Abstract
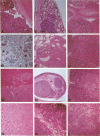
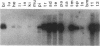
There is increasing evidence for the importance of events that govern and influence the interaction between the transformed cell and its host being ultimately responsible for the establishment of the cancer phenotype. To derive an animal model that will allow us to define some of these phenomena at the molecular level, we have chosen to induce the expression of a viral oncogene in all tissue types, with the hope of identifying sites that are more susceptible to malignant transformation. When the gene for simian virus 40 large tumor antigen (T antigen) was placed under the control of a major histocompatibility complex class I gene enhancer, the resulting transgenic mice not only developed choroid plexus papillomas, as seen with wild-type simian virus 40, but also lymphoid hyperplasia and multiple endocrine neoplasias. The development of lymphoid hyperplasia was preceded by an elevated level of expression of T antigen in these tissues at an early age. Surprisingly, the striking thymic hyperplasia has not been observed to progress toward malignancy. The multiple endocrine neoplasias developed later in life and involved the pancreas, pituitary, thyroid, adrenals, and testes. While not preceded by an elevated level of expression of T antigen, once endocrine tumors appeared they quickly progressed toward malignant growth. Although other tissues also exhibited a basal level of expression of the viral oncogene similar to that detected in endocrine tissues, they rarely developed tumors. This transgenic mouse model seems particularly suitable for a molecular understanding of events responsible for certain tissue types being so much more susceptible to neoplastic conversion, with others being so refractory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieberich C., Scangos G., Tanaka K., Jay G. Regulated expression of a murine class I gene in transgenic mice. Mol Cell Biol. 1986 Apr;6(4):1339–1342. doi: 10.1128/mcb.6.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis R. A., Dayal Y., Tischler A. S., Lee A. K., Wolfe H. J. Multiple endocrine neoplasia (MEN) syndromes: cellular origins and interrelationships. Int Rev Exp Pathol. 1986;28:163–215. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Wong C., Butel J. S. Differential ability of a T-antigen transport-defective mutant of simian virus 40 to transform primary and established rodent cells. Mol Cell Biol. 1985 May;5(5):1043–1050. doi: 10.1128/mcb.5.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987 Mar 27;235(4796):1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Ornitz D. M., Hammer R. E., Messing A., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987 Oct 9;238(4824):188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Messing A., Brinster R. L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985 Aug 1;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sugano S., Yamaguchi N. Two classes of transformation-deficient, immortalization-positive simian virus 40 mutants constructed by making three-base insertions in the T antigen gene. J Virol. 1984 Dec;52(3):884–891. doi: 10.1128/jvi.52.3.884-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]