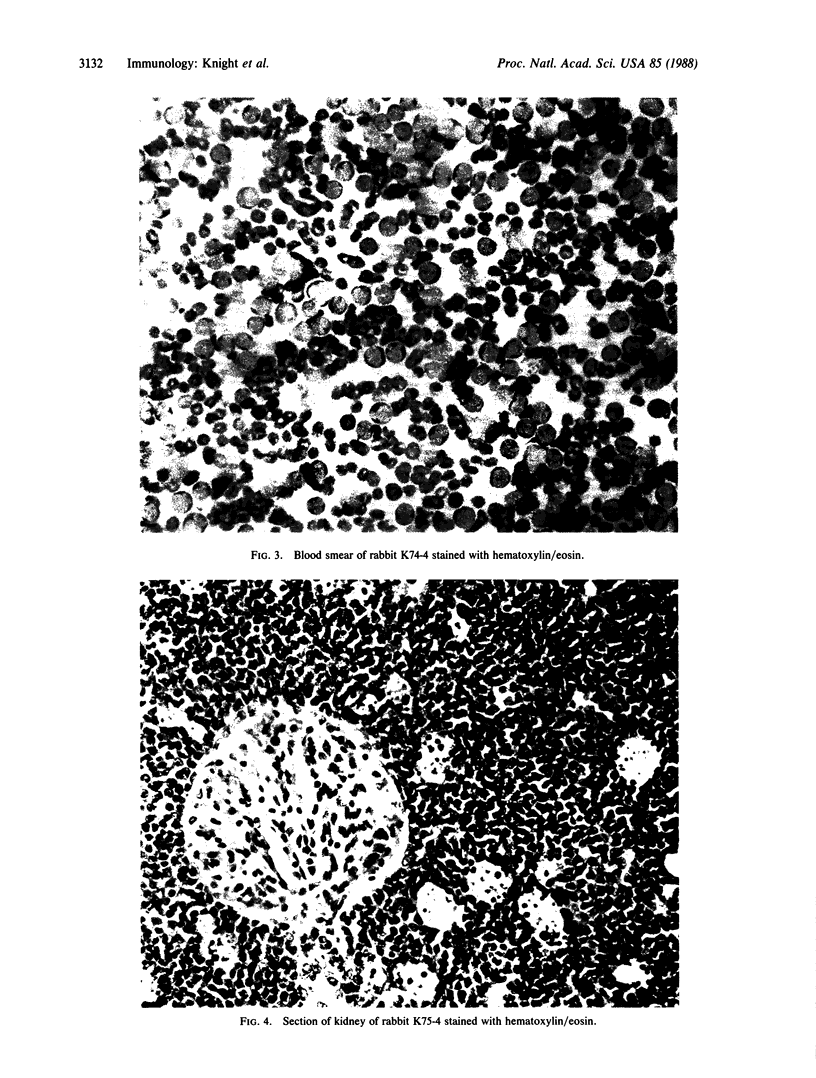
Transgenic rabbits with the rabbit c-myc oncogene fused with the rabbit immunoglobulin heavy chain enhancer region (E mu) DNA were developed by microinjecting pronuclei of single cell zygotes with the gene construct and implanting the microinjected eggs into pseudopregnant females. At age 17-20 days, 3 of 21 offspring born to seven females were found to have peripheral blood leukocyte counts of greater than 100,000 per mm3. Histology analyses showed extensive lymphocytic infiltration in the liver and kidney, indicating that these rabbits had a malignant lymphocytic leukemia. Genomic DNA analyses of thymus and peripheral blood lymphocytes revealed that the leukemic rabbits were transgenic and that both immunoglobulin heavy and kappa light chain genes were rearranged in the leukemic cells; thus, the leukemic cells are of B-cell lineage. One to four heavy and light chain gene rearrangements were observed, suggesting that the B-cell tumors were oligoclonal. Stable tissue culture cell lines from the bone marrow and peripheral blood of one of the transgenic rabbits have been developed. The development of B-cell leukemias in rabbits with the E mu-myc transgene contrasts with the development of B-cell lymphomas in mice carrying a similar transgene. The lymphomas in mice develop in adults and are monoclonal in origin. The leukemias in rabbits develop in juveniles, less than 3 weeks after birth, and appear oligoclonal in origin. The leukemias seem to develop in rabbit at a specific stage of development, and we suggest that a normal developmental signal may be involved in the oncogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada K., Magram J., Raphael K., Radice G., Lacy E., Costantini F. Specific expression of a foreign beta-globin gene in erythroid cells of transgenic mice. 1985 Mar 28-Apr 3Nature. 314(6009):377–380. doi: 10.1038/314377a0. [DOI] [PubMed] [Google Scholar]
- Cloyd G. G., Johnson G. R. Lymphosarcoma with lymphoblastic leukemia in a New Zealand white rabbit. Lab Anim Sci. 1978 Feb;28(1):66–69. [PubMed] [Google Scholar]
- Collins J. J., Black P. H., Strosberg A. D., Haber E., Bloch K. J. Transformation by simian virus 40 of spleen cells from a hyperimmune rabbit: evidence for synthesis of immunoglobulin by the transformed cells. Proc Natl Acad Sci U S A. 1974 Feb;71(2):260–262. doi: 10.1073/pnas.71.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L., Max E. E. Structural analysis of a rabbit immunoglobulin kappa 2 J-C locus reveals multiple deletions. Nucleic Acids Res. 1983 Dec 20;11(24):8877–8890. doi: 10.1093/nar/11.24.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie J. W., Bostock D. E., Walden N. B. Lymphoblastic leukaemia in a rabbit: a case report. Lab Anim. 1980 Jan;14(1):49–51. doi: 10.1258/002367780780943169. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Meier H., Crary D. D., Myers D. D., Norberg R. F., Laird C. W. Lymphosarcoma in the rabbit: genetics and pathology. J Natl Cancer Inst. 1970 Oct;45(4):719–729. [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Pursel V. G., Rexroad C. E., Jr, Wall R. J., Bolt D. J., Ebert K. M., Palmiter R. D., Brinster R. L. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985 Jun 20;315(6021):680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hannam-Harris A. C., Gordon J., Smith J. L. Immunoglobulin synthesis by neoplastic B lymphocytes: free light chain synthesis as a marker of B cell differentiation. J Immunol. 1980 Nov;125(5):2177–2181. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Knight K. L., Burnett R. C., McNicholas J. M. Organization and polymorphism of rabbit immunoglobulin heavy chain genes. J Immunol. 1985 Feb;134(2):1245–1250. [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Leder A., Pattengale P. K., Kuo A., Stewart T. A., Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986 May 23;45(4):485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Lobel S. A., Knight K. L. The role of rabbit Ia molecules in immune functions as determined with the use of an anti-Ia monoclonal antibody. Immunology. 1984 Jan;51(1):35–43. [PMC free article] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987 Mar 27;235(4796):1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Messing A., Hammer R. E., Pinkert C. A., Brinster R. L. Elastase I promoter directs expression of human growth hormone and SV40 T antigen genes to pancreatic acinar cells in transgenic mice. Cold Spring Harb Symp Quant Biol. 1985;50:399–409. doi: 10.1101/sqb.1985.050.01.050. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Shani M. Tissue-specific expression of rat myosin light-chain 2 gene in transgenic mice. Nature. 1985 Mar 21;314(6008):283–286. doi: 10.1038/314283a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Storb U., O'Brien R. L., McMullen M. D., Gollahon K. A., Brinster R. L. High expression of cloned immunoglobulin kappa gene in transgenic mice is restricted to B lymphocytes. Nature. 1984 Jul 19;310(5974):238–241. doi: 10.1038/310238a0. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]