Abstract
Background
Plasma high density lipoprotein (HDL) cholesterol (HDL-C) concentration is highly heritable but is also modifiable by environmental factors including physical activity. HDL-C response to exercise varies among individuals, and this variability may be associated with genetic polymorphisms in the key regulators of HDL metabolism including endothelial lipase (LIPG).
Methods
We examined associations between variants LIPG T111I (rs2000813) and LIPG i24582 (rs6507931), HDL and television viewing/computer use (“screen time”) as a marker for physical inactivity in a population with high prevalence of metabolic syndrome. Subjects consisted of 539 White men and 584 women (mean ± S.D., 49 ± 16 years) participating in the GOLDN study.
Results
We did not observe an association with either LIPG SNP or HDL independently of screen time. In multi-adjusted linear regression models, HDL interacted significantly with screen time as a continuous variable in LIPG i24582 subjects with TT genotype (P < 0.05). By dichotomizing screen time into high and low levels, we found significant genotype-associated differences in HDL in women but not men. When screen time was ≥2.6 h/day, the concentrations of total HDL-C, large HDL, large low density lipoprotein (LDL) were lower, the concentration of small LDL was higher and HDL and LDL particle sizes were smaller in subjects with LIPG i24582 TT compared to CT and CC subjects (P < 0.05).
Conclusions
We found a significant gene-physical inactivity interaction for HDL and some LDL measures for the LIPG i24582 polymorphism. Higher levels of physical activity may be protective for HDL-C concentrations and low activity detrimental in LIPG i24582 TT individuals, especially in women.
Keywords: LIPG, Endothelial lipase, HDL, Television, Physical activity
1. Introduction
Low concentration of HDL-C is a primary independent risk factor for cardiovascular disease (CVD). Reduced HDL-C concentration has become increasingly relevant in the context of growing prevalence of overweight and metabolic syndrome because these conditions are often associated with low HDL. Moreover, the distribution of HDL subfractions provides a more refined tool for predicting CVD risk than total HDL-C concentration. Distributions shifted towards larger particles confer greater protection [1].
Plasma HDL-C concentration is highly heritable, estimated at 50% [2], and many of its identified genetic contributors function in cholesterol metabolism [3]. Genetic variants of endothelial lipase (LIPG), a member of the triglyceride lipase family which also includes hepatic lipase and lipoprotein lipase, are associated with HDL-C concentration [4,5]. Endothelial lipase (LIPG) preferentially hydrolyzes phospholipids, and decreases HDL apolipoprotein through dose-dependent catabolism in an animal model [6].
Functional studies of LIPG in humans have demonstrated relationships between inflammation and LIPG [7,8]. Further, plasma LIPG is positively correlated with features characterizing the chronic inflammatory condition of metabolic syndrome. Obesity, triglycerides and hypertension were positively associated with LIPG concentration and HDL-C negatively associated with LIPG in overweight subjects [9].
In spite of the significant heritability of HDL-C concentration and the influence of traits such as obesity, HDL-C is modifiable through behavioral factors including physical activity, alcohol, smoking and diet [10]. While increased physical activity is associated with modestly increased HDL-C, and increased television viewing, a proposed measure of physical inactivity, is associated with decreased HDL-C [11], individual HDL-C responses to activity are highly variable [12].
Genetic variants may contribute to inter-individual variability in HDL-C response to exercise training as they do to baseline HDL-C [13]. Whether or not LIPG variants with a strong baseline association for HDL-C will interact with exercise training is unknown. LIPG T111I (Thr111Ile) and LIPG i24582 (T+2864C/In8) were among 20 LIPG SNPs identified through sequencing of LIPG in a small population with HDL-C > 90th percentile, suggesting that their influence on HDL-C may be substantial [5]. In a subsequent resequencing analysis in a larger, ethnically variable population, the association of both SNPs with HDL-C was confirmed. In that study, LIPG i24582 exhibited the strongest association with HDL-C compared to 6 other LIPG SNPs, and, along with another intronic SNP, defined a haplotype present in 24% of the population [15]. However, whether the LIPG i24582 association with HDL-C might be augmented by habitual physical activity is unknown. For the second SNP of interest, LIPG T111I, exercise training was previously shown to modulate HDL-C differentially according to genotype [14], and we sought to replicate those results. Therefore, in the current study, we examined associations between 2 LIPG variants, LIPG T111I and LIPG i24582, HDL and LDL cholesterol, and physical inactivity in a population with high prevalence of overweight and metabolic syndrome.
2. Methods
2.1. Study design and subjects
Study subjects were recruited from the NHLBI Family Heart Study [16] to participate in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) Study. The GOLDN study was designed to evaluate genetic factors that modulate dietary and fenofibrate responses, and its methods have been previously described [17]. Study sites were located in Minnesota and Utah and the predominantly White subjects were genetically similar. Study protocol approval was obtained from the Human Studies Committee of Institutional Review Board at the University of Minnesota, University of Utah, and Tufts University/New England Medical Center. All participants provided written informed consent. Questionnaires were used to collect demographic, lifestyle, medical history, medication and dietary data. Lifestyle data included the number of hours per day spent viewing television or using a computer (referred to subsequently as “screen time”) as a measure of physical inactivity.
2.2. Laboratory methods
Total concentrations, large and small particle concentrations, and particle size were measured for HDL and LDL cholesterol. HDL-C was measured using a cholesterol esterase, cholesterol oxidase reaction (Roche Diagnostics) on the Hitachi 911. Low density lipoprotein cholesterol was measured using a homogenous direct method on the Hitachi 911. Lipoprotein particle size and particle concentration were measured using proton nuclear magnetic resonance spectroscopy. Repeatability was assessed by analyzing duplicate samples from 5% of participants and was determined to be >90% for all lipids. Detailed laboratory methods, including definitions for particle size, have been previously described [17].
2.3. Genetic analysis
Genomic DNA was isolated from peripheral blood lymphocytes by standard methods. Endothelial lipase SNPs LIPG T111I (rs2000813) and LIPG I24582 (rs6507931) were genotyped using the ABI Prism TaqMan multiplex system (Applied Biosystems, Foster City, California). Linkage disequilibrium (LD) between the two SNPs was estimated as a correlation coefficient (r2) in unrelated subjects using Haploview software version 4.0 [18].
2.4. Statistical analyses
All continuous variables were examined graphically for normal distribution. The relationship between LIPG genotype and screen time on plasma lipids were evaluated by analysis of variance techniques. We used the generalized estimating equation (GEE) approach with exchangeable correlation structure implemented in the SAS GENMOD procedure to adjust for familial relationships. Interactions between screen time and LIPG polymorphisms were tested in a multi-adjusted interaction model controlling for potential confounders including age, alcohol, smoking, diabetes, hormone use in women, anti-lipemic medication, total energy, total fat intake, sugar intake and test site. The population mean value for screen time was used to dichotomize this variable into high and low levels. Screen time was also evaluated as a continuous variable by computing predicted values for each subject from the adjusted regression model and plotting the predicted values against screen time by genotype. Analyses were performed for the whole sample and separately by sex using SAS (Version 9.1 for Windows). A P value of 0.05 was considered significant.
3. Results
Subject characteristics are presented in Table 1. Abdominal obesity prevalence is higher and metabolic syndrome prevalence is lower in women than in men. Genotype frequencies for the two LIPG SNPs did not deviate from Hardy-Weinberg equilibrium expectations. Minor allele frequency for LIPG i24582 (C) was 0.45 and for LIPG T111I (A) was 0.27. Evaluation of linkage disequilibrium (LD) between the two SNPs revealed an r2 value of 0.257, indicating weak LD.
Table 1.
Demographic, anthropometric, biochemical, dietary and genotypic data, by sexa.
| Men (n = 539) | Women (n = 584) | P-Value | |
|---|---|---|---|
| Age (years) | 49.0 ± 16.4 | 48.2 ± 16.4 | 0.392 |
| BMI (kg/m2) | 28.5 ± 4.9 | 28.0 ± 6.2 | 0.129 |
| Abdominally obese, n (%)b | 214 (40.0) | 321 (55.1) | <.0001 |
| Metabolic syndrome, n (%)c | 217 (40.3) | 180 (30.8) | <.0001 |
| Waist (cm) | 100.8 ± 14.8 | 92.7 ± 17.2 | 0.0005 |
| LDL-C (mg/dL) | 123.2 ± 30.3 | 119.7 ± 32.1 | 0.202 |
| HDL-C (mg/dL) | 41.5 ± 9.8 | 52.2 ± 13.7 | <.0001 |
| Triglyceride (mg/dL) | 153.3 ± 142.3 | 125.3 ± 82.1 | <.0001 |
| Glucose (mg/dL) | 105.6 ± 20.8 | 97.8 ± 15.7 | <.0001 |
| Energy intake (kcal/day) | 2492.5 ± 1403.3 | 1767.3 ± 785.1 | <.0001 |
| Total fat (% of energy) | 35.9 ± 6.9 | 34.9 ± 6.9 | 0.013 |
| Total sugar (% of energy) | 12.3 ± 6.2 | 12.5 ± 6.9 | 0.472 |
| Screen time (h/day) | 2.8 ± 1.7 | 2.7 ± 1.6 | 0.339 |
| Alcohol user, n (%) | 266 (49.4) | 296 (50.7) | 0.590 |
| Smoker, n (%) | 42 (7.8) | 44 (7.6) | 0.162 |
| Diabetes, n (%) | 37 (6.9) | 52 (8.9) | 0.220 |
| Anti-lipemic medications, n (%) | 29 (5.4) | 13 (2.2) | 0.005 |
| LIPG i24582, n (%) | |||
| TT | 165 (30.8) | 166 (28.5) | |
| CT | 263 (49.2) | 288 (49.4) | |
| CC | 107 (20.0) | 129 (22.1) | |
| LIPG T111I, n (%) | |||
| GG | 282 (52.5) | 326 (55.9) | |
| AG | 224 (41.7) | 206 (35.3) | |
| AA | 31 (5.8) | 51 (8.8) | |
Values are mean ± S.D. or n (%).
Abdominal obesity: waist ≥102 cm in men, ≥88 cm in women.
Metabolic syndrome, definition from the 2005 National Cholesterol Education Program Adult Treatment Panel III guidelines.
We did not observe associations between plasma lipids and LIPG T111I independently of “screen time” (representing combined television viewing/computer use). For LIPG i24582, the minor C allele was associated with lower small LDL particle concentration (P = 0.042) in women but no significant associations were observed in men (Table 2). Interactions between physical inactivity and both SNPs were examined by dichotomizing screen time according to the mean population value and evaluating relationships to plasma lipids. We did not observe significant interactions between LIPG T111I and screen time for HDL or LDL.
Table 2.
Associations between LIPG i24582 genotype and lipid measures in women and men.
| Plasma lipidsa | Women |
Men |
||||
|---|---|---|---|---|---|---|
| TT (n = 166) | CT + CC (n = 417) | P-Value | TT (n = 165) | CT + CC (n = 370) | P-Value | |
| HDL-C (mg/dL) | 52.4 ± 1.8 | 53.4 ± 1.8 | 0.386 | 40.1 ± 1.3 | 40.4 ± 1.0 | 0.704 |
| Large HDL (mg/dL) | 22.5 ± 1.6 | 24.0 ± 1.7 | 0.207 | 13.8 ± 1.3 | 13.4 ± 1.1 | 0.604 |
| HDL size (nm) | 8.8 ± 0.1 | 8.9 ± 0.1 | 0.222 | 8.68 ± 0.05 | 8.65 ± 0.05 | 0.375 |
| LDL-C (mg/dL) | 129.7 ± 5.4 | 124.1 ± 5.3 | 0.056 | 126.1 ± 4.4 | 123.5 ± 4.1 | 0.332 |
| Large LDL (mg/dL) | 58.6 ± 8.6 | 61.6 ± 9.0 | 0.360 | 39.4 ± 4.7 | 38.4 ± 4.3 | 0.670 |
| Small LDL (mg/dL) | 61.4 ± 7.6 | 55.0 ± 8.0 | 0.042 | 69.9 ± 4.4 | 69.8 ± 4.3 | 0.958 |
| LDL size (nm) | 20.9 ± 0.2 | 21.0 ± 0.2 | 0.105 | 20.43 ± 0.1 | 20.39 ± 0.1 | 0.528 |
Means adjusted for age, alcohol, smoking, total energy, total fat intake, sugar intake, test site, waist circumference, anti-lipemic medication, hormone use in women, diabetes.
For the LIPG i24582 SNP, significant interactions for screen time and lipids were observed in women but not in men nor in the combined population (Table 3). Screen time was dichotomized based on the population mean for women into high (≥2.6 h/day) and low (<2.6 h/day) levels. Significant interaction terms between screen time and genotype in women were obtained for total HDL-C (P = 0.0003), large HDL particles (P = 0.0003), HDL particle size (P = 0.0007), large LDL particles (P = 0.001) and LDL particle size (P = 0.025) (Table 3). Adjustments for potential confounders included age, waist circumference, smoking, alcohol, diabetes, anti-lipemic medication, hormone use, test site, total energy, fat and sugar intake. For women with high screen time (≥2.6 h/day) reflecting a low level of physical activity, significant differences in lipid values were observed with lower concentrations of total HDL-C (P = 0.005), large HDL particles (P = 0.003), large LDL (P = 0.026), higher concentration of small LDL (P = 0.023) and smaller HDL size (P = 0.005) and LDL size (P = 0.025) in TT subjects compared to CC and CT subjects (Table 3). In women with low physical activity, TT genotype contributes 0.6% of the variance in total HDL-C. No significant differences in HDL or LDL between TT compared to CC and CT subjects were observed at low levels of screen time.
Table 3.
Interactions between LIPG i24582 and duration of screen time for HDL and LDL in women.
| Screen time (h/day) | Plasma lipidsa | TT (n = 166) | CC + CT (n = 417) | P for trend | P for interaction |
|---|---|---|---|---|---|
| <2.6 | HDL-C (mg/dL) | 57.2 ± 3.0 | 54.8 ± 2.9 | 0.062 | 0.0003 |
| ≥2.6 | 48.4 ± 2.4 | 52.6 ± 2.3 | 0.005 | ||
| <2.6 | Large HDL (mg/dL) | 24.9 ± 2.3 | 23.0 ± 2.2 | 0.166 | 0.0003 |
| ≥2.6 | 19.5 ± 2.08 | 24.1 ± 2.0 | 0.003 | ||
| <2.6 | HDL size (nm) | 8.9 ± 0.1 | 8.8 ± 0.1 | 0.168 | 0.0007 |
| ≥2.6 | 8.8 ± 0.1 | 8.9 ± 0.1 | 0.005 | ||
| <2.6 | LDL-C (mg/dL) | 125.4 ± 9.7 | 119.9 ± 9.3 | 0.142 | 0.959 |
| ≥2.6 | 136.0 ± 8.1 | 130.4 ± 7.8 | 0.190 | ||
| <2.6 | Large LDL (mg/dL) | 60.7 ± 8.7 | 54.7 ± 8.1 | 0.121 | 0.001 |
| ≥2.6 | 54.5 ± 11.7 | 65.9 ± 12.8 | 0.026 | ||
| <2.6 | Small LDL (mg/dL) | 60.9 ± 10.5 | 58.1 ± 10.0 | 0.423 | 0.117 |
| ≥2.6 | 67.7 ± 9.5 | 57.0 ± 10.9 | 0.023 | ||
| <2.6 | LDL size (nm) | 21.0 ± 0.2 | 20.9 ± 0.2 | 0.925 | 0.025 |
| ≥2.6 | 20.8 ± 0.2 | 21.1 ± 0.2 | 0.025 |
Means adjusted for age, alcohol, smoking, total energy, total fat intake, sugar intake, test site, waist circumference, anti-lipemic medication, hormone use in women, diabetes.
We further examined interactions between inactivity and LIPG genotype in women by evaluating total HDL-C at tertiles of screen time for TT subjects and for C allele carriers for LIPG i24582. For TT subjects only, HDL-C decreases significantly as screen time tertile increases (P for trend, 0.002, data not shown).
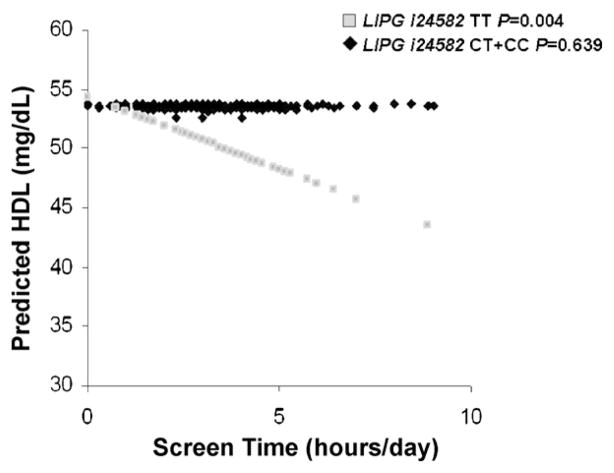
We also examined interactions between screen time as a continuous variable and LIPG i24582 on HDL in women. Predicted values for HDL-C are plotted against screen time for TT subjects (P = 0.004) and C allele carriers (P = 0.639) for LIPG i24582 in Fig. 1. As screen time increases, HDL-C is decreased significantly in TT subjects but not in subjects carrying the C allele.
Fig. 1.
Predicted values of total HDL-C in women by LIPG i24582 genotypes plotted against television viewing/computer use (“screen time”) (h/day) as a continuous variable. Predicted values for HDL-C were calculated from the regression model after adjustment for age, alcohol, smoking, total energy, total fat intake, sugar intake, test site, waist circumference, anti-lipemic medications, hormone use, and diabetes. P-values indicate the statistical significance of the adjusted regression coefficient for TT and CT or CC subjects.
4. Discussion
We have identified a significant interaction between the LIPG i24582 polymorphism and screen time in women, in which a high level of television viewing/computer use, a proposed marker for low physical activity, was associated with significant differences in HDL and LDL measures between LIPG i24582 genotypes. When screen time was ≥2.6 h/day, total HDL-C concentration, large HDL concentration, HDL size, large LDL concentration and LDL size were lower and small LDL concentration was higher in women with TT genotype compared to CT and CC genotype. Each of these interactions reflects a more atherogenic lipid profile for women lacking the C allele in the context of low levels of physical activity. Associations between a LIPG polymorphism and HDL measures were not apparent when the population was considered in its entirety, independently of screen time, and were not observed in men.
Considerable variability in individual HDL-C response to activity level or exercise training has been documented [12]. Genetic contributors to this variability strongly overlap with modulators of baseline HDL-C concentration including APOA1 and hepatic lipase (LIPC) [13,19]. In a previous study investigating relationships between LIPG, HDL and exercise, LIPG T111I was shown to be associated with altered baseline and exercise-associated increases in total HDL-C, with trends for altered HDL subfractions [14]. In the current study, no significant associations in HDL were observed with this SNP, but associations for another SNP, LIPG i24582, were observed for women with high screen time. Differences between the previous intervention study and current cross-sectional study could be related to population phenotypic differences and differences in study design. Specifically, in the previous study, subjects were limited to those with BMI < 37 mg/kg2, diabetics, smokers and those with CVD were all excluded, women were required to be at least 2 years past menopause, and subjects were not using medications which could alter lipoprotein concentrations. Further, intervention subjects also received extensive dietary instruction designed to shift them towards the American Heart Association Step 1 Diet, and weight loss was achieved in both genotype groups. In contrast, these exclusions and dietary interventions were not applied in the GOLDN population, where the high prevalence of overweight and metabolic syndrome may mask potential genotype interactions.
Mechanisms by which activity level could interact with endothelial lipase to modulate HDL are unclear but may involve inflammation, which is related to endothelial lipase in the endothelium and systemically [9,20–22]. Endothelial lipase concentrations are not available for our population, so we cannot evaluate relationships between inflammatory markers, endothelial lipase concentration or activity and LIPG genotype. However, growing evidence including upregulation of LIPG m-RNA and activity by inflammatory mediators, demonstration of endothelial lipase-mediated catabolism of HDL in an animal model and a positive association between endothelial lipase concentration and metabolic syndrome suggest that inflammation modulates endothelial lipase [9,21,22]. In contrast, exercise training attenuates the pro-inflammatory effects which are associated with overweight as demonstrated by interventions which decrease inflammatory markers [23]. However, without measurement of endothelial lipase concentration or activity, the proposed mechanisms remain theoretical.
An alternative and similarly hypothetical mechanistic explanation for the association between activity and endothelial lipase is suggested by two independent but related lines of research. In cultured endothelial cells, mechanical forces such as fluid shear stress increased endothelial lipase mRNA [24]. In support of this in vitro evidence, endothelial lipase mRNA expression was greater in hypertensive animals [25]. One possibility is that altered physical forces associated with elevated blood pressure could modify endothelial lipase concentration or activity. Altered endothelial shear stress may also result from exercise, and adaptations to these mechanical forces may alter gene expression and synthesis of mediators which regulate endothelial function [26–28]. In addition to local, physically mediated effects of exercise on the endothelium, exercise reduces blood pressure [29] which is often elevated in overweight subjects. Integration of these findings linking shear stress, exercise, hypertension, and endothelial lipase concentrations suggest complex interrelationships in which endothelial lipase-mediated HDL-C concentration could be a clinically important by-product of hypertension and a sedentary lifestyle.
The reasons that the observed associations and interactions were limited to women are unclear, but similar gender-specific results have been reported in other studies investigating television viewing and CVD risk. Television viewing of >20 h/week increased the risk of metabolic syndrome, in women but not men across all levels of physical activity [30]. In another study, HDL-C in women but not men was significantly negatively correlated with television viewing, implying a different relationship between television and metabolic risk according to gender [11]. Gender-specific differences in metabolic outcomes may reflect differences in complex behaviors. In women but not in men, television watching is positively associated with other sedentary behaviors and negatively associated with leisure time physical activity, implying that television is a better marker of overall physical activity in women than in men [31].
There are several limitations to the current cross-sectional study, one is its use of television viewing and computer use (screen time) as a marker of low physical activity, and we recognize that screen time and activity are not necessarily completely reciprocal. While others have confirmed associations between television viewing and HDL-C in women [11], television viewing may be confounded by factors such as altered dietary intakes. We have adjusted by nutritional factors reported to affect HDL-C to help address this limitation. Another limitation is our inability to determine whether endothelial lipase concentration or activity differs between C allele carriers and TT subjects of the LIPG i24582 variant, and whether exercise modifies endothelial lipase concentration or activity. Without evidence of altered endothelial lipase in association with genotype or response to activity, mechanisms proposed to explain our results remain theoretical. Ideally, these hypothetical mechanisms could be tested and the limitations of our cross-sectional design could be addressed by conducting a controlled exercise intervention comparing endothelial lipase and HDL-C concentrations across LIPG genotypes and at different activity levels. Further, the interactions we observed could be evaluated in additional populations.
In summary, we observed an association between LIPG i24852 and HDL and LDL concentrations and subfractions in women, which for HDL became apparent only when evaluated in light of low physical activity as reflected by high television/computer use. The overall health benefits of increased physical activity for inactive individuals are indisputable and wide-ranging, and these benefits may be mediated in part through reduced obesity. However, even within at-risk, overweight populations, higher levels of physical activity may be protective for HDL and LDL distributions and low activity detrimental, especially for individuals with the LIPG i24582 TT genotype. Knowledge of a genetically based increased atherogenic risk from a sedentary lifestyle could be used as a motivation tool to help selected individuals to adopt a more active lifestyle, but does not refute the overriding recommendation for greater physical activity in individuals of all genotypes.
5. Conclusions
We have identified an interaction between LIPG variant i24582 and physical inactivity which may contribute to inter-individual variability in HDL response to exercise. At low levels of activity, individuals with LIPG i24582 TT genotype may experience increased risk of a more atherogenic lipid profile characterized by lower total HDL-C, lower large HDL, smaller HDL particles, smaller LDL particles and higher concentration of small LDL. Communication of genetic information to those individuals who are at increased risk of dyslipidemia due to a sedentary lifestyle may enhance compliance to interventions intended to decrease that risk. However, these results do not diminish the public health message that increased physical activity is generally beneficial, regardless of genotype.
Acknowledgments
Funding: Supported by the National Institutes of Health, National Institute on Aging, Grant Number 5P01AG023394-02 and NIH/NHLBI grant number HL54776 and NIH/NIDDK DK075030 and contracts 53-K06-5-10 and 58–1950-9–001 from the U.S. Department of Agriculture Research Service. MJ is supported by a grant from the Fulbright-Spanish Ministry of Education and Science (reference 2007-1086). C. Smith is supported by T32 DK007651-19.
Footnotes
Conflict of interest
None of the authors report any conflicts of interest.
References
- 1.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2000;90(2):89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 2.Kathiresan S, Manning AK, Demissie S, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nettleton JA, Steffen LM, Ballantyne CM, Boerwinkle E, Folsom AR. Associations between HDL-cholesterol and polymorphisms in hepatic lipase and lipoprotein lipase genes are modified by dietary fat intake in African American and White adults. Atherosclerosis. 2007;194(2):e131–40. doi: 10.1016/j.atherosclerosis.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ordovas JM. Endothelial lipase: a new member of the family. Nutr Rev. 1999;57(9 Pt 1):284–7. doi: 10.1111/j.1753-4887.1999.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 5.deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation. 2002;106(11):1321–6. doi: 10.1161/01.cir.0000028423.07623.6a. [DOI] [PubMed] [Google Scholar]
- 6.Maugeais C, Tietge UJ, Broedl UC, et al. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108(17):2121–6. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 7.Kojma Y, Hirata K, Ishida T, et al. Endothelial lipase modulates monocyte adhesion to the vessel wall. A potential role in inflammation. J Biol Chem. 2004;279(52):54032–8. doi: 10.1074/jbc.M411112200. [DOI] [PubMed] [Google Scholar]
- 8.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase is increased in vivo by inflammation in humans. Circulation. 2008;117(5):678–85. doi: 10.1161/CIRCULATIONAHA.107.707349. [DOI] [PubMed] [Google Scholar]
- 9.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 2006;3(2):e22. doi: 10.1371/journal.pmed.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison RC, Zhang Y, Qureshi MM, et al. Investigators of the NHLBI Family Heart Study. Lifestyle determinants of high-density lipoprotein cholesterol: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2004;147(3):529–35. doi: 10.1016/j.ahj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt MD, Cleland VJ, Thomson RJ, Dwyer T, Venn AJ. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol. 2008;18(5):378–86. doi: 10.1016/j.annepidem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56(4):444–50. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Ruano G, Seip RL, Windemuth A, et al. Apolipoprotein A1 genotype affects the change in high density lipoprotein cholesterol subfractions with exercise training. Atherosclerosis. 2006;185(1):65–9. doi: 10.1016/j.atherosclerosis.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Halverstadt A, Phares DA, Ferrell RE, et al. High-density lipoprotein-cholesterol, its subfractions, and responses to exercise training are dependent on endothelial lipase genotype. Metabolism. 2003;52(11):1505–11. doi: 10.1016/s0026-0495(03)00284-1. [DOI] [PubMed] [Google Scholar]
- 15.Mank-Seymour AR, Durham KL, Thompson JF, Seymour AB, Milos PM. Association between single-nucleotide polymorphisms in the endothelial lipase (LIPG) gene and high-density lipoprotein cholesterol levels. Biochim Biophys Acta. 2004;1636(1):40–6. doi: 10.1016/j.bbalip.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Higgins M, Province M, Heiss G, et al. NHLBI Family Heart Study: objectives and design. Am J Epidemiol. 1996;143(12):1219–28. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 17.Corella D, Arnett DK, Tsai MY, et al. The –256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53(6):1144–52. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 18.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Teran-Garcia M, Santoro N, Rankinen T, et al. Hepatic lipase gene variant –514C>T is associated with lipoprotein and insulin sensitivity response to regular exercise: the HERITAGE Family Study. Diabetes. 2005;54(7):2251–5. doi: 10.2337/diabetes.54.7.2251. [DOI] [PubMed] [Google Scholar]
- 20.Bartels ED, Nielsen JE, Lindegaard ML, et al. Endothelial lipase is highly expressed in macrophages in advanced human atherosclerotic lesions. Atherosclerosis. 2007;195(2):e42–9. doi: 10.1016/j.atherosclerosis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Feingold KR, Memon RA, Moser AH, Shigenaga JK, Grunfeld C. Endotoxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis. 1999;142(2):379–87. doi: 10.1016/s0021-9150(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 22.Jin W, Sun GS, Marchadier D, et al. Endothelial cells secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ Res. 2003;92(6):644–50. doi: 10.1161/01.RES.0000064502.47539.6D. [DOI] [PubMed] [Google Scholar]
- 23.Kadoglou NP, Perrea D, Iliadis F, et al. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care. 2007;30(3):719–21. doi: 10.2337/dc06-1149. [DOI] [PubMed] [Google Scholar]
- 24.Hirata K, Ishida T, Matsushita H, Tsao PS, Quertermous T. Regulated expression of endothelial cell-derived lipase. Biochem Biophys Res Commun. 2000;272(1):90–3. doi: 10.1006/bbrc.2000.2747. [DOI] [PubMed] [Google Scholar]
- 25.Shimokawa Y, Hirata K, Ishida T, et al. Increased expression of endothelial lipase in rat models of hypertension. Cardiovasc Res. 2005;66(3):594–600. doi: 10.1016/j.cardiores.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Gan L, Doroudi R, Hagg U, et al. Differential immediate-early gene responses to shear stress and intraluminal pressure in intact human conduit vessels. FEBS Lett. 2000;477(1–2):89–94. doi: 10.1016/s0014-5793(00)01788-9. [DOI] [PubMed] [Google Scholar]
- 27.Ganguli A, Persson L, Palmer IR, et al. Distinct NF-[kappa]B regulation by shear stress through Ras-dependent[kappa]B[alpha] oscillations: real-time analysis of flow-mediated activation in live cells. Circ Res. 2005;96(6):626–34. doi: 10.1161/01.RES.0000160435.83210.95. [DOI] [PubMed] [Google Scholar]
- 28.van Thienen JV, Fledderus JO, Dekker RJ, et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72(2):231–40. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa-Takata K, Ohta TH. How much exercise is required to reduce blood pressure in essential hypertensives: a dose–response study. Am J Hypertens. 2003;16(8):629–33. doi: 10.1016/s0895-7061(03)00895-1. [DOI] [PubMed] [Google Scholar]
- 30.Chang PC, Li TC, Wu MT, et al. Association between television viewing and the risk of metabolic syndrome in a community-based population. BMC Public Health. 2008;8:193. doi: 10.1186/1471-2458-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama T, Healy GN, Dunstan DW, Salmon J, Owen N. Is television viewing time a marker of a broader pattern of sedentary behavior? Ann Behav Med. 2008;35(2):245–50. doi: 10.1007/s12160-008-9017-z. [DOI] [PubMed] [Google Scholar]