Summary
SWAP-70-like adapter of T cells (SLAT) is a recently identified guanine nucleotide exchange factor (GEF) for Cdc42 and Rac1, which is highly expressed in both thymocytes and peripheral T cells. Here, we present and discuss findings resulting from biochemical and genetic analyses aimed at unveiling the role of SLAT in CD4+ T-cell development, activation, and T-helper (Th) cell differentiation. Slat−/− mice display a developmental defect at one of the earliest stages of thymocyte differentiation, the double negative 1 (DN1) stage, leading to decreased peripheral T-cell numbers. Slat−/− peripheral CD4+ T cells demonstrate impaired T-cell receptor/CD28-induced proliferation and IL-2 production. Moreover, SLAT positively regulates the development of Th1 and Th2 inflammatory responses by controlling Ca2+ /NFAT signaling. SLAT is also a positive regulator of the recently emerging Th subset, i.e., Th17 cells, as evidenced by its critical role in Th17 cell-mediated central nervous system inflammation. Furthermore, TCR engagement induces SLAT translocation to the immunological synapse, a process mediated by its Lck-dependent phosphorylation, which thereafter facilitates the triggering of SLAT GEF activity towards Cdc42 and Rac1, leading to NFAT activation and Th1/Th2 differentiation. Future work will aim to dissect the interacting partners of SLAT and may thus shed light on the poorly understood events that coordinate and link actin cytoskeleton reorganization to Ca2+ signaling and gene transcription in T cells.
Keywords: SWAP-70-like adapter of T cells, guanine nucleotide exchange factor, T-cell receptor, nuclear factor for activated T cells, calcium, T-helper cell differentiation
Introduction
Signals from the T-cell receptor (TCR) play a critical role in T-cell development, activation, differentiation, survival, and death. During T-cell development in the thymus, signals from the pre-TCR and the TCR control developmental progression (1, 2). From the earliest thymic stage, the double negative (DN) stage, signals from the pre-TCR, resulting from the assembly of a TCRβ chain with a surrogate α chain, pTα, allow the survival of DN thymocytes, their proliferation, and differentiation into CD4+ CD8+ double positive (DP) thymocytes as well as participating in allelic exclusion completing the so-called ‘β selection’. At this stage, successful rearrangement of TCR Vα and Jα genes results in the synthesis of TCR α chains and assembly of the mature αβTCR on the cell surface. Furthermore, TCR signals are critically involved in both positive and negative selection in the thymus giving rise to CD4+ or CD8+ single positive (SP) cells and eliminating potentially autoreactive T cells from the repertoire. TCR signals also control the fate of naive T cells that migrate to the periphery, i.e. their survival and the maintenance of their numbers (homeostasis) (3, 4). TCR engagement by specific antigen-major histocompatibility complex (MHC) molecules presented by antigen-presenting cells (APCs) is central to the effective induction of an antigen-specific T-cell response. Antigen exposure thus results in proliferation and subsequent differentiation into CD4+ or CD8+ effector T cells. On one hand, CD4+ T-helper (Th) cells differentiate into three subsets of effector cells, Th1, Th2, and Th17, based on their distinct cytokine expression profiles and their subsequent immune regulatory functions (5–8). Th1 cells mainly secrete interleukin-2 (IL-2) and interferon-γ (IFN-γ) and mediate defense against infection by intracellular pathogens; Th2 cells secrete IL-4, IL-5, IL-6, IL-13, and IL-10 and mediate predominantly humoral immunity and allergic responses (9). The balance between Th1 and Th2 subsets determines susceptibility to disease states: development of excess Th2 cells can lead to allergy and asthma, while an overactive Th1 response can lead to autoimmunity (9). Lastly, Th17 cells produce IL-17, IL-17F, and IL-22, all of which regulate tissue inflammatory responses, and are critical for enhancing host protection against extracellular bacteria and fungi (6–8), which are not efficiently cleared by Th1 and Th2 responses. Th17 cells have been shown to have critical functions in the pathogenesis of a variety of organ-specific autoimmune inflammatory diseases, e.g. collagen-induced arthritis (CIA) (10), experimental autoimmune encephalomyelitis (EAE) (11, 12), or psoriasis (13). On the other hand, CD8+ T cells become cytotoxic cells and can efficiently kill target cells presenting the agonist peptide. Antigen-specific signals may also result in T-cell differentiation into long-lived memory cells.
T-cell activation is not a simple bimolecular event (i.e. a single receptor binding its ligand) but rather involves multiple interactions at the cell surface, including costimulatory molecules (e.g. CD28), adhesion molecules (e.g. integrins), and signaling from cytokine or chemokine receptors. The sum of these events, along with the qualitative difference in TCR signals and the nature of the APC, dictates the outcome of the antigen-specific T-cell response.
At a cellular level, for activation to occur, the formation of a stable conjugate between an antigen-specific T cell and a cognate peptide-bearing APC and the reorganization of molecules at the cell surface are required. The induction of T-cell activation is accompanied by large-scale rearrangements of the T-cell cytoskeleton, reorientation of the T-cell microtubule organizing center (MTOC), and the segregation of surface receptors, adapter proteins, and signaling molecules at the T cell/APC interface, also called the immunological synapse (IS) (14–17). Protein segregation within the IS was initially described as forming a supramolecular activation cluster (SMAC) comprised of a central region (c-SMAC) containing TCR and associated signaling proteins and a peripheral ring (p-SMAC) containing integrins [such as leukocyte-function-associated antigen-1 (LFA-1)] and adhesion molecules (17). However, several parameters including the nature of the T cell and the APC, costimulatory interactions, and the in vivo environment in which the interaction takes place, can dramatically influence IS architecture. The precise function of the IS in T-cell activation is still a matter of controversy, as the IS is thought to play a role both in triggering, sustaining, and terminating TCR signaling (16–19).
On a molecular level, signaling from the TCR αβ heterodimer depends critically on its non-covalently associated signal transduction components, the CD3γ, δ, and ε and ζ polypeptides (20, 21). In particular, these proteins have cytoplasmic domains bearing immunoreceptor tyrosine-based activation motifs (ITAMs), which each contain tandem tyrosine residues spaced 8–12 amino acids apart. Engagement of the TCR results in rapid activation of Src-family kinases Lck and Fyn, which phosphorylate the ITAM tyrosine residues. The phospho-ITAMs in turn recruit the tyrosine kinase ζ-associated protein of 70 kDa (ZAP-70) or the related Syk kinase via interactions with their tandem Src homology 2 (SH2) domains. This binding allows phosphorylation of ZAP-70 by Lck and, subsequently, its activation. Subsequently, many other proteins are also tyrosine phosphorylated, ultimately leading to the activation of a number of signaling pathways, including a rise in intracellular Ca2+ concentration (Ca2+ flux), activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) cascades, and activation of multiple transcription factors, notably nuclear factor for activated T cells (NFAT), activated protein-1 (AP-1), and nuclear factor κB (NF-κB). Although NFAT, NF-κB, and AP-1 share a number of properties (i.e. DNA binding domains and nuclear translocation upon TCR stimulation) and must be coordinately activated to stimulate IL-2 gene transcription, these transcription factors are regulated by discrete signaling cascades and differ remarkably in their function, resulting in distinct functional out-comes (22–24). In this review, we focus specifically on what we have learned recently about the function of SLAT, a novel TCR-regulated protein linking the actin cytoskeleton to NFAT/Ca2+ signaling, which is ultimately involved in the differentiation of naive CD4+ T cells into distinct Th subsets.
SLAT and SWAP-70: a new class of GEFs for Rho-family GTPases
SLAT isolation
Using a differential display approach to identify Th2-associated genes, we isolated a cDNA encoding a putative protein of 630 amino acids showing a significant (45%) amino acid homology with mouse SWAP-70 (see below) and, thus, termed it SWAP-70-like adapter of T cells (SLAT) (25). Concomitantly, a yeast two-hybrid interaction analysis of a human lymph node cDNA library aimed at identifying potential partners of the lymphoid-restricted transcription factor interferon regulatory factor-4 (IRF-4) led to the identification of a novel cDNA, hence termed IRF-4-binding protein (IBP) (26), encoding the human orthologue of Def-6, which has previously been shown to be expressed in myeloid progenitors but to be downregulated in differentiated macrophages, granulocytes, and erythrocytes (27). More recently, Def-6 has also been detected in skeletal muscle, where it influences myoblast differentiation by interacting with the skeletal muscle integrin chain α7A (28). Furthermore, SLAT has been shown to be expressed in murine peritoneal and bone marrow-derived macrophages (BMMs), in which it modulates Fcγ receptor-mediated phagocytosis (29). It turned out that the amino acid sequence of human IBP also displays a significant sequence homology to SWAP-70 and was, in fact, the human homolog of SLAT, based on a comparison of the predicted amino acid sequence showing that human and murine IBP display 92% identity and 95% similarity over the entire sequence.
SWAP-70: structural features and function
SWAP-70 is predominantly expressed in activated mature B lymphocytes, although low levels can also be detected in mast cells and embryonic fibroblasts (30–33). SWAP-70 contains a putative EF-hand Ca2+ -binding domain, a coiled-coil region, three nuclear localization signals (NLSs), a nuclear export signal, a pleckstrin-homology (PH) domain, which is involved in membrane targeting through binding of phosphatidylino-sitol-3,4,5-triphosphate (PIP3), and a domain weakly homologous to Dbl homology (DH) domains (30, 31, 33, 34).
Although its sequence contains three potential NLSs, in small resting B cells, SWAP-70 is mainly found in the cytoplasm (33). Upon BCR stimulation, SWAP-70 translocates to the nucleus, where it was found to be a component of an enzyme complex called SWAP, also composed of B23 (nucleophosmin), C23 (nucleolin), and poly(ADP-ribose) polymerase (PARP), which promotes the recombination of immunoglobulin (Ig) switch regions in B cells (33). SWAP-70, which is only expressed in B lymphocytes that had been induced to undergo Ig class switch and in switching B-cell lines (in contrast to B23, C23, and PARP, which are expressed in most cells), has a weak affinity for DNA, binds ATP, and is considered to be the specific recruiting element that assembles the switch recombinase from universal components. SWAP-70 is also critical for CD40-dependent switching to the IgE isotype (35). In activated, class-switching B-cell cultures, SWAP-70 is also found to be associated with membrane IgG but not IgM, an association mediated by its PH domain (33).
The DH domain is a hallmark of proteins that function as guanine nucleotide exchange factors (GEFs) for Rho-family guanosine triphosphatases (GTPases) (36). GEFs activate their target GTPases by catalyzing their conversion from the GDP-bound inactive state to the GTP-bound active state (36). Accordingly, SWAP-70 was found to be a Rac-GEF, which, independently of Ras, transduces signals from tyrosine kinase receptors to Rac, and, in turn, plays a role in specific processes during remodeling of the actin cytoskeleton, such as membrane ruffling [possibly via binding to non-muscle F-actin (37)] and lamellipodia formation (34, 38, 39). Recently, Pearce et al. (39) reported a role for SWAP-70 in B-cell adhesion and migration into secondary lymphoid organs. Swap-70−/− B cells rolled and adhered normally yet accumulated in lymph node (LN) high endothelial venules, preventing their entry into LNs (39). This defect was due to an aberrant regulation of integrin-mediated adhesion during attachment, inducing defective polarization and leading to the lack of uropod formation or lamellipodia stabilization at a defined region. In addition to its role in B-cell activation, Ig class switching, migration, and homing, SWAP-70 also plays a role in T-cell-dependent immune response, particularly in the formation and function of germinal centers (GCs) (40). Indeed, SWAP-70 deficiency uncouples GC formation from T-dependent antibody and long-lived plasma cell production and causes extrafollicular generation of high-affinity plasma cells but does not adequately support the memory response (40).
In contrast to B cells, SWAP-70 is confined to the cytoplasm in mast cells, where it plays a role in development into mature mast cells and FcεRI-mediated degranulation (41). Furthermore, SWAP-70 regulates mast cell FcεRI-mediated signaling events such as LAT phosphorylation and Akt/PKB and p38 MAPK activation (42), which are important for the control of mast cell-dependent anaphylaxis. Finally, Swap-70−/− bone marrow mast cells (BMMCs) also display impaired c-kit receptor signaling (38). Swap-70−/− BMMCs are impaired in Ca2+ flux, in proper membrane translocation, and activity of Akt kinase, and in c-kit-induced translocation of Rac1 and Rac2, which are required for mast cell activation, cell–cell adhesion, and migration.
Expression and structural features of SLAT
Unlike SWAP-70, SLAT is abundant in thymocytes as well as in peripheral T cells (25, 26, 43) and translocates from the cytoplasm to the plasma membrane (PM), specifically to the IS, upon antigen stimulation (25, 44, 45). However, the significant sequence homology between SWAP-70 and SLAT is further reflected at the level of the domain structure of the SLAT protein (Fig. 1).
Fig. 1. Domain structure of SWAP-70-like adapter of T cells (SLAT).
Sequence analysis predicts the existence of a number of domains, which are shown in the figure. Numbers above the scheme refer to amino acid residues. A comparison of the ITAM-like sequence of SLAT with the consensus ITAM is also shown. Gray boxes indicate conserved amino acids in the ITAM. The ITAM-like domain contains negatively charged amino acids at conserved positions, the first YxxL/I motif, a spacer sequence of seven amino acids, and a second Tyr-containing sequence, YxxK, in which a lysine residue replaces the leucine/isoleucine typically found in ITAMs. The two tyrosines (Y) of this ITAM-like sequence, Tyr-133 and Tyr-144, are phosphorylated in a Lck-dependent manner upon TCR stimulation. The PH domain has the ability to bind PIP3 but is not required for TCR-induced translocation of SLAT to the membrane/IS. The DH domain has catalytic GEF activity towards Cdc42 and Rac1. The functionality and relevance of the putative EF hand domain and the nuclear localization signal (NLS) have yet to be determined.
SLAT harbors at its N-terminus a potential Ca2+ binding EF-hand domain, whose function remains elusive, followed by an imperfect ITAM-like sequence. This ITAM sequence contains negatively charged amino acids at conserved positions, the first YxxL/I motif, a spacer sequence of seven amino acids, and a second Tyr-containing sequence, YxxK, in which a lysine residue replaces the leucine/isoleucine typically found in ITAMs (Fig. 1). TCR/CD28 costimulation was found to lead to rapid Lck-dependent phosphorylation of Tyr-133 and -144 in this sequence in Jurkat T cells and in primary murine T cells (45). The relevance of this phosphorylation event for SLAT function in T cells is discussed later. In contrast, another report identified instead Tyr-210 as a substrate for Lck phosphorylation by in vitro Lck kinase assays in non-T (293T) cells, i.e., not in the context of TCR stimulation (44).
SLAT next contains a PH domain, shown to be able to bind the phosphatidylinositol 3-kinase (PI3K) product PIP3 (44, 46). Gupta et al. (44) further described an Lck-dependent binding of PIP3 to Arg-236 of the PH domain, whereas Oka et al. (46) identified several basic amino acids in the β1/β2 loop of the PH domain, namely Lys-225 and Arg-226/230/231, as being critical for PIP3 binding but without any requirement for Lck activity. The PH domain is then flanked at its C-terminal side by a DH domain, exhibiting a TCR-regulated GEF activity towards Cdc42 and Rac1 (44, 45, 47), which are major regulators of TCR-mediated cytoskeleton dynamics. Of a particular note here is the arrangement of the PH–DH domains, which in both SLAT and SWAP-70 is the reverse of that observed in the best-characterized GEFs for Rho GTPases (known as Dbl family proteins), wherein the DH domain is invariably flanked by a C-terminal PH domain (36). Moreover, the DH domains of SLAT and SWAP-70 do not exhibit any statistically significant amino acid sequence homology to that of other known Dbl family members such as Vav1 and Tiam1. These structural and functional features thus define SLAT and SWAP-70 as members of a new subfamily of GEFs for Rho GTPases. In addition to its role as an upstream activator of Rho GTPases, SLAT interacts and cooperates with activated Rac1 to induce a change in cell shape, most likely independently of its GEF activity (46). The regions of SLAT interacting with activated Rac have been further mapped to two separate segments: one central part between amino acid residues 315 and 407, which did not show any homology to other Rac-interacting proteins, and an N-terminal region containing a stretch of residues weakly homologous to the Rac-binding domain conventionally found in Rac-interacting proteins, i.e. Leu-18, Leu-31, and Val-33. Finally, the predicted amino acid sequence contains a potential NLS, whose functionality and relevance have not been elucidated.
SLAT expression and its structural features suggest that it may play a novel and crucial role in TCR signaling by activating a subset of Rho-family GTPases, hence influencing T-cell responses in a unique manner. To address the role of SLAT in vivo, we and others used homologous recombination in embryonic stem cells to disrupt the Slat gene to generate SLAT-deficient (Slat−/−) mice (43, 45, 48). The rest of this review focuses on what we have recently learned about the regulation and function of SLAT in T-cell physiology, i.e. T-cell development, activation/signaling, and Th differentiation.
SLAT and T-cell development
Slat−/− mice are viable, fertile, and grossly normal (43, 48). The overall thymic architecture is normal, with the DP thymocytes residing in the cortex while the CD4+ and CD8+ SP thymocytes are concentrated in the medulla. Analysis of thymic development showed that the overall thymus size and cellularity was reduced by about twofold in Slat−/− mice by comparison to wildtype (WT) mice (43). Moreover, Slat−/− and WT mice had similar proportions of DN, DP, and SP thymocytes, although the absolute number of each thymic subset was decreased by approximately 50%, suggesting a defect in differentiation and/or expansion of an early progenitor (43). This defect in thymic development was further traced to impaired expansion of the DN1 population, as evidenced by a specific reduction of bromodeoxyuridine (BrdU) incorporation into this subset (43), which contains the earliest intrathymic progenitors. The differentiation and proliferation of DN subsets are mainly governed by complex interrelationships between Notch, c-kit, and IL-7R signaling pathways (49, 50), raising a question about the role of SLAT in this signaling network, apart from its TCR-dependent function in the periphery. Specifically, c-kit/stem cell factor interactions have been shown to be involved in the earliest stages of thymopoiesis by promoting the proliferation of these immature thymocytes (51), i.e. the stage affected by Slat deletion, suggesting that SLAT may regulate c-kit signaling in the very immature thymocyte compartment. In this regard, it is interesting to note that SWAP-70, the homolog of SLAT, has been shown to regulate c-kit signaling in BMMCs (38).
SLAT and T-cell activation
In the periphery, we observed a mild CD4+ and CD8+ T-cell lymphopenia in both spleen and lymph nodes (LNs) of Slat−/− mice (43), likely occurring as a direct consequence of the decreased thymic output. However, histological examination did not reveal any abnormality in splenic architecture in Slat −/− mice, indicating that SLAT is not required to maintain a normal lymphoid tissue organization.
Regarding the impact of SLAT deficiency on T cell activation, we found that Slat−/− peripheral CD4+ T cells displayed impaired TCR/CD28-induced proliferation and IL-2 production (43). However, phorbol myristate acetate plus ionomycin treatment bypassed this defect, demonstrating that SLAT is part of a signaling pathway downstream of the TCR but upstream of protein kinase C (PKC) and/or Ca2+ signaling that is necessary for full T-cell activation, in agreement with its recruitment to IS upon antigen stimulation. Interestingly, the absence of SLAT has no discernible impact on TCR-induced CD25 upregulation, and the proliferative defect of Slat−/− CD4+ T cells could be overcome by adding exogenous IL-2, implying that this defect is due to altered TCR signaling and not IL-2R signaling.
SLAT, a key component of NFAT/Ca2+ signaling
Proximal TCR signaling events
Examination of TCR/CD28-mediated early signaling events revealed that SLAT is mostly dispensable for activation of early signaling events, with the exception of ERK1/2 and p38 MAPK pathways, whose TCR/CD28-mediated activation was reduced in the absence of SLAT (43, 48). Indeed, after TCR/CD28 stimulation, Slat−/− CD4+ T cells demonstrate intact activation of ZAP-70, linker for activation of T cells (LAT), phospholipase Cγ1 (PLCγ1), Akt, glycogen synthase 3β (GSK3β), and JNK, as well as normal NF-κB activation, while the magnitude and duration of ERK1/2 and p38 activation were modestly reduced by comparison with their WT counterparts. However, Slat−/− CD4+ T cells failed to sustain intracellular Ca2+ influx upon TCR/CD28 ligation, leading consequently to defective translocation of NFAT to the nucleus (43). Another indication for the involvement of SLAT in NFAT activation was the demonstration that overexpression of SLAT in Jurkat T cells dramatically potentiated TCR-mediated transcriptional activation of NFAT but not of AP-1 or NF-κB (45).
Ca2+ signaling
TCR triggering induces a biphasic rise in [Ca2+]i that consists of an early transient rise, resulting from the release of Ca2+ from the endoplasmic reticulum (ER), which serves as the primary trigger for a second phase of sustained Ca2+ mobilization, resulting from the opening of Ca2+ channels in the PM (52, 53). The initial Ca2+ release is mediated by inositol-1,4,5-triphosphate (IP3), a second messenger that binds and activates the IP3 receptor (IP3R), an ER Ca2+ channel. The tyrosine kinases Lck and ZAP-70 indirectly raise IP3 levels by activating PLCγ1, which hydrolyzes PIP2 into membrane-bound diacylglycerol and soluble IP3. IP3 binding is mandatory for channel activity, but other levels of regulation can increase channel sensitivity. In particular, another tyrosine kinase, Fyn, binds to the IP3R, induces its tyrosine phosphorylation, and thereby further prolongs intracellular Ca2+ release (54, 55). The early rise in [Ca2+]i triggers the second Ca2+ wave by opening the Ca2+ release-activated Ca2+ (CRAC) channels (56), such as Orai/CRACM (57), thereby allowing Ca2+ influx from the extracellular space. Two RNA interference (RNAi)-mediated interference screens in Drosophila and HeLa cells revealed that stromal interaction molecule 1 (STIM1) plays a role in the activation of CRAC channels (58, 59). STIM1 is a single-pass transmembrane protein localized in the ER membrane, which contains a Ca2+-binding EF-hand domain facing the ER lumen. STIM proteins were therefore postulated to act as sensors of Ca2+ concentration in the ER. The exact mechanism by which STIM proteins activate CRAC channels in the PM is not fully understood. After store depletion, however, STIM proteins form oligomers in the ER membrane and redistribute into discrete ‘puncta’, as a consequence of the dissociation of Ca2+ from the EF-hand domain (59), and then move to regions of ER–PM apposition that colocalize with clusters of Orai1-containing CRAC channels and sites of localized Ca2+ influx in the PM (60–62). Although STIM1 was reported to localize in the PM in addition to the ER (63), it is not clear whether this STIM fraction is involved in CRAC channel activation. Currently available data favor a model in which clusters of STIM1 in the ER act in trans on the CRAC channel localized in the PM. Ca2+ signaling then activates NFAT proteins that are heavily phosphorylated and reside in the cytoplasm in resting cells; when cells are stimulated, NFATs are dephosphorylated by calcineurin, a calmodulin-dependent serine/threonine phosphatase, and translocate to the nucleus, where they can form transcriptionally active complexes (64).
We analyzed the molecular basis of the defect in Ca2+ signaling in Slat−/− mice in more detail (43). The failure of TCR/CD28 crosslinking and subsequent extracellular Ca2+ addition to induce a significant increase in [Ca2+]i in Slat−/− T cells when extracellular Ca2+ was chelated strongly suggests that the primary Ca+ defect in Slat−/− T cells resides in the receptor-induced release of Ca2+ from intracellular stores, which is required for subsequent Ca2+ influx.
However, as noted above, no defect in TCR-induced PLCγ1 phosphorylation (and, hence, activation) or in IP3 generation was observed in Slat−/− T cells (43), suggesting that SLAT acts downstream of PLCγ1-dependent IP3 production to regulate Ca2+ release from the ER. A number of issues relating to the role of SLAT in controlling [Ca2+]i remain unresolved. It is tempting to speculate that SLAT could regulate ER Ca2+ channels or sensors, e.g., the IP3R (52) or STIM1 (63, 65), or interact with other cytosolic proteins to allow efficient Ca2+ release (66).
SLAT, a regulator of actin cytoskeleton and IS formation
The SLAT DH domain possesses TCR-stimulated GEF activity towards Rac1 and Cdc42 (44, 45, 47). Consistent with the well-documented ability of Rho-family GTPases to control the dynamics of the actin cytoskeleton (67), SLAT has been reported to transduce TCR signals to and through the actin cytoskeleton. In fact, in the absence of SLAT, TCR-induced actin polymerization is reduced, a defect compensated by the expression of a constitutively active form of Rac2 in Slat−/− T cells (48), thus linking the ability of SLAT to activate Rho GTPases to TCR-mediated cytoskeletal reorganization. Furthermore, in the absence of SLAT, antigen-specific IS formation and stabilization is also impaired (48), likely as a consequence of the TCR-mediated cytoskeletal remodeling defect, thus leading in turn to altered T-cell effector function.
SLAT, an actor in the interplay between NFAT/Ca2+ signaling and actin dynamics
Actin reorganization, Ca2+/NFAT, and gene expression in T cells
Despite the importance of Rho GTPase-mediated actin cytoskeleton reorganization in assembly of the IS and in controlling early events preceding gene transcription (such as migration, integrin activation, or T-APC conjugate formation) (68–70), the pathways linking actin rearrangement to Ca2+ /NFAT signaling and downstream changes in gene expression are far less clear.
There is growing evidence showing that the actin cytoskeleton plays a dual role in changes in T-cell function, first by enhancing T-cell activation by promoting conjugate formation and assembly of signaling complexes but also by downregulating activation by facilitating internalization of the TCR. Indeed, cytochalasin D (CytoD) treatment of T cells stimulated with superantigen-pulsed APCs results in diminished Ca2+ flux, whereas stimulation with anti-CD3 antibodies results in a prolonged Ca2+ response (71). Similarly, although one study showed that CytoD treatment impairs activation of the IL-2 gene promoter (72), another study reported that at low doses of CytoD, Ca2+ signaling was prolonged and IL-2 production increased (73). Second, the finding that CytoD treatment impairs TCR-stimulated NFAT but not NF-κB or AP-1 activation (74) suggests that the actin cytoskeleton does not globally regulate all TCR-stimulated signaling pathways. Lastly, antigen receptor-stimulated NFAT activation was previously found to require Rac1 activity in mast cells (75).
SLAT-mediated, Cdc42/Rac1-dependent NFAT activation
Several lines of evidence strikingly support the notion that the GEF activity of SLAT is cardinal for SLAT-induced Ca2+ /NFAT activation (45). First, latrunculin B, an inhibitor of actin polymerization, abrogated TCR-dependent, SLAT-mediated NFAT activation (45). Second, a dominant-negative Cdc42, and to a lesser extent Rac1, mutant blocked SLAT-dependent NFAT activation and, conversely, transduction of Slat−/− CD4+ T cells with constitutively active Cdc42 restored NFAT nuclear translocation and Ca2+ mobilization (45), thus making a strong case for a causal role of the GEF activity of SLAT in inducing its Ca2+-promoting activities.
Several Cdc42/Rac1 effectors can selectively regulate Ca2+/NFAT signaling. Numerous studies indicated that Wiskott–Aldrich syndrome protein (WASP), a specific interacting partner and downstream effector of Cdc42, plays a key role in actin cytoskeleton rearrangement as well as in NFAT activation via its association with WIP [WAVE (WASP-family verprolin homologous protein)-interacting protein], preventing in turn WASP degradation (76–78). Similarly, the WAVE2 complex regulates actin cytoskeleton reorganization but is also required for NFAT (but not NF-κB or AP-1) activation, reflecting its essential role in CRAC-mediated Ca2+ entry (79). Finally, Pak1 (p21-activated kinase 1) kinase, a common Rac1 and Cdc42 effector, is involved in NFAT activation via its Lck-mediated phosphorylation and resulting association with the adapter Nck (80).
Accumulating evidence shows that Rho GTPases regulate gene transcription through diverse distal effector elements of the TCR pathway, including MAPKs p38 and ERK (80–84), whose activation is also decreased in Slat−/− T cells. Notably, Yablonski et al. (80) demonstrated that Rac/Cdc42 could influence NFAT by activating ERK through its target Pak1 in T cells. Furthermore, Rac2−/− T cells show many similarities to T cells from Slat−/− mice, namely, altered p38 and ERK activation, defective Ca2+ mobilization, cytoskeleton reorganization, and cellular activation (83). Cdc42 has also been recently shown to specifically modulate ERK activation (85). In addition, other studies provide evidence for a functional coupling of NFAT transcriptional activity to Ras/MAPK–ERK kinase (MEK)/ERK activation (86–88). Thus, Ras activation through MEK1–ERK was associated with increased NFATc4 nuclear translocation and transcriptional activity in cardiac myocytes (87). This result is also consistent with an observation made in T lymphocytes, whereby Vav signaling was coupled to Ras-MEK–ERK activation, which, in turn, promoted NFAT activation (88). Likewise, dominant negative or constitutively active MEK1 mutants blocked and induced NFAT activation in T lymphocytes, respectively (86). In agreement with these reports, SLAT-mediated NFAT activation was totally abrogated by an ERK inhibitor (45). These findings prompt us to suggest that the defective activation of MAPKs exhibited by Slat−/− T cells may result from impaired Rho GTPase function and that SLAT may thus fulfill its role in NFAT activation through a specific Rac1/Cdc42-ERK pathway. Altogether, the identification of downstream effectors involved in Cdc42/Rac1-dependent, SLAT-mediated NFAT activation may shed some light on the unresolved mechanism that links actin polymerization to Ca2+ /NFAT signaling.
SLAT and T-helper cell differentiation
Given that SLAT is a key regulator of the NFAT pathway (43), presumably via its GEF activity (45), lessons learned from mice deficient for the two NFAT family members most highly expressed in lymphocytes, i.e. NFATc1 and NFATc2, or mice deficient for GEF-induced effectors, are of special interest to understanding the role of SLAT in immune functions in vivo. NFATc1 deficiency results in an impaired Th2 response by decreasing IL-4 production (89), whereas NFATc2 deficiency enhances Th2 development by prolonging IL-4 transcription (90, 91). Interestingly, mice lacking both NFAT isoforms have a profound defect in the production of both Th1 and Th2 cytokines (92). Moreover, transgenic mice expressing a dominant-negative form of NFAT were shown to be resistant to Th2-mediated allergic pulmonary inflammation (93). Lastly, Rac2 activates Th1-specific signaling (94) and CD4+ T cells from Wiskott–Aldrich syndrome patients (mutated in WAS gene encoding WASP) display defective Th1 response due to impaired NFATc2 activation (95), establishing a link between activation of Rho family GTPases, NFAT activation, and Th differentiation. In addition, the proximal promoter of the human Il17A gene contains two NFAT-binding sites, which appear to be important for IL-17 expression (96). Lastly, NR2F6, a nuclear zinc-finger orphan receptor, has been shown to interfere with the DNA binding of NFAT:AP-1 (but not NF-κB) and, subsequently, transcriptional activity of the NFAT-dependent Il-17A cytokine promoter (97). Together, these findings show the emerging role of NFAT in Th17 differentiation and function.
SLAT and Th2 responses
Th functions were tested in vivo in Slat−/− C57BL/6 mice to determine whether biochemical defects observed in Slat−/− T cells, i.e. alteration of Ca2+ /NFAT signaling, translate into impaired T-helper responses in vivo (43). As expected, Slat−/− mice were grossly impaired in their ability to mount a Th2-mediated lung inflammatory response (43). This phenotype was evidenced by reduced airway eosinophilia and levels of Th2 specific cytokines (IL-4/5) in the bronchoalveolar lavage (BAL) fluid from primed Slat−/− mice as well as a reduction in pulmonary inflammation characterized by a drastically diminished perivascular and peribronchial infiltration of eosinophils and mononuclear cells (43). Additionally, immunized Slat−/− mice displayed a drastic reduction in the hyperplasia of goblet cells and mucus production relative to that observed in primed WT mice (43).
In our earlier study, ectopic SLAT expression has been shown to have a moderate positive effect on IL-4 production (25). This study showed an inducible association of SLAT with ZAP-70, which may competitively reduce the association of ZAP-70 with TCR-ζ, consequently dampening the overall strength of TCR signaling. Based on studies showing that Th2 cell activation is associated with inefficient ZAP-70 activation (98–101), this study provided a potential mechanism for the ability of SLAT to positively regulate Th2 cell development and/or expansion, namely, via inhibition of ZAP-70-dependent signaling. Furthermore, another report using dominant-negative Ras transgenic mice, in which TCR-induced ERK activity and Th2 development were severely impaired, showed that the ERK pathway was necessary for Th2 development (102). Altogether, potential crosstalk or synergy between ZAP-70, Ca2+ /NFAT, and MAPK signaling pathways could underlie the defect in Th2 responses observed in Slat−/− mice.
SLAT, Th1/Th17 responses, and autoimmunity
The response of Slat−/− mice in a Th1-mediated lung inflammatory model was also defective. Severely reduced numbers of lymphocytes and neutrophils were found in the BAL fluid from primed Slat−/− C57BL/6 mice as well as decreased level of IFNγ and lack of cellular infiltration/inflammation in the airways (43).
In apparent contrast, Fanzo et al. (48) paradoxically found that aging (>5-month old) and preferentially female Slat−/− mice on a mixed C57BL/6 × 129 background spontaneously developed a lupus-like autoimmune syndrome characterized by the accumulation of effector/memory T cells and IgG+ B cells, proteinuria, glomerulonephritis, profound hyper-gammaglobulinemia, and autoantibody production. Moreover, antigen-experienced T cells from Slat−/− mice exhibited a significant resistance to apoptosis, most likely accounting for the accumulation of effector/memory T cells and, in return, for the development of the autoimmune disorder observed in aged Slat−/− mice. The peripheral T-cell homeostasis defect observed in Slat−/− mice (43) might be related to the spontaneous lupus-like syndrome reported in this study (48). Indeed, several studies showed that lymphopenia can predispose mice to several autoimmune diseases such as insulin-dependent diabetes mellitus, systemic lupus erythematosus, colitis, or rheumatoid arthritis (103, 104). The compensatory homeostatic proliferation, normally a consequence of such lymphopenia, has been reported to drive the development of autoimmunity via the rapid expansion of autoreactive short-lived memory-like T cells within the depleted niche (105, 106). However, the biologic relevance of the lupus-like disease observed in that study (48) is unclear, given the mixed background of the mice and the finding that epistatic interactions between the non-autoimmune-prone strains 129 and C57BL/6 can induce a lupus-like disease (107).
For over a decade, IFNγ-secreting Th1 cells were thought to be the population central to the pathogenesis of autoimmunity, considering the clear association of the Th1 effector phenotype to diseases like rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. In the respective animal models, however, the loss of the major Th1 cytokines, IFNγ, IL-12, and IL-18, surprisingly did not hamper disease development (108–111). In fact, IFNγ and IL-12 deficiency led to an even more severe inflammation in CIA and EAE. As a result of these discoveries, the simplistic notion that Th1 cells and their respective cytokines are the sole regulators of autoimmunity had to be revised. Recently, it has been extensively shown that a third Th subset, i.e., Th17 cells, has critical functions in the pathogenesis of a variety of organ-specific autoimmune and inflammatory diseases (112, 113), including EAE (11, 12), CIA (10), and psoriasis (13). We showed that Slat−/− mice on a C57BL/6 background were resistant to EAE induction, displaying both dramatically reduced onset and severity of the disease (114). This defective in vivo response was correlated with a lack of demyelination of the spinal cord’s white matter and reduced encephalitogenic CD4+ T-cell infiltration into the central nervous system as well as impaired Th1 and Th17 responses in both the central nervous system and draining LNs. Consistent with a key role of SLAT in autoimmunity, naive Slat−/− CD4+ T cells were unable to differentiate properly into Th17 cells in vitro under TGF-β- and IL-6-dependent polarizing conditions, indicating that SLAT acts as a positive regulator of Th17 differentiation (114). In contrast to our study, a recent publication (115) reported that Slat−/− BALB/c mice crossed to DO11.10 transgenic mice expressing an MHC class II-restricted ovalbumin-specific TCR spontaneously and rapidly develop rheumatoid arthritis-like joint disease and large vessel vasculitis associated with enhanced IL-17 and IL-21 production. Nevertheless, these observations, made in the setting of a restricted TCR repertoire (in the DO11.10 TCR transgenic background), have not been confirmed in mice expressing an intact polyclonal TCR repertoire, as young Slat−/− BALB/c mice do not develop obvious signs of arthritis (115), questioning the physiological and pathological relevance of findings based on the study of Slat−/− mice on a TCR transgenic background.
SLAT-mediated NFAT activation and T-helper cell differentiation
To further dissect the role of SLAT in Th cell differentiation, a number of in vitro studies have examined the ability of Slat−/− naive CD4+ T cells to differentiate under defined culture conditions. Bécart et al. (43) showed that SLAT was required for efficient Th1 and Th2 differentiation in vitro. Moreover, the addition of ionomycin could (i) restore a proper nuclear translocation of NFAT and (ii) rescue decreased production of both IL-4 and IFNγ in Slat−/− T cells (43), indicating that SLAT regulates early TCR signaling (prior to Th1/Th2 commitment) and controls lung inflammation by promoting nuclear localization of both NFATc1 and NFATc2 induced by early TCR/CD28 instructive signals. In contrast, Fanzo et al. (48) failed to observe a defect in IL-4 production by unskewed or Th2-polarized Slat−/− CD4+ T cells and observed that the defect in IFNγ production under neutral conditions was rescued by addition of IL-12 to the primary culture. Although the Slat−/− mice used in these two studies were of the same origin, they differ notably by their genetic background; thus, Bécart et al. (43) used Slat−/− mice on a homogenous C57BL/6 background, whereas Fanzo et al. (48) studied Slat−/− mice on a mixed 129 × C57BL/6 genetic background, providing a likely explanation for these discrepancies.
Regulation of SLAT-dependent NFAT-mediated Th differentiation: structure-function analysis
Although SLAT clearly plays a crucial role in Ca2+ /NFAT-mediated Th1/2 differentiation of CD4+ T cells (43), the underlying molecular mechanisms have remained elusive. In this regard, SLAT possesses several prominent TCR-inducible features, such as Lck-dependent tyrosine phosphorylation (44, 45), activation-induced translocation to the IS/PM (25, 44, 45), and GEF activity towards Cdc42 and Rac1 (44, 45, 47), all of which may be relevant for NFAT activation. Based on previous reports emphasizing the role of other actin regulatory proteins in Ca2+ /NFAT signaling, the GEF activity of SLAT is likely to be important for these events. SLAT tyrosine phosphorylation may also control its GEF activity, as it is the case for other DH-containing proteins (e.g. Vav1). The translocation of SLAT to the membrane/IS may also be potentially regulated by its PH domain and play a crucial role in SLAT-mediated T-cell activation and, thus, possibly in downstream NFAT activation.
Further structure–function analysis and the study of the regulation of SLAT subcellular localization provided insights into the mechanisms by which SLAT coordinates NFAT activation and Th differentiation. This work has been carried out by examining the function and localization of SLAT mutants (deleted for specific domains or carrying defined point mutations) overexpressed in Jurkat T cells, or expressed in primary Slat−/− CD4+ T cells using retroviral transduction to establish the physiological relevance of the findings (45). SLAT-transfected Jurkat T cells showed a dose-dependent increase in NFAT reporter activity, which was abrogated by cyclosporine A, an inhibitor of calcineurin, confirming the involvement of SLAT in modulating TCR-induced NFAT transcriptional activity (45). However, SLAT was dispensable for TCR-mediated NFκB and AP-1 activation (45).
Tyrosine phosphorylation of SLAT
Consistent with findings that IS assembly and translocation of signaling molecules to the membrane/IS greatly influence T-cell activation (16), a strict correlation between the structural features required for SLAT membrane/IS recruitment and its ability to activate NFAT was observed; both events required the Lck-dependent phosphorylation of Tyr-133 and Tyr-144 (45). In contrast, Tyr-210, shown to be an Lck substrate in pervanadate-stimulated 293T cells (44), was not required for TCR-induced NFAT activation (45). Of note, phosphorylation of Tyr-133/144 was required but not sufficient to induce NFAT activation as a SLAT mutant mimicking phosphorylation of these residues (Y133/144D) still needed TCR stimulation to activate NFAT and translocate to the cell membrane (45).
Independence of SLAT function on its PH domain
Strikingly, the combined PH–DH domains or the DH domain alone were not sufficient, and PI3K-dependent binding of the PH domain to PIP3 was not even required, for SLAT translocation to the IS and, in return, for NFAT activation and proper Th1/2 differentiation (45). Thus, a point mutation of Arg-236 (R236C), shown to be critical for binding of PIP3 to the SLAT PH domain (44), as well as pretreatment with the PI3K inhibitor LY294002 had no effect on antigen-induced SLAT translocation to the membrane or IS, NFAT activation, or Th1/2 differentiation of CD4+ T cells.
Relevance of SLAT recruitment to membrane for its function
The fact that the isolated catalytic domain of SLAT, the DH domain, which displays GEF activity in COS-7 cells by itself (45), failed to translocate to IS and activate NFAT raised the question whether the IS translocation of SLAT was a prerequisite for NFAT activation. In this regard, unlike the native DH domain alone, constitutive targeting of the DH domain to the PM, accomplished by fusing the N-terminal acylation sequence of Lck to the SLAT DH domain (MyrDH) and, thus, bypassing TCR-induced SLAT translocation to the IS/membrane, enhanced TCR-stimulated NFAT activation and rescued the Th differentiation defect of Slat−/− CD4+ T cells (45). This finding demonstrates that mislocalization of the SLAT DH domain in the cytosol upon TCR engagement underlies its inability to activate NFAT and substantiates the conclusion that membrane/IS association of SLAT is a prerequisite for NFAT activation. Enforced membrane targeting of the isolated DH domain per se was not sufficient to activate NFAT but still required TCR stimulation, indicating that additional TCR-induced signals, beyond the mere membrane/IS translocation of SLAT, are required for its proper function.
Role of SLAT GEF activity in NFAT activation
We further found that the MyrDH-induced NFAT activation was dependent on functional Cdc42 activity (45), as indicated by the ability of a dominant-negative Cdc42 mutant to block SLAT-dependent NFAT activation and, conversely, the restoration of NFAT nuclear translocation, Ca2+ mobilization, and Th1 differentiation in Slat−/− CD4+ T cells by a transduced, constitutively active Cdc42 protein. This causality between activation of Rho family GTPases and Th differentiation is consistent with reports showing that (i) Rac2 activates Th1-specific signaling (94) and (ii) CD4+ T cells from Wiskott–Aldrich syndrome patients display defective Th1 response because of impaired NFATc2 activation (95). Altogether, these findings establish a causal link between the GEF activity of SLAT and the resulting activation of Cdc42 on one hand and its critical function in promoting activation of the Ca2+ /NFAT signaling pathway and, consequently, Th differentiation, on the other.
These data raised the question of the regulation of SLAT GEF activity specifically involved in NFAT activation. A recent study proposed a model for regulation of SLAT GEF activity, reminiscent of that for Vav GEF activity, based on a tyrosine phosphorylation-dependent relief of an intramolecular inhibitory interaction (44). In this model, the N-terminal region of SLAT would serve a regulatory function by maintaining SLAT in a closed conformation until a stimulus-induced phosphorylation relieves this autoinhibitory interaction. This would expose the PH domain allowing it to bind PIP3 and leading to full activation of SLAT by enabling its DH domain to interact with target Rho GTPases. This model is based on findings showing that deletion of the N-terminal region of SLAT renders it constitutively active and capable of activating Rac1 and Cdc42 in COS-7 or 293T cells (44); however, the behavior of this mutant in T cells has not been studied. Nevertheless, as the SLAT DH domain alone failed to induce TCR-regulated, Cdc42/Rac1-mediated NFAT activation (45), the regulatory mechanism accounting for SLAT GEF activity in the context of NFAT activation in T cells appears to be more complex. In particular, our recent findings (45) prompted us to speculate that the TCR-induced, Lck-dependent tyrosine phosphorylation of SLAT combined with its segregation to the membrane/IS are the mandatory key steps in stimulating its GEF activity, which is necessary, in turn, for proper NFAT activation.
Speculative model for SLAT-mediated NFAT activation and Th1/2 differentiation
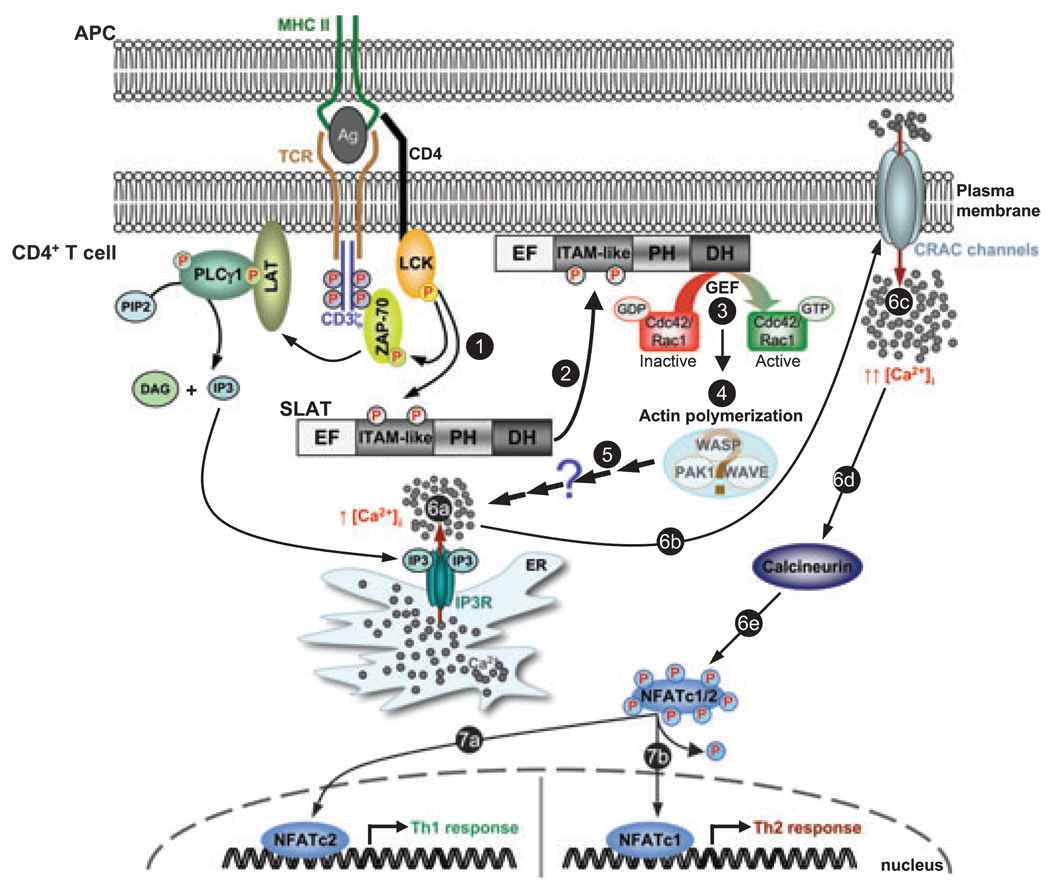
Based on our findings, we can propose the following regulatory model to account for the TCR-induced localization and function of SLAT in the course of T-cell activation and differentiation (Fig. 2). First, Lck-mediated phosphorylation of SLAT creates a binding site(s) for an undefined critical TCR-regulated adapter protein (implicating a potential adapter function for SLAT), and/or induces a conformational change in SLAT. As a result of this modification, SLAT is recruited to the IS, where it can access and activate Rho GTPases, which thereafter induce reorganization of the actin cytoskeleton, and subsequently lead to Ca2+ /NFAT activation and Th1/2 differentiation.
Fig. 2. A hypothetical model of SWAP-70-like adapter of T cells (SLAT) function in Ca2+ /NFAT signaling and Th cell differentiation.
TCR stimulation leads to the sequential activation of protein tyrosine kinases such as Lck and ZAP-70, which phosphorylate adapter proteins such as LAT. Consequently, phosphorylated LAT binds the SH2 domain of PLCγ1, resulting in the phosphorylation/activation of PLCγ1. PLCγ1 catalyses the hydrolysis of the membrane phospholipid PIP2 to IP3 and DAG. IP3 binding to its receptor (IP3R) in the ER triggers Ca2+ release from intracellular stores, which, in return, triggers the opening of plasma membrane CRAC channels. Ca2+ influx through CRAC channels and elevated intracellular Ca2+ concentration activate Ca2+-dependent enzymes such as calcineurin, which dephosphorylates cytoplasmic NFATc1/2. Dephosphorylated NFATc1/2 translocate to the nucleus to induce gene transcription and influence Th cell responses; NFATc1 and NFATc2 positively regulate Th2 and Th1 responses, respectively. In this scenario, TCR engagement induces SLAT phosphorylation on Tyr-133 and Tyr-144 in its ITAM-like sequence (step 1), an event required for its translocation to the plasma membrane/IS (step 2). The exact mechanism mediating this relocalization remains elusive but does not involve the SLAT PH domain. Once at the membrane, additional TCR signals stimulate SLAT GEF activity towards Cdc42 and Rac1 (step 3), leading to activation of actin-regulatory proteins, e.g., WASP, WAVE, or PAK1, which promote actin polymerization (step 4). SLAT-mediated Cdc42/Rac1-dependent actin reorganization thereafter promotes Ca2+ release from intracellular stores (step 6a) and, consequently, Ca2+ influx (steps 6b-c) and NFAT activation (steps 6d-e), by acting downstream of IP3 production (step 5). The exact mechanism underlying this function is currently unknown. SLAT-mediated, Ca2+-dependent NFAT activation (steps 6a-c) controls Th1/Th2 commitment, depending upon the NFAT isoform involved (steps 7a and 7b, respectively).
Concluding remarks and perspectives
TCR signaling triggers interlinked pathways involving a plethora of proteins including tyrosine and serine/threonine kinases, adapter proteins, and cytoskeleton-regulating proteins, leading to highly regulated and fine-tuned responses. In this complex network, SLAT has been identified as a member of a new class of Rho GTPases specific for Cdc42 and Rac1 (44, 45). Studies focusing on CD4+ T-cell responses attributed to SLAT a pivotal role in the regulation of Th1, Th2, and Th17 inflammatory responses by controlling Ca2+ /NFAT signaling through its role in actin cytoskeleton reorganization and Cdc42/Rac1 activation (25, 43, 45). Although recent work improved our understanding of the molecular basis of SLAT function in CD4+ T cells, important questions remain to be answered.
SLAT has been shown to translocate to the membrane/IS upon TCR/CD28 stimulation in a Lck-dependent but PH-independent manner (45). A major question emerging from this finding relates to the tyrosine phosphorylation-dependent mechanism that selectively recruits SLAT to the IS and the identification of proteins mediating this recruitment. Thus, further biochemical studies will aim to define potential SLAT-binding partners following TCR-mediated SLAT phosphorylation on Tyr-133 and Tyr-144 within its ITAM-like motif and accounting for its translocation to the IS. Phosphorylation of these tyrosine residues may generate binding sites for SH2 domain-containing proteins such as Syk or ZAP-70, initiating a cascade of signaling pathways resulting in the formation of an IS-localized signalosome. In this regard, SLAT and ZAP-70 have been shown to interact upon TCR stimulation (25). Furthermore, SLAT phosphorylation may also induce interaction with adapters, such as LAT or Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76), whose function is to link proximal and distal signaling events downstream of the TCR by nucleating a multi-molecular signaling complex that facilitates activation of intermediate signaling cascades such as those stimulated by Rho GTPases.
The SLAT DH domain clearly contains GEF activity towards Cdc42 and Rac1. However, the DH domain residue(s) essential for the GEF activity has not been identified, a task rendered difficult by the fact that the DH domain of SLAT lacks significant primary amino acid sequence homology with ‘conventional’ DH domains or even with the DH domain of its close relative, SWAP-70. Furthermore, as SLAT controls NFAT activation supposedly via its GEF-mediated Cdc42 activation and, hence, via cytoskeleton actin reorganization, future studies exploring the signaling events downstream of SLAT GEF activity may shed light on the poorly understood events that coordinate and link actin cytoskeleton reorganization to Ca2+ signaling and gene transcription in T cells.
Recent reports focused on the role of SLAT in CD4+ T-cell activation and Th1/Th2/Th17 differentiation. However, SLAT is broadly expressed in the peripheral T-cell compartment; thus, its role in other T-cell subsets or states of activation such as regulatory T cells, CD8+ T cells, or memory T cells may be of major interest to broaden our understanding of SLAT function in T-cell physiology.
Beyond these fundamental questions, strategies designed to selectively block the function of SLAT in T cells (e.g. by inhibiting the translocation of SLAT to the IS or, more directly, its GEF activity) may provide an efficient means of blocking the Ca2+ /NFAT signaling machinery in T cells and hence be therapeutically useful for the treatment of NFAT-dependent autoimmune and inflammatory diseases, especially given the predominant and abundant expression of SLAT in T cells (http://biogps.gnf.org/?referer=symatlas#goto=gene-report& id=50619). In that regard, SLAT may turn out to be an attractive drug target with potentially similar importance to that of the recently discovered T-cell CRAC channel, Orai1 (116–118) and the Ca2+ sensor, STIM1 (58, 63). Lastly, additional work is required to define the precise role that SLAT plays in T-cell development through its selective action in DN1 thymocytes. Altogether, resolution of these outstanding questions is likely to firmly establish SLAT as a novel and clinically relevant player in the complex network of T-cell activation and function.
Acknowledgements
We thank members of the Altman laboratory for helpful comments and discussion. This work was supported by National Institutes of Health grants R01 AI68320 (A.A) and fellowships from Fondation pour la Recherche Medicale (S.B), Diabetes & Immune Disease National Research Institute (S.B), and Philippe Foundation (S.B). This is publication number 1050 from La Jolla Institute for Allergy and Immunology.
References
- 1.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki S, Saito T. Molecular basis for pre-TCR-mediated autonomous signaling. Trends Immunol. 2007;28:39–43. doi: 10.1016/j.it.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 4.Freitas AA, Rocha B. Peripheral T cell survival. Curr Opin Immunol. 1999;11:152–156. doi: 10.1016/s0952-7915(99)80026-0. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy KM. T lymphocyte differentiation in the periphery. Curr Opin Immunol. 1998;10:226–232. doi: 10.1016/s0952-7915(98)80253-7. [DOI] [PubMed] [Google Scholar]
- 10.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 11.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupfer A, Dennert G, Singer SJ. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J Mol Cell Immunol. 1985;2:37–49. [PubMed] [Google Scholar]
- 15.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 16.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 17.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 18.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 20.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 21.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 22.Serfling E, et al. NFAT and NF-kappaB factors-the distant relatives. Int J Biochem Cell Biol. 2004;36:1166–1170. doi: 10.1016/j.biocel.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Abbas AK, Sen R. The activation of lymphocytes is in their CARMA. Immunity. 2003;18:721–722. doi: 10.1016/s1074-7613(03)00143-2. [DOI] [PubMed] [Google Scholar]
- 24.Round JL, Humphries LA, Tomassian T, Mittelstadt P, Zhang M, Miceli MC. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat Immunol. 2007;8:154–161. doi: 10.1038/ni1422. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, et al. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, et al. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 27.Hotfilder M, Baxendale S, Cross MA, Sablitzky F. Def-2, -3, -6 and -8, novel mouse genes differentially expressed in the haemopoietic system. Br J Haematol. 1999;106:335–344. doi: 10.1046/j.1365-2141.1999.01551.x. [DOI] [PubMed] [Google Scholar]
- 28.Samson T, et al. Def-6, a guanine nucleotide exchange factor for Rac1, interacts with the skeletal muscle integrin chain alpha7A and influences myoblast differentiation. J Biol Chem. 2007;282:15730–15742. doi: 10.1074/jbc.M611197200. [DOI] [PubMed] [Google Scholar]
- 29.Mehta H, Glogauer M, Becart S, Altman A, Coggeshall KM. Adaptor protein SLAT modulates Fcgamma receptor-mediated phagocytosis in murine macrophages. J Biol Chem. 2009;284:11882–11891. doi: 10.1074/jbc.M809712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borggrefe T, Masat L, Wabl M, Riwar B, Cattoretti G, Jessberger R. Cellular, intracellular, and developmental expression patterns of murine SWAP-70. Eur J Immunol. 1999;29:1812–1822. doi: 10.1002/(SICI)1521-4141(199906)29:06<1812::AID-IMMU1812>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Borggrefe T, Wabl M, Akhmedov AT, Jessberger R. A B-cell-specific DNA recombination complex. J Biol Chem. 1998;273:17025–17035. doi: 10.1074/jbc.273.27.17025. [DOI] [PubMed] [Google Scholar]
- 32.Masat L, et al. Mapping of the SWAP70 gene to mouse chromosome 7 and human chromosome 11p15. Immunogenetics. 2000;51:16–19. doi: 10.1007/s002510050003. [DOI] [PubMed] [Google Scholar]
- 33.Masat L, et al. Association of SWAP-70 with the B cell antigen receptor complex. Proc Natl Acad Sci U S A. 2000;97:2180–2184. doi: 10.1073/pnas.040374497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinohara M, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 35.Borggrefe T, Keshavarzi S, Gross B, Wabl M, Jessberger R. Impaired IgE response in SWAP-70-deficient mice. Eur J Immunol. 2001;31:2467–2475. doi: 10.1002/1521-4141(200108)31:8<2467::aid-immu2467>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 37.Ihara S, Oka T, Fukui Y. Direct binding of SWAP-70 to non-muscle actin is required for membrane ruffling. J Cell Sci. 2006;119:500–507. doi: 10.1242/jcs.02767. [DOI] [PubMed] [Google Scholar]
- 38.Sivalenka RR, Jessberger R. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol Cell Biol. 2004;24:10277–10288. doi: 10.1128/MCB.24.23.10277-10288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce G, et al. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nat Immunol. 2006;7:827–834. doi: 10.1038/ni1365. [DOI] [PubMed] [Google Scholar]
- 40.Quemeneur L, Angeli V, Chopin M, Jessberger R. SWAP-70 deficiency causes high-affinity plasma cell generation despite impaired germinal center formation. Blood. 2008;111:2714–2724. doi: 10.1182/blood-2007-07-102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross B, et al. SWAP-70-deficient mast cells are impaired in development and IgE-mediated degranulation. Eur J Immunol. 2002;32:1121–1128. doi: 10.1002/1521-4141(200204)32:4<1121::AID-IMMU1121>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 42.Sivalenka RR, Sinha M, Jessberger R. SWAP-70 regulates mast cell FcepsilonRI-mediated signaling and anaphylaxis. Eur J Immunol. 2008;38:841–854. doi: 10.1002/eji.200737597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becart S, et al. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J Clin Invest. 2007;117:2164–2175. doi: 10.1172/JCI31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S, et al. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J Biol Chem. 2003;278:43541–43549. doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 45.Becart S, Balancio AJ, Charvet C, Feau S, Sedwick CE, Altman A. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oka T, Ihara S, Fukui Y. Cooperation of DEF6 with activated Rac in regulating cell morphology. J Biol Chem. 2007;282:2011–2018. doi: 10.1074/jbc.M605153200. [DOI] [PubMed] [Google Scholar]
- 47.Mavrakis KJ, McKinlay KJ, Jones P, Sablitzky F. DEF6, a novel PH-DH-like domain protein, is an upstream activator of the Rho GTPases Rac1, Cdc42, and RhoA. Exp Cell Res. 2004;294:335–344. doi: 10.1016/j.yexcr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Fanzo JC, et al. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J Clin Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robey EA, Bluestone JA. Notch signaling in lymphocyte development and function. Curr Opin Immunol. 2004;16:360–366. doi: 10.1016/j.coi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Di Santo JP, Rodewald HR. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr Opin Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- 51.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 52.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 53.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 54.Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks AR. Regulation of the type 1 inositol 1,4,5-trisphosphate receptor by phosphorylation at tyrosine 353. J Biol Chem. 2004;279:16311–16316. doi: 10.1074/jbc.M400206200. [DOI] [PubMed] [Google Scholar]
- 55.Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- 56.Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 57.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 58.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 65.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 67.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 68.Cannon JL, Burkhardt JK. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol Rev. 2002;186:90–99. doi: 10.1034/j.1600-065x.2002.18609.x. [DOI] [PubMed] [Google Scholar]
- 69.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, et al. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 71.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holsinger LJ, et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 73.Rivas FV, O’Keefe JP, Alegre ML, Gajewski TF. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol Cell Biol. 2004;24:1628–1639. doi: 10.1128/MCB.24.4.1628-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nolz JC, Fernandez-Zapico ME, Billadeau DD. TCR/CD28-stimulated actin dynamics are required for NFAT1-mediated transcription of c-rel leading to CD28 response element activation. J Immunol. 2007;179:1104–1112. doi: 10.4049/jimmunol.179.2.1104. [DOI] [PubMed] [Google Scholar]
- 75.Turner H, Gomez M, McKenzie E, Kirchem A, Lennard A, Cantrell DA. Rac-1 regulates nuclear factor of activated T cells (NFAT) C1 nuclear translocation in response to Fcepsilon receptor type 1 stimulation of mast cells. J Exp Med. 1998;188:527–537. doi: 10.1084/jem.188.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silvin C, Belisle B, Abo A. A role for Wiskott-Aldrich syndrome protein in T-cell receptor-mediated transcriptional activation independent of actin polymerization. J Biol Chem. 2001;276:21450–21457. doi: 10.1074/jbc.M010729200. [DOI] [PubMed] [Google Scholar]
- 77.Huang W, Ochs HD, Dupont B, Vyas YM. The Wiskott-Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-kappa B (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement. J Immunol. 2005;174:2602–2611. doi: 10.4049/jimmunol.174.5.2602. [DOI] [PubMed] [Google Scholar]
- 78.Dong X, Patino-Lopez G, Candotti F, Shaw S. Structure-function analysis of the WIP role in T cell receptor-stimulated NFAT activation: evidence that WIP-WASP dissociation is not required and that the WIP NH2 terminus is inhibitory. J Biol Chem. 2007;282:30303–30310. doi: 10.1074/jbc.M704972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nolz JC, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yablonski D, Kane LP, Qian D, Weiss A. A Nck-Pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 1998;17:5647–5657. doi: 10.1093/emboj/17.19.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frost JA, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H, Leitenberg D, Li B, Flavell RA. Deficiency of small GTPase Rac2 affects T cell activation. J Exp Med. 2001;194:915–926. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang K, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 85.Marques CA, et al. An immune escape screen reveals Cdc42 as regulator of cancer susceptibility to lymphocyte-mediated tumor suppression. Blood. 2008;111:1413–1419. doi: 10.1182/blood-2007-05-089458. [DOI] [PubMed] [Google Scholar]
- 86.Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 87.Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem. 2001;276:3524–3530. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 88.Villalba M, Hernandez J, Deckert M, Tanaka Y, Altman A. Vav modulation of the Ras/ -MEK/ERK signaling pathway plays a role in NFAT activation and CD69 up-regulation. Eur J Immunol. 2000;30:1587–1596. doi: 10.1002/1521-4141(200006)30:6<1587::AID-IMMU1587>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 89.Ranger AM, et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 90.Hodge MR, Ranger AM, Charles de la Brousse F, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 91.Xanthoudakis S, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 92.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 93.Diehl S, Krahl T, Rinaldi L, Norton R, Irvin CG, Rincon M. Inhibition of NFAT specifically in T cells prevents allergic pulmonary inflammation. J Immunol. 2004;172:3597–3603. doi: 10.4049/jimmunol.172.6.3597. [DOI] [PubMed] [Google Scholar]
- 94.Li B, et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–2222. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 95.Trifari S, et al. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol. 2006;177:7451–7461. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- 96.Liu XK, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J Biol Chem. 2004;279:52762–52771. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 97.Hermann-Kleiter N, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29:205–216. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamura T, et al. Early activation signal transduction pathways of Th1 and Th2 cell clones stimulated with anti-CD3. Roles of protein tyrosine kinases in the signal for IL-2 and IL-4 production. J Immunol. 1995;155:4692–4701. [PubMed] [Google Scholar]
- 99.Hannier S, Bitegye C, Demotz S. Early events of TCR signaling are distinct in human Th1 and Th2 cells. J Immunol. 2002;169:1904–1911. doi: 10.4049/jimmunol.169.4.1904. [DOI] [PubMed] [Google Scholar]
- 100.Singh RA, et al. Th1 and Th2 deviation of myelin-autoreactive T cells by altered peptide ligands is associated with reciprocal regulation of Lck, Fyn, and ZAP-70. J Immunol. 1999;163:6393–6402. [PubMed] [Google Scholar]
- 101.Faith A, Akdis CA, Akdis M, Joss A, Wymann D, Blaser K. An altered peptide ligand specifically inhibits Th2 cytokine synthesis by abrogating TCR signaling. J Immunol. 1999;162:1836–1842. [PubMed] [Google Scholar]
- 102.Yamashita M, et al. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci U S A. 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gleeson PA, Toh BH, van Driel IR. Organ-specific autoimmunity induced by lymphopenia. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 104.Koh WP, Chan E, Scott K, McCaughan G, France M, Fazekas de St Groth B. TCR-mediated involvement of CD4+ transgenic T cells in spontaneous inflammatory bowel disease in lymphopenic mice. J Immunol. 1999;162:7208–7216. [PubMed] [Google Scholar]
- 105.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 106.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol. 2005;26:5–8. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Bygrave AE, et al. Spontaneous autoimmunity in 129 and C57BL/ 6 mice-implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 110.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 111.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 112.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 113.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 114.Canonigo-Balancio AJ, Fos C, Prod’homme T, Bécart S, Altman A. SLAT/Def6 plays a critical role in the development of Th17 cell-mediated experimental autoimmune encephalomyelitis. J Immunol. 2009 doi: 10.4049/jimmunol.0902573. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Q, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 118.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]