Abstract
Under conditions of nutrient stress, cells switch to a survival mode catabolizing cellular and tissue constituents for energy. Proline metabolism is especially important in nutrient stress because proline is readily available from the breakdown of extracellular matrix (ECM), and the degradation of proline through the proline cycle initiated by proline oxidase (POX), a mitochondrial inner membrane enzyme, can generate ATP. This degradative pathway generates glutamate and α-ketoglutarate, products that can play an anaplerotic role for the TCA cycle. In addition the proline cycle is in a metabolic interlock with the pentose phosphate pathway providing another bioenergetic mechanism. Herein we have investigated the role of proline metabolism in conditions of nutrient stress in the RKO colorectal cancer cell line. The induction of stress either by glucose withdrawal or by treatment with rapamycin, stimulated degradation of proline and increased POX catalytic activity. Under these conditions POX was responsible, at least in part, for maintenance of ATP levels. Activation of AMP-activated protein kinase (AMPK), the cellular energy sensor, by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), also markedly upregulated POX and increased POX-dependent ATP levels, further supporting its role during stress. Glucose deprivation increased intracellular proline levels, and expression of POX activated the pentose phosphate pathway. Together, these results suggest that the induction of proline cycle under conditions of nutrient stress may be a mechanism by which cells switch to a catabolic mode for maintaining cellular energy levels.
Keywords: proline oxidase, nutrient stress, rapamycin, AMPK
Recent research has emphasized metabolism and bioenergetics and their role in complex diseases such as cancer. However these studies have mainly focused on the pathways for glycolysis and oxidative phosphorylation (Dang & Semenza 1999; Fox et al. 2005). Alternative metabolic pathways are also important especially under conditions of nutrient limitation since they can contribute in maintaining bioenergetics for survival (Pan & Mak 2007). Proline metabolism can be particularly important during nutrient stress because proline is readily available from the breakdown of extracellular matrix (ECM), and its degradation results in ATP generation (Yeh & Phang, 1988). The first step in degradation of proline is its conversion to Δ1-pyrroline-5-carboxylate (P5C) which is catalyzed by proline oxidase (POX), a.k.a. proline dehydrogenase, a mitochondrial inner membrane enzyme (Fig. 1) (Phang, 1985). This conversion of proline to P5C results in the generation of electrons which are donated to mitochondrial electron transport through flavine adenine dinucleotide (FAD) to generate ATP. P5C thus formed can be transported to the cytosol and reduced back to proline by the enzyme P5C reductase. This cycling of proline and P5C through the proline cycle (Phang, 1985) forms a redox couple which interacts with the NADPH/NADP+ redox couple and forms a metabolic interlock with the pentose phosphate pathway (Hagedorn & Phang, 1983). Further, P5C is in equilibrium with its tautomeric form i.e. glutamic-γ-semialdehyde, which is degraded to glutamate by P5C dehydrogenase and can enter the TCA cycle. Alternately it can be converted to ornithine to enter the urea cycle. Thus proline metabolism is closely linked to various pathways that are important in redox regulation and bioenergetics.
Fig. 1. Schematic Representation of proline metabolism.
The first step in the degradation of proline (PRO) is its conversion to pyrroline-5-carboxylate (P5C), by proline oxidase (POX). P5C can be reduced back to proline by P5C-reductase (P5CR) or it can be converted to glutamate by the enzyme, pyrroline-5-carboxylate dehydrogenase (P5CD) and can enter the TCA cycle or alternately it can be converted to ornithine by ornithine aminotransferase (OAT) and can enter the urea cycle.
Proline oxidase has been demonstrated to play a role in apoptosis; a screening study identified PRODH (the gene encoding POX) as a p53-induced gene (Polyak et al., 1997). Earlier work in our laboratory has demonstrated that cytotoxic agents induce POX and hyperexpression of POX in cancer cells was sufficient to initiate apoptosis due to its ability to generate reactive oxygen species (ROS) (Donald et al., 2001; Ferri & Kroemer, 2001; Liu et al., 2005; Pandhare et al., 2006; Liu et al., 2008). The cycling of P5C and proline between mitochondria and cytosol can transfer reducing potential which contributes to ATP generation. However, when there is cytotoxic stress, this process results in the generation of ROS. The role of POX-induced ROS in apoptosis is well documented, but there have been no studies showing the significance of ATP generation by proline metabolic pathway especially under conditions of nutrient or energy stress that forces cells to conserve energy and activate catabolic energy generating processes. This could be particularly relevant in the context of the tumor microenvironment. Since rapidly growing tumor cells are deficient in circulating nutrients as well as hypoxic as they grow beyond their blood supply; they alter their metabolism and switch to a catabolic mode. Cellular constituents are degraded for producing energy as a temporary survival mechanism. The degradation of proline could be one of the survival mechanisms, since proline metabolism is linked to various pathways important in cellular bioenergetics. We considered that proline could play a role because unlike other amino acids proline has a unique structure. It lacks the primary amino group and therefore cannot be metabolized by transamination or decarboxylation. Instead a distinct set of enzymes have evolved for the metabolism of proline (Phang, 1985). Further, these enzymes are differentially localized in cells. Thus, proline metabolism can be activated under stress conditions providing accessory mechanisms for bioenergetic and redox regulation. Furthermore, proline is one of the most abundant amino acids and more importantly it is a large constituent of proteins in the cellular microenvironment. The ECM is composed predominately of collagen (80%) and 25% of collagen residues are either proline or hydroxyproline (Ii et al., 2006). As the ECM is degraded by MMP, proline is released. Therefore, we hypothesized that depending on the metabolic mode; proline can either be used for protein synthesis or oxidized in the mitochondria for energy production. Due to the uniqueness of the proline cycle coupled to its ability to generate ATP during degradation of proline, we considered that the proline cycle may be important for survival under conditions of stress.
Materials and Methods
Reagents
Rapamycin and 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) were from EMD Biosciences (San Diego, USA). Dehydroproline was from Sigma. The phospho-AMPK (Thr-172) and AMPK antibodies were from Cell Signaling Technology (Beverly, MA); anti-actin antibody was from Sigma. The anti-POX antibody was prepared in the lab and has been described elsewhere (Liu et al., 2005)
Cell Culture
The colorectal cancer cell line RKO was obtained from ATCC and cultured in DMEM medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT), penicillin and streptomycin, 2 mM glutamine (Quality Biologicals, Gaithersburg, MD) at 37°C and 5% CO2. For experiments involving glucose deprivation RKO cells were cultured in DMEM containing dialyzed fetal bovine serum and L-glutamine without glucose and then supplemented with glucose at the concentrations indicated. Prior to resuspension in media with glucose at various concentrations, cells were washed twice in glucose-free media. The DLD-1 human colon cancer cells are from America Type Culture Collection (Manassas, VA, USA). The generation of the DLD-1 Tet-Off POX cells has been previously described (Donald et al., 2001). The cells were maintained in McCoy's 5A medium (Quality Biological, Gaithersburg, MD, USA) supplemented with 10 % fetal bovine serum (HyClone Laboratories, Logan, UT, USA) in the presence of 0.4 mg/ml G418, 0.25 mg/ml hygromycin B and 20 ng/ml doxycycline (DOX).
POX Enzyme Assay
After treatment cells were rinsed and scraped in cold PBS, pelleted, and resuspended in cold sucrose buffer [0.25 M sucrose, 3.5 mM Tris, and 1 mM EDTA (pH 7.4)]. Suspensions were then sonicated for 20 s at a setting of 25% (Branson Sonifier 450; Branson Ultrasonics Corp., Danbury, CT). Total protein was determined using the BCA protein assay (Pierce). Pyrroline-5-carboxylate (P5C) formed was detected using a spectrophotometric method. Briefly, P5C formed from the substrate proline was reacted with O-aminobenzaldehyde (OAB) and the resultant OAB-P5C complex was quantified. A 200 μl reaction mixture containing KPO4 0.1 M, pH 7.2, OAB 0.12 mg/ml, cytochrome c 0.012 mg/ml, proline 0.5 mM and cell extract containing 50 μg protein was incubated for 30 min at 37 °C. The reaction was terminated by addition of 20 μl of OAB (10 mg/ ml in 6N HCl). The samples were centrifuged and the absorbance of the supernatants was measured at 440 nm. All the reactions were performed in triplicates and proper protein controls were included for each measurement. A standard calibration curve was generated using P5C and the P5C formed (nM/min/μg protein) was determined.
Measurement of ATP by Luciferase Assay
ATP levels were measured by the luciferin/luciferase method using an ATP Determination Kit (Molecular Probes), following the instructions of the manufacturer. Cells were lysed with 1X Passive Lysis Buffer (Promega). 10 μL of lysate was added to the ATP Determination Kit reaction mix for a total volume of 110 μl. Luminescence was determined directly after the addition of the lysate to the reaction and was quantified in a 20/20 Luminometer (Turner Designs, Sunnyvale, CA). ATP concentrations in experimental samples were calculated by using an ATP standard curve generated from known concentrations of ATP.
Western Blotting
Cell lysates were prepared and quantified according to established methods. Equal amounts of cell lysates were electrophoresed on SDS-polyacrylamide gels and transferred to nitrocellulose membrane using a semi-dry blotter (Bio-Rad). Membranes were blocked using Tris-buffered saline with 5% nonfat milk (pH 8.0; Sigma). Blots were then probed with the appropriate primary antibody in blocking buffer overnight at 4 °C. Incubation with secondary anti-mouse or anti-rabbit IgG antibodies conjugated to horseradish peroxidase (1:2000) was performed at room temperature for 1 hour. All blots were washed in Tris-buffered saline with Tween 20 (pH 8.0; Sigma) and developed using the enhanced chemiluminescence (ECL) procedure (Amersham, Biosciences, Piscataway, NJ). Blots were routinely stripped by Encore Blot Stripping Kit (Novus Molecular, Inc., San Diego, CA) and reprobed with anti-actin monoclonal antibody (Sigma) (1:2000) to serve as loading controls.
Measurement of Intracellular Proline levels
RKO cells were grown in the appropriate medium after which cells were rinsed and scraped in cold PBS, pelleted, and resuspended in PBS. Suspensions were lysed by sonication and the cellular debris were removed by centrifugation (9000xg). The supernatants were transferred to a boiling water bath, and intracellular amino acids were extracted by boiling for 10 min. After centrifugation (5 min, 4°C, 15,000xg), the supernatant was free of proteins, and intracellular proline was determined as described (Bates, 1973; Chen et al., 2006). Briefly, 200 μl of the supernatant was incubated with 200 μl of acid-ninhydrin (0.25 g ninhydrin dissolved in 6 ml glacial acetic acid and 4 ml 6 M phosphoric acid) and 200 μl of glacial acetic acid for 1 h at 100°C. The reaction was stopped by incubation on ice, and the mixture was extracted with 400 μl toluene. The toluene phase was separated, and absorbance at 520 nm was used to determine the concentration of proline. All the reactions were performed in triplicates and a standard curve ranging in concentrations of 0.005- 0.1 mM proline was generated for determining proline concentration of samples.
Determination of MMP Activation by Gelatin Zymography
RKO were cells were treated in serum-free medium with different concentrations of glucose. The medium was concentrated using 10kDa Amicon Ultra-4 spin columns and equal protein loads were used for zymography. Gelatinase zymography was performed in 10% Novex pre-cast polyacrylamide gel (Invitrogen) in the presence of 0.1% gelatin. After electrophoresis, the gel was washed 5 times in zymogram wash buffer followed by 3 washes in incubation buffer and then incubated for 48 h at 37°C in incubation buffer before being stained with coomassie blue (G-250) for visualization of activation. After destaining (30% methanol, 1% formic acid), areas void of blue stain indicated areas of enzyme activity. Molecular markers were used to identify MMP2/9. Protein standards were run concurrently and approximate molecular weights were determined.
Measurement of Pentose Phosphate Pathway Activity
Cells were grown in 25-cm2 plastic flask in 4 ml of growth medium containing either Rapamycin (10 nmol/L) or different glucose concentrations as indicated. After treatment on the morning of the experiment, the medium was removed and replaced with 2 ml of Earle's balanced salt solution with specifically labeled 14C glucose substrate. The flasks were sealed with a serum stopper containing a plastic centerwell (Kontes), and incubated at 37°C for 2 hours. At the end of incubation, 0.3 ml of 6 N H2SO4 was injected through the stopper into the medium and the flasks were placed horizontally so that the acidified medium was in contact with the cell monolayer. After 10 minutes of acid treatment, the flasks were placed vertically and 0.3 ml of Hyamine was injected through the stopper into the well. The trapping of carbon dioxide by Hyamine was completed with the flasks in a Dubnoff shaker at room temperature for 45 minutes. The well containing the Hyamine and trapped CO2 was transferred into a scintillation vial with 12 ml of Aquasol, 0.2 ml of glacial acetic acid and the amount of radioactivity was quantitated by liquid scintillation spectrometry.
Results
Nutrient stress activates POX catalytic activity
To investigate whether POX plays any role in the nutrient sensing pathway we initiated this study by mimicking a starvation signal using the mTOR inhibitor rapamycin in RKO colorectal cancer cells. Since mTOR coordinates cell growth to the availability of extracellular nutrients and its inhibition mimics a condition of nutrient or energy stress. RKO cells were treated with rapamycin, a specific inhibitor of mTOR, at a concentration of 10 nmol/L and its effect on POX catalytic activity was measured. Rapamycin treatment caused a marked increase in POX catalytic activity (Fig. 2Aa, Fig. 2Ab). A time-dependent and dose-dependent increase in POX activity indicated that proline degradation may be playing a role under conditions when mTOR is inhibited.
Fig. 2. Nutrient stress induced by rapamycin treatment and glucose withdrawal induces POX.

(A)(a) RKO cells were treated with rapamycin (10nmol/L) or DMSO in control for various time periods and the POX catalytic activity was measured. Glucose concentration was 5 mM. The results are expressed as mean ± S.E. for three separate experiments. (b) RKO cells were exposed to medium containing rapamycin at various concentrations for 24 hours; the cell lysates were harvested, and the POX catalytic activity was determined. The results are expressed as mean ± S.E. for three separate experiments. **, p < 0.005 is for comparison of rapamycin-treated cells vs untreated cells. (B) RKO cells were exposed for 24 hours to medium with decreasing glucose concentrations from 5mM (control) to 0.01mM and; (a) the levels of endogenous POX protein were determined by Western blotting; the bands of POX were analyzed by densitometry. (b) the POX catalytic activity was measured and (c) RKO cells were treated with medium containing 0.05 mM glucose and collected at various time points. The cell lysates were harvested, and the POX catalytic activity was determined. The results are expressed as mean ± S.E. for three separate experiments. **, p < 0.005 in comparison of glucose-deprived cells vs control cells.
Further, we investigated the impact of nutrient limitation on induction of POX activity. RKO cells were stressed by withdrawing glucose from the medium and the effect on POX was monitored. As the concentration of glucose decreased in the medium (Fig. 2Ba), an increase in POX expression was observed along with an increase in POX activity similar to the effect of rapamycin. There was almost a 5-fold increase in POX enzyme activity as the concentration of glucose decreased (Fig. 2Bb). A time-dependent increase in POX activity was also seen in the presence of 0.05 mM glucose with maximum activity occurring after 12 hrs of treatment (Fig. 2Bc). Thus induction of nutrient stress conditions either by rapamycin treatment or by glucose withdrawal induces the activation of POX and may lead to increased degradation of proline.
Parallel to the induced POX catalytic activity the intracellular ATP levels are maintained under conditions of stress
To check whether the induced POX catalytic activity has any effect on cellular energy levels in the nutrient starved cells, we measured the intracellular ATP levels using a luciferase method. Interestingly, even at concentrations of glucose as low as 0.05 and 0.01 mM, ATP levels were maintained and did not significantly decrease (Fig. 3A) suggesting that the metabolism of alternative substrates, such as the degradation of proline by POX, may be contributing in maintaining ATP. Additionally, when we checked ATP levels in cells treated with rapamycin at 10 nmol/L, we observed that ATP levels rebounded by 9 h of treatment to a level above controls and plateaued by 12 h (Fig. 3B). Paralleling the increase in POX activity, the increase in ATP with rapamycin treatment suggested that POX may be contributing to maintenance of ATP levels. This result indicates that even in the presence of complete medium, the inhibition of mTOR by rapamycin shuts down energy consuming processes, e.g. protein synthesis, and switches to an ATP conserving mode. In the glucose starved cells the maintenance of the ATP levels also suggests that the cells have switched to an ATP conserving mode similar to rapamycin in which POX may be contributing by inducing the degradation of proline (Fig 3A).
Fig. 3. Effect of Rapamycin and glucose withdrawal on intracellular ATP levels.
RKO cells were exposed to medium with A) decreasing glucose concentrations from 5mM (control) to 0.01mM; B) rapamycin (10nmol/L) or DMSO in control for various time period and C) rapamycin (10nmol/L) in the presence and absence of 10 mM proline/dehydroproline (DHP). The cells were lysed and the intracellular ATP levels were measured using a luciferase based assay. The results are expressed as mean ± S.E. for three separate experiments. Statistical comparisons were as follows: for B, * *, p < 0.005 in comparison of rapamycin-treated versus respective controls; for C, * *, p < 0.005 for rapamycin-treated samples compared to controls without rapamycin; + +, p < 0.005 in comparison of samples treated with dehydroproline (DHP) versus corresponding samples without DHP.
To further demonstrate the involvement of proline degradation in maintaining the cellular energy levels we used the POX inhibitor dehydroproline (DHP). DHP is a substrate analogue of proline and competitively inhibits the activity of POX. ATP levels in the rapamycin treated cells were monitored in the presence and absence of proline and DHP. As shown in figure 3C, the addition of proline had only a modest effect on ATP. However, inhibition of POX by DHP markedly decreased ATP levels either with or without added proline. The modest effect of added proline is most likely due to adequate endogenous sources. This result directly demonstrates that ATP maintenance in the face of rapamycin treatment is, at least in part, due to increased activity of POX.
Activation of AMPK induces POX enzymatic activity
Another protein important in sensing the metabolic state of the cell and influencing the activity of mTOR is AMPK. The activation of AMPK by phosphorylation indirectly results in the inhibition of mTOR. AMP levels rise as the cellular ATP: ADP ratio declines and this rise in AMP leads to activation of AMPK for maintaining cellular energy balance. Since activation of AMPK also downregulates mTOR and our earlier results showed that inhibition of mTOR leads to induction of POX activity, we considered whether activation of AMPK can also induce POX. We first checked the phosphorylation of AMPK following glucose withdrawal in RKO cells. As the concentration of glucose decreases there was activation of AMPK by phosphorylation (Fig. 4A). To further confirm that activation of AMPK can also indirectly induce POX activity we used a synthetic AMPK activator 5-aminoimidazole-4-carboxamide riboside (AICAR). AICAR is a cell-permeable compound, converted intracellularly to AICAR monophosphate or ZMP. ZMP is an analogue of AMP and mimics its effects on AMPK signaling. Figure 4Ba shows that treatment with AICAR at 0.5 mM results in the time-dependent increase in phosphorylation of AMPK. Using AICAR treatment, we monitored POX expression and catalytic activity. We observed that AICAR treatment at 0.5 mM induced a time-dependent increase in the expression of POX along with increase in POX activity (Fig. 4Bb). A dose-dependent increase in POX activity was obtained with AICAR up to a concentration of 1 mM (Fig. 4C). This result indicated that the induction of POX catalytic activity under conditions of glucose deprivation may be mediated via AMPK for maintaining cellular energy levels.
Fig. 4. The activation of POX under glucose deprived conditions may be mediated by AMPK.

A) RKO cells were exposed to medium with decreasing glucose concentrations from 5mM (control) to 0.01mM and the activation of AMPK by phosphorylation at Thr 172 was monitored by western blot analysis. (B) RKO cells were treated with AICAR (0.5 mM) or DMSO in control and collected at various time points; (a) western blot analysis was used to monitor the activation of AMPK by phosphorylation at Thr 172 and the levels of endogenous POX protein; the bands of phosphorylated-AMPK and POX were analyzed by densitometry. (b) the POX catalytic activity in cells treated with AICAR (0.5 mM) for various durations.was measured. (C) RKO cells were exposed to medium containing AICAR at various concentrations for 24 hours; the cell lysates were harvested, and the POX catalytic activity was determined. (D) RKO cells were treated with AICAR (0.5 mM) in the presence and absence of glucose and the POX catalytic activity was determined. The results are expressed as mean ± S.E. for three separate experiments. * *, p < 0.005 in comparison of AICAR-treated cells versus untreated cells.
To further assess the role of increased POX activation in the AMPK response to energy stress, we monitored the effect of AICAR on POX activity in cells stressed by glucose depletion. POX catalytic activity was induced both by glucose-free medium and by AICAR treatment (Fig. 4D). The increase in POX activity in coordination with AMPK was shown by the additive effect on POX activity by AICAR and glucose depletion in glucose-free medium in the presence of AICAR. Presumably, alternative pathways were a source of energy which was superseded by the addition of AICAR.
Proline functions as a stress substrate that is made available under conditions of nutrient stress
If energy or nutrient stress activates the degradation of proline as shown by our results, then pathways that will result in the generation of proline as substrate for POX also should be activated for maintaining cellular energy levels. The ECM is rich in proline and hydroxyproline, which can be made available by degradation of ECM. Therefore, we checked the degradation of ECM by monitoring activation of matrix metalloproteinases (MMP) under conditions of glucose stress. The activation of MMP-9/MMP-2 was monitored by gelatin zymography. Both MMP-9 and MMP-2 activities were detected in serum free conditioned medium. As the concentration of glucose was lowered in the medium the activities of both enzymes were increased (Fig. 5A). Maximum increase in activity was observed in the complete absence of glucose in the medium.
Fig. 5. Effect of glucose withdrawal on MMP-2/ -9 activity and intracellular proline levels.

Aa) RKO cells were cultured for 24 hours in serum-free medium with different concentrations of glucose from 5mM (control) to 0.01mM. After treatment the medium was concentrated and equal amounts of protein were analyzed for activation of MMP-2 and MMP–9 by zymography; (b) The graph shows the densitometric analysis of MMP-2 and MMP-9 bands from Fig 5Aa. Cells were exposed to medium with B) decreasing glucose concentrations as indicated; and C) 0.01 mM glucose and collected at various times. After treatment all the cells were harvested and the intracellular proline levels were measured as described in “Materials and Methods.” * *, p < 0.005 in comparison of glucose-deprived cells versus controls.
Although glucose depletion resulted in the activation of MMP-9/MMP-2, additional verification was required. ECM degradation supposedly made proline available as a stress substrate. Therefore, we measured intracellular proline levels under conditions of glucose stress using an acid-ninhydrin assay. As the levels of glucose decreased to 1 mM and lower in the medium, intracellular proline increased almost 2-fold (Fig. 5B). A time-dependent increase in proline levels was observed when the initial concentration of glucose in the medium was 0.01 mM (Fig. 5C) indicating that even in the face of increased POX activity there was an increased availability of proline as substrate.
Effect of POX induction on the pentose phosphate pathway
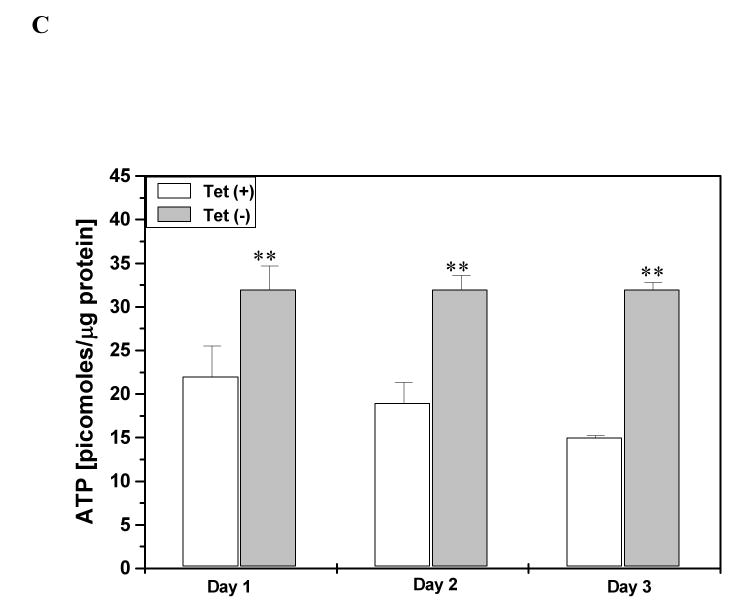
The proline degradative pathway sequentially generates P5C, glutamate and α-ketoglutarate and thereby can play an anaplerotic role for the TCA cycle (Phang, 1985). However, this is not the only bioenergetic contribution by the proline metabolic pathway especially since the TCA cycle is compromised in tumor cells. The proline cycle and its metabolic interlock with the pentose phosphate pathway provide an alternative mechanism for energy production (Hagedorn & Phang, 1983). This interlock provides a pathway for metabolizing glucose to generate reduced pyridine nucleotide (NADPH); the reducing equivalents are shuttled into mitochondria for ATP generation by the cycling of proline. To determine whether such a metabolic interlock is operative in our current study, we measured pentose phosphate pathway activity by monitoring the conversion of 1-14C-glucose to 14CO2 in RKO cells treated with rapamycin and found that concomitant to the increase in POX catalytic activity, the pentose phosphate activity was increased 2-fold (Fig. 6A). To further corroborate this finding, we used the DLD-1 colorectal cancer cells stably transfected with the POX gene under the control of a tetracycline-off promoter (Donald et al., 2001). In these cells the maximum induction of POX activity after removal of tetracycline is obtained after 24 hours. The induction of POX increased the pentose phosphate pathway more than 5-fold (Fig. 6B), and this increase in activity was observed over a range of low glucose concentrations. By contrast, glycolysis as measured by the production of 3H2O from 5-H3-glucose was only modestly increased (25%) (data not shown). Furthermore, parallel to the increase in pentose phosphate activity, cellular ATP levels increased with induction of POX after 24 hours as compared to the uninduced cells in presence of 0.05 mM glucose (Fig. 6C) and the effect on ATP could be observed upto 72 hours (1-3 days). Thus, the activation of POX supplements bioenergetics, and this is due, in part, by activation of the pentose phosphate pathway.
Fig. 6. The effect of POX induction on the pentose phosphate pathway.
A) RKO cells were exposed to medium with and without rapamycin 10 nmol/L and 14CO2 was collected as described in “Materials and Methods”. B) DLD-POX cells were cultured without doxycycline to induce POX. Control cells were cultured in the presence of doxycycline (20 ng/ml). Cells cultured under both conditions were incubated with glucose-1-14C at concentrations of glucose shown and 14CO2 collected as above. Activities are shown as glucose utilized per hour per mg cell protein. The results are expressed as mean ± S.E. for three separate experiments. C) DLD-POX cells were cultured in medium containing 0.05 mM glucose; with and without doxycycline to induce POX. The cells were lysed and the intracellular ATP levels were measured using a luciferase based assay. The results are expressed as mean ± S.E. for three separate experiments. Statistical comparisons for A: * *, p < 0.005 in comparison versus control; for B & C, * *, p < 0.005 in comparison of cells with POX induced (tet -) versus POX uninduced (tet +).
Discussion
As cancer cells grow, invade and metastasize, they encounter an ever-changing microenviroment. Outgrowing their blood supply, they face not only hypoxia but also a diminishing supply of growth factors, glucose and other nutrients (Dang & Semenza, 1999). Due to their intrinsic plasticity, cancer cells can adapt their metabolic pathways and thrive in spite of stress conditions (Hammerman et al., 2004). During hypoxia, the central transcriptional regulator HIF-1α is required for induction of a variety of genes encoding erythropoietin, VEGF and glycolytic enzymes to stimulate angiogenesis and increase glycolysis (Lando et al., 2003). This mechanism elaborately explains how tumors survive under oxygen limitation; however, they do not satisfactorily explain how cancer cells survive under avascular conditions in which hypoxia is associated with nutrient insufficiency. Furthermore, as a result of the isolation from blood supply, energy production in the hypoxic microenvironment by increased glycolysis might be insufficient because glucose also is limited. It may be essential that these cells use alternative energy sources (Pan & Mak, 2007). Proline can serve as such a “stress substrate” because of its availability and unique metabolism. First, POX donates proline-derived electrons directly into the electron transport chain (Phang, 1985). Further, the cycling of proline and P5C can transfer reducing potential into mitochondria to generate ATP (Hagedorn & Phang, 1986), or ROS (Liu, 2005; White et al., 2007) and the metabolism of proline and its metabolites can regulate redox balance (Merill et al., 1989). Since proline metabolism responds to stress conditions and is associated with various energy pathways, we hypothesized that it might also respond under conditions of nutrient or energy stress. Based on these observations, we proposed that proline metabolism can contribute to energy maintenance under conditions of nutrient depletion or fuel source limitation.
Our data shows that in low-nutrient conditions, the degradation of proline is induced by increasing POX catalytic activity both in rapamycin-treated and glucose-depleted cells. It is known that under conditions of nutrient stress, cells maintain energy levels by inducing catabolic processes that will generate ATP. For example, in response to glucose deprivation, drug- and radiation-resistant cancer cells use fatty acids to support mitochondrial oxygen consumption (Harper et al., 2002). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer cells (Liu, 2006). Although oxidation of fatty acids is primarily used to sustain bioenergetics under low nutrient conditions, at least in tissue culture, other sources present in the tumor microenvironment under avascular conditions could also contribute to maintenance of cellular energy levels and serve as alternative or stress substrate. Therefore we considered that the degradation of proline may be an important mechanism cells utilize for maintaining ATP. This indeed seems to be the case since parallel to the increase in POX activity the intracellular ATP levels were relatively maintained in glucose starved cells, and in fact, rapamycin-treated cells, even in the presence of glucose increased their ATP. The functional contribution of proline degradation was shown by the decrease in ATP in presence of dehydroproline, the inhibitor of POX. This result directly demonstrated that the ATP elevating effects may, at least in part, be due to increased POX activity.
Various factors may be involved in regulating the induction of POX activity; we considered the involvement of the intracellular energy sensor AMPK. An increase in intracellular AMP allosterically activates LKB1 which in turn activates AMPK (Gwinn et al., 2008) to maintain energy balance within cells under stress conditions such as glucose deprivation, hypoxia, and ischemia (Hardie, 2003). AMPK activation represses anabolic pathways that consume energy such as protein synthesis and simultaneously stimulates catabolic pathways that produce ATP including the degradation of proline. In our study, parallel to the activation of AMPK by glucose withdrawal, POX catalytic activity was induced suggesting that it may be AMPK-dependent. Kato et al. showed that AMPK plays a critical role in tolerance of cancer cells to nutrient deprivation and tumor formation (Kato et al., 2002). In their study they demonstrated that expression of AMPK α1 and α2 increased with glucose deprivation in pancreatic cancer cells, and the prolonged survival of these cells was mainly AMPK-dependent. In another study Hashimoto et al. have shown that the survival of Hep2 and HF cells can be prolonged by activation of AMPK by AICAR under glucose-deprived conditions (Hashimoto et al., 2002). Pharmacologic activation of AMPK by AICAR resulted in a dramatic induction of POX activity directly showing the effect of AMPK activation on POX. Our results were further corroborated by the additive effect obtained on POX activity in glucose-free medium in the presence of AICAR. In response to glucose depletion it has been shown that activation of AMPK by AICAR in Akt-expressing cells is sufficient to maintain cell survival by inducing fatty acid oxidation to provide mitochondria with sufficient substrates to sustain oxidative phosphorylation and bioenergetics (Buzzai et al., 2005). AMPK activation acts as a metabolic checkpoint and blocks cells from energy-consuming progress through the cell cycle (Gwinn et al., 2008) and simultaneously stimulates catabolic pathways that produce ATP including the degradation of proline. AMPK plays a key role as a metabolic checkpoint which is analogous to the DNA damage checkpoint regulated by p53 (Gwinn et al., 2008), and interestingly, both AMPK and p53 markedly activate POX.
Considering that the proline metabolic pathway contributes to survival under conditions of energy/ nutrient stress, the availability of proline as a stress substrate for generating ATP is an important question. An important source for free proline comes from the degradation of ECM that is composed predominately of collagen (80%) and containing about 25% of proline and hydroxyproline (Ii et al., 2006). ECM degradation under conditions of genotoxic, nutrient or inflammatory stress is mediated by the activation of MMPs. Rapamycin has been shown to exert direct antifibrotic activities by markedly upregulating the expression of interstitial collagenase (MMP-1) (Poulalhon et al., 2006). Studies by Burke et al. showed that tumor associated macrophages express high levels of MMP-7 protein in hypoxic areas of human breast carcinomas and suggested that it could be important for survival and functioning of macrophages under hypoxic conditions (Burke et al., 2003). With glucose depletion we observed the activation of MMPs -2 and -9 and this may be a survival strategy under stress conditions for mediating the degradation of ECM so that a supply of free proline is made available for maintaining bioenergetics through the cycling of proline. And indeed, parallel to the activation of MMPs, an increase in intracellular proline levels occurred, indicating that free proline is made available as a substrate in response to nutrient stress. In the context of a microenvironmental source of stress substrate under the aforementioned avascular conditions, the ECM/proline source has a marked advantage over fatty acids (and glutamine) which require delivery by the circulation. This process may be termed “ecophagy,” the consumption of substrates available in the tumor microenvironment.
Another temporary survival strategy which cancer cells are known to exploit is autophagy, which can provide amino acids for adaptive protein synthesis and ATP to fuel bioenergetic needs. Amino acids from degraded proteins can also be substrates for mitochondrial oxidation through the TCA cycle (Lum et al., 2005). Proline can be oxidized to glutamate which can enter the TCA cycle as dicarboxylic acids (α-ketoglutarate) (Fig. 1), and can be partially oxidized through TCA reactions, or converted to acetyl CoA for complete oxidation. With glucose deprivation we observed induction of autophagy, which can again result in the generation of free proline that may be reutilized to sustain bioenergetics (data not shown). However, the degradation of ECM by MMP to provide free proline and hydroxyproline, the process we have termed “ecophagy” may accompany autophagy. Another important energy generating mechanism is provided by the metabolic interlock between the proline cycle with the pentose phosphate pathway which could be specially exploited when the supply of glucose is limited. Our results showed that the pentose phosphate pathway is activated when POX activity is induced under glucose deprivation and the glucose-derived reducing potential in the form of NADPH increased ATP levels via the cycling of proline. Therefore, activation of POX augments bioenergetics not only by supplying carbons to the TCA cycle but also by the recruitment of the pentose phosphate pathway linked by the proline cycle to supplement bioenergetics.
Thus, the degradation of proline may be an alternative energy source for survival especially in the context of the microenvironment of rapidly growing tumors to sustain periods of reduced metabolite supply. Although proline degradation is not an efficient source for generating ATP, its small contribution could be significant in the metabolic adaptation of cancer cells in response to nutrient limitation. At first, this role of proline metabolism may seem paradoxical to the established role of proline oxidation in generating ROS and inducing apoptosis (Liu et al., 2005). Although the increased donation of electrons to electron transport may generate ROS as well as ATP, the oxidation of proline may be channeled according to the specific stress. The work of White et al. (White et al., 2007) using a recombinant monofunctional bacterial proline dehydrogenase has shown that the flavine adenine dinucleotide (FAD) at the active site can transfer proline-derived electrons directly to reduce oxygen forming superoxide. Furthermore, an adjacent alpha-helix may be conformationally shifted to block the access of FAD to solvent oxygen. The mechanism mediating this switch remains unknown. Nevertheless, we propose that under nutrient stress, the oxidation of proline is channeled toward ATP. However, if survival becomes impossible, a switch is activated to couple the oxidation of proline to the generation of ROS for apoptosis.
Acknowledgments
This work was supported by fedral funds from the NCI, National Institutes of Health, under contract NO1-CO-12400 and by a grant from the Intramural Research Program of the National Institues of Health, NCI, Center for Cancer Research.
The abbreviations used are
- POX
proline oxidase
- P5C
pyrroline-5-carboxylate
- AMPK
AMP activated protein kinase
- AICAR
5-aminoimidazole-4-carboxamide ribonucleoside
- MMP
matrix metalloproteinase
References
- Bates L. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- Chen C, Wanduragala S, Becker DF, Dickman MB. Tomato QM-like protein protects Saccharomyces cerevisiae cells against oxidative stress by regulating intracellular proline levels. Appl Environ Microbiol. 2006;72:4001–4006. doi: 10.1128/AEM.02428-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–1815. [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mohaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn CH, Phang JM. Transfer of reducing equivalents into mitochondria by the interconversions of proline and Δ-1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1983;225:95–101. doi: 10.1016/0003-9861(83)90010-3. [DOI] [PubMed] [Google Scholar]
- Hagedorn CH, Phang JM. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986;248:166–174. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- Hammerman PS, Fox CJ, Thompson CB. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem Sci. 2004;29:586–592. doi: 10.1016/j.tibs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A, Kupperman J, Newell EW, Bespalov JA, Wallace SS, Liu Y, Rogers JR, Gibbs GL, Leahy JL, Camley RE, Melamede R, Newell MK. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J. 2002;16:1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kato K, Imamura K, Kishimoto A, Yoshikawa H, Taketani Y, Esumi H. 5-Amino-4-imidazolecarboxamide riboside confers strong tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent fashion. Biochem Biophys Res Commun. 2002;290:263–267. doi: 10.1006/bbrc.2001.6193. [DOI] [PubMed] [Google Scholar]
- Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–790. doi: 10.1046/j.1432-1033.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Borchert GL, Donald SP, Surazynski A, Hu CA, Weydert CJ, Oberley LW, Phang JM. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis. 2005;26:1335–1342. doi: 10.1093/carcin/bgi083. [DOI] [PubMed] [Google Scholar]
- Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- Liu Y, Borchert GL, Surazynski A, Phang JM. Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene. 2008 doi: 10.1038/onc.2008.322. 2008, September 15 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Merrill MJ, Yeh GC, Phang JM. Purified human erythrocyte pyrroline-5-carboxylate reductase. Preferential oxidation of NADPH. J Biol Chem. 1989;264:9352–9358. [PubMed] [Google Scholar]
- Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE. 2007;381:14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- Pandhare J, Cooper SK, Phang JM. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J Biol Chem. 2006;281:2044–2052. doi: 10.1074/jbc.M507867200. [DOI] [PubMed] [Google Scholar]
- Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, Mauvid A, Verrecchia F. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045–33052. doi: 10.1074/jbc.M606366200. [DOI] [PubMed] [Google Scholar]
- White TA, Krishnan N, Becker DF, Tanner JJ. Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J Biol Chem. 2007;282:14316–14327. doi: 10.1074/jbc.M700912200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh GC, Phang JM. Stimulation of phosphoribosyl pyrophosphate and purine nucleotide production by pyrroline 5-carboxylate in human erythrocytes. J Biol Chem. 1988;263:13083–13089. [PubMed] [Google Scholar]