Abstract
Major discoveries have been made in the recent past in the genetics, biochemistry and neuropathology of frontotemporal lobar degeneration (FTLD). TAR DNA-binding protein 43 (TDP-43), encoded by the TARDBP gene, has been identified as the major pathological protein of FTLD with ubiquitin-immunoreactive (ub-ir) inclusions (FTLD-U) with or without amyotrophic lateral sclerosis (ALS) and sporadic ALS. Recently, mutations in the TARDBP gene in familial and sporadic ALS have been reported which demonstrate that abnormal TDP-43 alone is sufficient to cause neurodegeneration. Several familial cases of FTLD-U, however, are now known to have mutations in the progranulin (GRN) gene, but granulin is not a component of the TDP-43- and ub-ir inclusions. Further, TDP-43 is found to be a component of the inclusions of an increasing number of neurodegenerative diseases. Other FTLD-U entities with TDP-43 proteinopathy include: FTLD-U with valosin-containing protein (VCP) gene mutation and FTLD with ALS linked to chromosome 9p. In contrast, chromosome 3-linked dementia, FTLD-U with chromatin modifying protein 2B (CHMP2B) mutation, has ub-ir, TDP-43-negative inclusions. In summary, recent discoveries have generated new insights into the pathogenesis of a spectrum of disorders called TDP-43 proteinopathies including: FTLD-U, FTLD-U with ALS, ALS, and a broadening spectrum of other disorders. It is anticipated that these discoveries and a revised nosology of FTLD will contribute toward an accurate diagnosis, and facilitate the development of new diagnostic tests and therapeutics.
Keywords: amyotrophic lateral sclerosis, frontotemporal dementia, frontotemporal lobar degeneration, granulin, motor neuron disease, TARDBP, TDP-43, ubiquitin, valosin-containing protein
Introduction
Frontotemporal lobar degeneration (FTLD) is used here as an umbrella term to include both a clinical syndrome and one of the neuropathological entities [1– 3]. FTLD is a focal, non-Alzheimer form of dementia, clinically characterized as either behavioral or aphasic variants [1,2,4]. Most commonly, the behavioral or frontal variant is characterized by behavioral dysfunction and change in personal and social conduct. The aphasic variant includes a non-fluent form called progressive non-fluent aphasia, and a fluent form called semantic dementia. Typically, the patient with FTLD does not have an amnestic syndrome, at least in the early stage of the disease, which distinguishes FTLD clinically from Alzheimer’s disease (AD) [5], but there are exceptions [6]. Focal dementias account for up to 20% of presenile dementia cases [7], and FTLD is the second most frequent form of dementia in people under the age of 65 years after AD [8]. FTLD may occur alone or in combination with amyotrophic lateral sclerosis (ALS), parkinsonism, or corticobasal syndrome during the course of the disease.
ALS is the most common adult-onset progressive and, ultimately fatal, motor neuron disease (MND). Like FTLD, ALS encompasses a range of clinicopathological entities [9]. ALS is used here to describe signs of upper and lower motor neuron degeneration with a progressive spread of signs within a region or to other regions as defined by the El Escorial World Federation of Neurology Criteria [10]. The overlap between dementia and ALS is demonstrated by the presence of cognitive and behavioral dysfunction in up to 50% of ALS patients [10–16], indicating a spectrum of clinical phenotypes that relate to common neuropathological lesions [17–19]. Further evidence for a clinical overlap between FTLD and ALS is the occurrence of progressive aphasia [20,21] and the presence of frontotemporal atrophy [22] in patients with ALS. Incidence rates of FTLD with ALS vary often as a consequence of referral bias and differing diagnostic criteria. Several studies have shown that ALS patients with frontotemporal impairment have significantly shorter survival compared with FTLD patients [23,24]. However, the clinical diagnosis of FTLD may only be considered after other potential causes of dementia (e.g. small and/or large vessel disease), systemic conditions (e.g. hypothyroidism, B-vitamin deficiency), tumors, and substance abuse have been excluded. This review will highlight a number of important advances in our understanding of the molecular genetics, biochemistry, and neuropathology of FTLD that have occurred within the recent past.
Genetic studies
FTLD is a genetically complex disorder, with multiple genetic factors contributing to the disease. A positive family history with an autosomal dominant pattern of inheritance and high penetrance is usually found in one quarter to one half of patients [25–30]. Recently, several genes and a locus on chromosome 9p have been linked to familial FTLD with ubiquitin-immunoreactive, tau-negative inclusions (FTLD-U): genetic defects include mutations in the chromatin modifying protein 2B (CHMP2B gene), the cause of chromosome 3-linked FTLD [31], and mutations in the valosin-containing protein (VCP) gene, a cause of chromosome 9-linked FTLD [32,33]. Locus heterogeneity for FTLD and ALS is indicated by the presence of other genetic loci at 9p [34,35]. Recently, the major genetic cause of familial FTLD-U linked to chromosome 17 was identified as mutations in the progranulin (GRN) [36,37] gene. This discovery was soon replicated by the identification of other GRN mutations in the HDDD2 [38], PPA1 and PPA3 [39], and HDDD1 [40] families. Although null mutations, mainly nonsense and frameshift mutations resulting in premature stop codons, in GRN were the first to be identified and the causal mechanism underlying FTLD-U, some of the families described have missense mutations which are predicted to alter trafficking, protein folding, or processing leading to a GRN protein haploinsufficiency [36,37]. Thus, a spectrum of mutations in GRN leads to loss of functional protein, the primary etiology of FTLD with GRN mutation.
This genetic heterogeneity is also reflected in clinical variability. FTLD with GRN mutation has been described in families with corticobasal syndrome [41–43] and in familial and sporadic patients from large FTLD cohorts [44–48]. In 37 patients with a single gene defect, Arg493X, in 30 families with FTLD, there was a variable age at onset (range 44–69 years) and clinical diagnoses included: frontotemporal dementia, primary progressive aphasia, corticobasal syndrome, and Alzheimer’s disease [47]. In contrast, there is phenotypical homogeneity in the PPA1 and PPA3 families, at least in the initial stage of disease [39]. Also, FTLD with GRN A9D, a missense mutation located in the signal peptide, has been described in a family with corticobasal syndrome [43] as well as in another family (HDDD2) with prominent behavioral and language dysfunction [38]. Thus, FTLD with GRN mutation is both clinically, neuropathologically, and genetically heterogeneous [36–50]. Most of the mutations reported to date, but not all, lead to premature termination of the coding sequence and nonsense mediated decay (NMD) resulting in functional loss of one allele. The overall effect of these changes is likely to be a significant reduction in the growth modulator activity of GRN. The various GRN mutations predict at least two disease mechanisms – the partial loss of functional GRN (haploinsufficiency) [36,37] or functional loss caused by mis-trafficking of mutant protein [38,49]. Further studies are required to expand the spectrum of FTLD phenotypes with the several GRN mutations that have now been reported [36–50]. (For the current list of pathogenic mutations in FTLD refer to: http://www.molgen.ua.ac.be/FTDMutations/).
FTLD-U accounts for 5–15% of all dementia disorders [51]. Mutations in the microtubule-associated protein tau (MAPT) gene on chromosome 17q21 which, coincidentally, is in close proximity to the GRN gene, are known to be responsible for 10–20% of familial FTLD [52]. The progranulin gene (GRN) is mutated in 5–10% of patients with FTLD and in about 20% of patients with familial FTLD [53], similar to that of FTLD with MAPT mutation [52].
The recent discovery of pathogenic missense mutations in a highly conserved glycine-rich, heterogeneous ribonucleoprotein interacting domain of the TARDBP gene (Fig. 1) in autosomal dominant ALS families and sporadic cases confirms the importance of TDP-43 in the pathogenesis of ALS and demonstrates that defects in TARDBP are sufficient to cause TDP-43 proteinopathy [54–57]. In none of these families, or in any of the sporadic cases, was there evidence of FTLD. As additional families are identified, more heterogeneous clinical phenotypes may emerge. As the glycine-rich domain (Fig. 1) is a ‘hot spot’ for the mutations reported so far, dysregulation of mRNA splicing may be the functional consequence of these gene defects. A great deal of work has yet to be done to elucidate the normal function of TDP-43 and the cellular pathways disrupted by abnormal protein caused by mutations in TARDBP. As some familial FTLD-U cases do not have GRN, VCP, CHMP2B, or TARDBP mutations, it is likely that additional causal genes and genetic risk factors for FTLD-U exist.
Figure 1.
Mutations in the TAR DNA-binding protein 43 (TDP-43) encoded by the TARDBP gene in familial (FALS) and sporadic amyotrophic lateral sclerosis (SALS). Schematic diagram of functional domains and mutations in the coding region are indicated using the amino acid numbering of the 414 amino acid protein. RRM1, RNA recognition motif 1; RRM2, RNA recognition motif 2; NL, nuclear localization signal; NE, nuclear export signal; hnRNP, heterogeneous nuclear ribonucleoprotein interaction domain. TARDBP mutations: autosomal-dominant FALS (black), SALS (green).
Neuropathology
FTLD comprises a neuropathologically heterogeneous group of neurodegenerative diseases, which share the common feature of preferential degeneration of the frontal and temporal lobes [1,4]. FTLD pathology can be broadly divided into two main classes, based on abnormal accumulation of hyperphosphorylated tau protein: those with tau-immunoreactive (tau-ir) neuronal and/or glial inclusions called tauopathies and those with ubiquitin-immunoreactive (ub-ir), tau-negative inclusions, called FTLD-U. FTLD-U is the most common entity within this group [58]. ALS is also accompanied by a wide range of neuropathological features in which both cortical (upper motor neuron), and either brainstem motor neurons or anterior horn cells (lower motor neuron) are involved with a signature lesion: abnormal accumulation of insoluble proteins, which are ubiquitinated, in the cytoplasm of degenerating motor neurons [59]. Ub-ir inclusions are observed more commonly in sporadic ALS (SALS), but they are also seen in familial ALS (FALS) with Cu/Zn superoxide dismutase (SOD1) gene mutations [60,61]. Until recently, ubiquitin immunohistochemistry (IHC) was the only method to detect abnormal protein aggregates in FTLD-U, FTLD-U with ALS, and SALS. In this spectrum of diseases, the ubiquitinated inclusions contain neither tau, nor α-synuclein, nor neuronal intermediate filament protein epitopes.
Recently, TAR DNA-binding protein 43 (TDP-43) [18,19], was identified as the major component of inclusions of sporadic and familial FTLD-U, with and without ALS, and SALS (Fig. 2) [60,62]. However, the absence of pathological TDP-43 in familial cases harboring SOD1 gene mutations implies that motor neuron degeneration may result from a different mechanism in those cases [60]. It remains to be seen if TDP-43 may be useful in differentiating SOD1-related ALS from SALS [60].
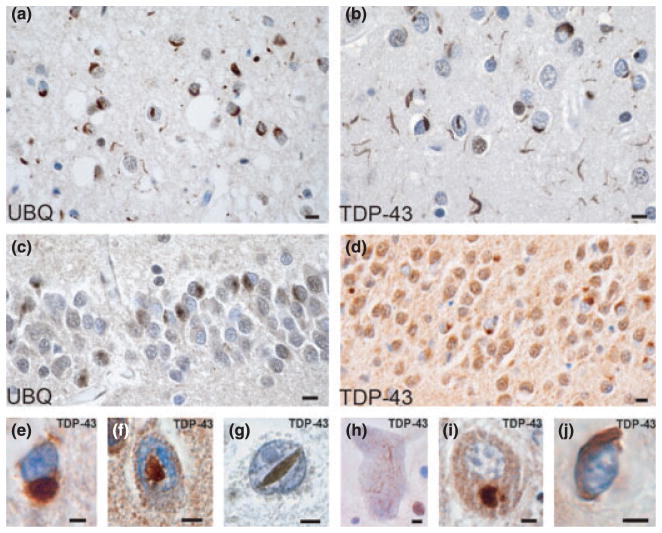
Figure 2.
TDP-43 proteinopathy in FTLD-U. Adjacent sections of superficial frontal neocortex showing neuronal cytoplasmic inclusions (NCIs), dystrophic neurites (DNs), and isolated neuronal intranuclear inclusions (NIIs), stained for both ubiquitin (a) and TDP-43 (b). NCIs in the dentate granule cells stain for ubiquitin (c) and TDP-43 (d). Neuronal and glial inclusions include: NCI (e), round and lentiform NIIs (f, g); skein-like (h) and compact round (i) NCIs in lower motor neurons; and a glial cytoplasmic inclusion (GCI) (j). (a, c) ubiquitin immunohistochemisty; (b, d, e–j) TDP-43 immunohistochemistry. Bars 10 μm (a–d); 5 μm (e–j). Source: Cairns et al. [60] with permission from the American Society for Investigative Pathology.
Neuropathologically, the TDP-43 proteinopathies are characterized by ubiquitin- and TDP-43-ir neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs), dystrophic neurites (DNs) and, in cases of MND, glial cytoplasmic inclusions (GCIs) [18,19,63–67]. The pathological inclusions of these disorders may be distinguished from other protein folding diseases which are characterized by abnormal aggregates of tau, α-synuclein, β-amyloid, neuronal intermediate filament proteins, or expanded polyglutamine repeats. TDP-43 proteinopathy is characterized biochemically by the presence of relatively insoluble ubiquitinated TDP-43-containing inclusions of which TDP-43 is abnormally phosphorylated and cleaved to produce C-terminal fragments [18,19,62,64–66]. The variability in the distribution and morphology of ub-ir or TDP-43-ir inclusions in FTLD-U has led to the development of a classification of FTLD-U into four pathological subtypes based on the morphology of the inclusion, its location within the cell, and the density and distribution in the frontal and/or temporal lobes (Fig. 3) [3,62,67]. These different pathological subtypes correlate, although imperfectly, with the molecular genetic and clinical phenotype [68]. Alternative schemes have been proposed and additional studies are required to determine their validity [62,68]. The discovery of TDP-43 as a novel misfolded protein links a spectrum of diseases including FTLD, ALS, Alzheimer’s disease [69], Lewy body disease [70] and Guam parkinsonism-dementia complex [71] by a common molecular pathology called TDP-43 proteinopathy. The identification of this protein as a major or minor component of the pathological inclusions of a spectrum of neurodegenerative disease provides new clues into the pathogenesis of protein aggregation, and it presents new targets for therapeutic intervention where none exists.
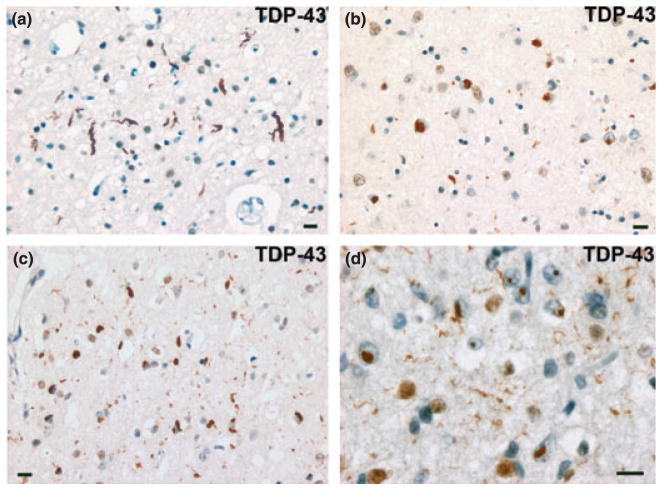
Figure 3.
FTLD-U subtypes 1–4. (a) Type 1 is characterized by long dystrophic neurites (DNs) in laminae II/III with relatively few neuronal cytoplasmic inclusions (NCIs) and no neuronal intranuclear inclusion (NII). (b) Type 2 has numerous NCIs, relatively few DNs, and no NII. (c) Type 3 has numerous NCIs and DNs and an occasional NII. (d) Type 4 pathology is characterized by numerous NIIs and DNs but few NCIs. TDP-43 immunohistochemistry. Bar 10 μm (a–d). Source: Cairns et al. [60] with permission from the American Society for Investigative Pathology.
TDP-43 proteinopathy is also a signature feature of other rare familial diseases. Inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD) is a rare autosomal dominant disorder caused by a mutation in the VCP gene [32,33,62]. IBMPFD is a distinct subtype of FTLD-U with numerous DNs and NIIs [33,62]. As with cases of FTLD-U with GRN mutation, the ubiquitinated inclusions are not primarily composed of the mutated protein VCP, but rather TDP-43 [62]. Recently, a new genetic locus on chromosome 9p for familial ALS with or without FTLD has been described [34,35]. Neuropathology of some of these families reveals the characteristic lesions of FTLD-U: ub-ir and TDP-43-ir NCIs, DNs, and NIIs indicating that another gene locus on chromosome 9 may precipitate TDP-43 proteinopathy [3].
FTLD linked to chromosome 3 is an FTLD-U with mutation in the CHMP2B gene and is an exception to the FTLD-U entities described above [31]. Early reports of this kindred described the neuropathology as ‘dementia lacking distinctive histopathology’ (DLDH). Subsequently, improved IHC methods revealed ub-ir, but TDP-43-negative, granular NCIs in frontal neo-cortex and hippocampus [62,72]. Thus, rarely, FTLD-U may not be a TDP-43 proteinopathy. In the light of these recent immunohistochemical, biochemical, and genetic advances, the Consortium for Frontotemporal Lobar Degeneration has developed an algorithm to facilitate diagnosis and revised neuropathological diagnostic criteria (Table 1) [3]. These criteria will be of value to the practicing clinician and provide a foundation for clinical, clinico-pathological, and mechanistic studies and in vivo models of the pathogenesis of FTLD.
Table 1.
Neuropathologic diagnostic criteria for FTLD
|
CHMP2B, charged multivesicular body protein 2B gene; FTLD, frontotemporal lobar degeneration; FTLD-U, FTLD with ubiquitin-positive, tau-, α-synuclein-, TDP-43-, and neuronal intermediate filament protein-negative inclusions; MAPT, microtubule-associated protein tau gene; MND, motor neuron disease; neurofibrillary tangle dementia, also called tangle predominant form of senile dementia; GRN, granulin gene; TDP-43, TAR DNA-binding protein 43; VCP, valosin-containing protein gene. Reprinted from Ref. [3] with permission of the Editor.
Clinical evaluation
In addition to being the causative gene defect in autosomal dominantly inherited FTLD with GRN mutation, a mutation in GRN may rarely occur in supposedly sporadic FTLD-U. Both behavioral and aphasic forms of FTLD can be associated with these gene defects [45–47,68], although the behavioral variant is the most common clinical phenotype associated with FTLD with GRN mutation [46,47]. Magnetic resonance imaging and 18-fluoro-deoxyglucose positron emission tomography helps discriminate AD from FTLD [73–77]. Patients with GRN mutations have predominant frontal, temporal and, to lesser extent, parietal atrophy and hypometabolism with a right-sided predominance and this probably relates to the predominance of behavioral symptoms [74]. However, language dysfunction in patients with FTLD with GRN mutation show a left-sided predominance of atrophy on imaging [45,78].
Several FTLD with GRN mutation families have been described: hereditary dysphasic disinhibition dementia families 1 and 2 (HDDD1 and 2) [79,80] and other kindreds with a similar clinical phenotype [37,75], UBC-17 [50,81], and aphasic families described by Mesulam et al. [39] and Snowden et al. [82]. Interestingly, the HDDD1 and HDDD2 kindreds are characterized pathologically by FTLD-U and additional AD-type pathology, which distinguishes them from other reported families with no or little coexisting neurodegenerative disease [36,37]. Another family with the same GRN A9D mutation has been reported in an individual with corticobasal syndrome [43] indicating, again, clinical heterogeneity associated with the same mutation. Unlike most other FTLD-U with GRN mutation families [47], the HDDD1 mutation carriers also had AD-type early-memory loss which correlated with coexisting AD pathology [40]. The overlap between FTLD-U and AD in familial cases is important as 23% of AD cases show FTLD-U type TDP-43 pathology [67].
Recent discoveries in the molecular genetics, biochemistry, and neuropathology of FTLD have transformed our understanding of this hitherto enigmatic group of diseases. TDP-43 has been identified as the major pathological protein of a spectrum of diseases which includes FTLD-U, FTLD-U with ALS, and sporadic ALS, and a minor component of the inclusions of a widening spectrum of other diseases. The physiological function of TDP-43 in the brain is currently unknown; however, it is normally localized to the nucleus of neurons and some glial cells [19,64,65]. Although the specific role of TDP-43 in neurodegeneration remains speculative, some studies indicate that this protein is directly involved in the pathogenesis of SALS, ALS with dementia and FTLD-U [18,19,60,61]. The most common FTLD is FTLD-U and accounts for more than one half of all FTLD entities in most studies [51,52]. Ubiquitin and TDP-43 immunohistochemistry may be used to distinguish four subtypes of FTLD-U which correlate with genotype and neuropathological phenotype [62,67], but mutation analysis will be required to determine the genetic cause in familial cases. Additional studies of larger groups of cases are required to determine the utility of the FTLD-U subtypes that have been described. The identification of different mutations in the GRN gene in HDDD kindreds [38,40] links these families to other FTLD-U families in which GRN mutations have been identified [36,37,43,50,68], but the HDDD kindreds appear to be distinctive both clinically and pathologically. Further studies are required to describe more accurately the clinical and neuropathological heterogeneity associated with different gene defects that result in FTLD-U.
The identification of the causative gene defect in a patient with FTLD and/or ALS may provide some insight into clinical and neuropathological variation that may be present. Clinical studies of ALS, FTLD and FTLD with ALS have found significant clinical overlap, indicating a spectrum of phenotypes, suggesting that they represent different manifestations of the same neurodegenerative disorder [17,83]. The clinical overlap between ALS and FTLD has also been illustrated in a study of 36 FTLD patients, five of whom met clinical and electrophysiological criteria for definite ALS and an additional one-third met criteria for possible ALS [15]. In contrast, in 100 consecutive ALS patients, frontal executive deficits were present in half of ALS patients, many of whom met research criteria for FTLD [16]. In patients with bulbar onset ALS, however, the incidence of FTLD has been reported as high as 48% [14]. The presence of TDP-43 proteinopathy in cases with sporadic ALS, ALS with FTLD, and FTLD links clinical phenotypes by a common molecular pathology. The absence of pathological TDP-43, however, in ALS with SOD1 mutation may partially explain why therapeutic strategies, shown to be effective in SOD1 mouse models, have not been effective in clinical trials of patients with sporadic ALS [83–85].
Conclusion
FTLD and ALS are clinically, genetically, and neuropathologically heterogeneous. FTLD can no longer be considered a rare group of disorders as it accounts for 5–10% of dementia cases in most dementia centers. Although ALS and FTLD phenotypes may be distinguished in the clinic, it is not usually possible to determine which neuropathological entity is responsible for the clinical presentation. In familial cases, molecular genetics may identify the causative gene but, at present, there is no effective treatment. Two distinct FTLD entities have been linked to chromosome 17: FTLD-U with GRN mutation and FTLD with microtubule-associated protein tau (MAPT) mutation. TDP-43 is the major pathological protein of the motor neuron inclusions found in FTLD-U, FTLD-U with ALS, SALS, FALS, but not FALS with SOD1 mutation, and G-PDC, and is a minor component of the inclusions of other disorders including the neurofibrillary tangles of AD, Lewy bodies of Parkinson’s disease and dementia with Lewy bodies, and the ubiquitinated inclusions of some cases of hippocampal sclerosis. TDP-43 proteinopathies are distinct from most other neurodegenerative disorders in which protein misfolding leads to brain amyloidosis, as pathologic TDP-43 forms neuronal and glial inclusions lacking the features of brain amyloid deposits [62]. Both HDDD kindreds are examples of FTLD-U with GRN mutation which links them to other FTLD-U with GRN mutation families. However, the HDDD kindreds appear to have coexisting memory deficits and AD-type pathology and as many as 20% AD cases have pathology similar to FTLD-U indicating that these coexisting diseases may be more frequent than previously realized. Also, TDP-43 proteinopathy may be a component of a growing number of inclusions of different neurodegenerative diseases and further studies are needed to determine the prevalence of this proteinopathy. Additional clinicopathological correlations are required to discern the relationship between genotype, neuropathology, and clinical phenotype more closely.
A rare form of familial FTLD-U is caused by mutations in the VCP gene, while other cases of FTLD with ALS are linked to chromosome 9p. The majority of inclusions of sporadic and familial FTLD-U with GRN and VCP mutations contain TDP-43 protein, but FTLD-U with CHMP2B mutation appears to be an exception with ub-ir positive and TDP-43-negative inclusions. Also, familial cases of ALS with SOD1 mutations have ub-ir, but TDP-43-negative inclusions. These data indicate that most, but not all, familial cases of FTLD or ALS are TDP-43 proteinopathies. Thus, FTLD-U and SALS represent two ends of a spectrum of disorders that are united by a common pathogenic mechanism TDP-43 proteinopathy which is reinforced by recent discoveries of pathogenic mutations in TARDBP in some FALS. These dramatic new insights into the molecular genetics and neuropathology of FTLD-U and ALS have led to a revised nosology of FTLD. These advances will contribute toward an accurate diagnosis, foster clinicopathological studies, and facilitate the quest for biomarkers and rational therapeutics.
Acknowledgments
We thank the clinical, genetic, pathology, and technical staff of the collaborating centers for making information and tissue samples available for this study and we thank the families of patients whose generosity made this research possible. This work was supported by NIH (National Institute on Aging) grants (P01-AG03991, P50-AG05681, U01-AG16976), Fulbright Grant 68428174 (RML) and CAPES.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 3.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathologica. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 5.Liscic RM, Storandt M, Cairns NJ, Morris JC. Clinical and psychometric distinction of frontotemporal and Alzheimer dementias. Archives of Neurology. 2007;64:535–540. doi: 10.1001/archneur.64.4.535. [DOI] [PubMed] [Google Scholar]
- 6.Graham A, Davies R, Xuereb J, et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain. 2005;128:597–605. doi: 10.1093/brain/awh348. [DOI] [PubMed] [Google Scholar]
- 7.Neary D, Snowden JS, Mann DM. Classification and description of frontotemporal dementias. Annals of the New York Academy of Sciences. 2000;920:46–51. doi: 10.1111/j.1749-6632.2000.tb06904.x. [DOI] [PubMed] [Google Scholar]
- 8.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 9.Strong MJ, Kesavapany S, Pant HC. The pathobiology of ALS: a proteinopathy? Journal of Neuropathology and Experimental Neurology. 2005;64:649–664. doi: 10.1097/01.jnen.0000173889.71434.ea. [DOI] [PubMed] [Google Scholar]
- 10.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuyama Y. Presenile dementia with motor neuron disease in Japan: clinicopathological review of 26 cases. Journal of Neurology, Neurosurgery and Psychiatry. 1984;47:953–959. doi: 10.1136/jnnp.47.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N. Frontal lobe dementia and motor neuron disease. Journal of Neurology, Neurosurgery and Psychiatry. 1990;53:23–32. doi: 10.1136/jnnp.53.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massman PJ, Sims J, Cooke N, Haverkamp LJ, Appel V, Appel SH. Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 1996;61:450–455. doi: 10.1136/jnnp.61.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portet F, Cadilhac C, Touchon J, Camu W. Cognitive impairment in motor neuron disease with bulbar onset. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders. 2001;2:23–29. doi: 10.1080/146608201300079382. [DOI] [PubMed] [Google Scholar]
- 15.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 16.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60:1094–1097. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. Journal of Neuropathology and Experimental Neurology. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. [DOI] [PubMed] [Google Scholar]
- 18.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochemical and Biophysical Research Communications. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 19.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 20.Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Annals of Neurology. 1993;33:200–207. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya K, Ozawa E, Fukushima J, et al. Rapidly progressive aphasia and motor neuron disease: a clinical, radiological, and pathological study of an autopsy case with circumscribed lobar atrophy. Acta Neuropathologica. 2000;99:81–87. doi: 10.1007/pl00007411. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya K, Ikeda K, Haga C, et al. Atypical amyotrophic lateral sclerosis with dementia mimicking frontal Pick’s disease: a report of an autopsy case with a clinical course of 15 years. Acta Neuropathologica. 2001;101:625–630. doi: 10.1007/s004010000336. [DOI] [PubMed] [Google Scholar]
- 23.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA, Knopman DS, Whitwell JL, et al. Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- 25.Gunnarsson LG, Dahlbom K, Strandman E. Motor neuron disease and dementia reported among 13 members of a single family. Acta Neurologica Scandinavica. 1991;84:429–433. doi: 10.1111/j.1600-0404.1991.tb04983.x. [DOI] [PubMed] [Google Scholar]
- 26.Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Archives of Neurology. 1999;56:817–822. doi: 10.1001/archneur.56.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird T, Knopman D, van Swieten J, et al. Epidemiology and genetics of frontotemporal dementia/Pick’s disease. Annals of Neurology. 2003;54(Suppl 5):S29–S31. doi: 10.1002/ana.10572. [DOI] [PubMed] [Google Scholar]
- 28.Polvikoski TM, Murray A, Harper PS, Neal JW. Familial motor neurone disease with dementia: phenotypic variation and cerebellar pathology. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1516–1520. doi: 10.1136/jnnp.74.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosso SM, Donker KL, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 30.Martinaud O, Laquerriere A, Guyant-Marechal L, et al. Frontotemporal dementia, motor neuron disease and tauopathy: clinical and neuropathological study in a family. Acta Neuropathologica. 2005;110:84–92. doi: 10.1007/s00401-005-1028-2. [DOI] [PubMed] [Google Scholar]
- 31.Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nature Genetics. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 32.Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature Genetics. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 33.Forman MS, Mackenzie IR, Cairns NJ, et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. Journal of Neuropathology and Experimental Neurology. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Morita M, Al Chalabi A, Andersen PM, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- 35.Vance C, Al Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain. 2006;129:868–876. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- 36.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 37.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee O, Pastor P, Cairns NJ, et al. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Annals of Neurology. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesulam M, Johnson N, Krefft TA, et al. Progranulin mutations in primary progressive aphasia- the PPA1 and PPA3 families. Archives of Neurology. 2007;64:314–322. doi: 10.1001/archneur.64.1.43. [DOI] [PubMed] [Google Scholar]
- 40.Behrens MI, Mukherjee O, Tu PH, et al. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer Disease and Associated Disorders. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- 41.Benussi L, Binetti G, Sina E, et al. A novel deletion in progranulin gene is associated with FTDP-17 and CBD. Neurobiology of Aging. 2008;29:427–435. doi: 10.1016/j.neurobiolaging.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Masselis M, Momeni P, Meschino W, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain. 2006;129:3115–3123. doi: 10.1093/brain/awl276. [DOI] [PubMed] [Google Scholar]
- 43.Spina S, Murrell J, Huey E, et al. Corticobasal syndrome associated with the A9D progranulin mutation. Journal of Neuropathology and Experimental Neurology. 2007;66:892–900. doi: 10.1097/nen.0b013e3181567873. [DOI] [PubMed] [Google Scholar]
- 44.Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Human Molecular Genetics. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 45.Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a progranulin mutation. Annals of Neurology. 2006;60:374–380. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. Journal of Neuropathology and Experimental Neurology. 2006;66:142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 47.Rademakers R, Baker M, Gass J, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C–>T (Arg493X) mutation: an international initiative. Lancet Neurology. 2007;6:857–868. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- 48.Spina S, Murrell JR, Huey ED, et al. Clinicopathologic features of frontotemporal dementia with progranulin sequence variation. Neurology. 2007;68:820–827. doi: 10.1212/01.wnl.0000254460.31273.2d. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee O, Wang J, Gitcho M, et al. A Molecular characterization of novel progranulin (GRN) mutations in frontotemporal dementia. Human Mutation. 2008;29:512–521. doi: 10.1002/humu.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie IR, Baker M, Pickering-Brown S, et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda M, Ishikawa T, Tanabe H. Epidemiology of frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders. 2004;17:265–268. doi: 10.1159/000077151. [DOI] [PubMed] [Google Scholar]
- 52.Rademakers R, Cruts M, van Broeckhoven C. Epidemiology of frontotemporal dementia and related tauopathies. Human Mutation. 2004;24:277–295. doi: 10.1002/humu.20086. [DOI] [PubMed] [Google Scholar]
- 53.Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathology and Applied Neurobiology. 2004;30:369–373. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 54.Gitcho MA, Baloh RH, Chakraverty S, et al. TDP-43 A315T mutation in familial motor neuron disease. Annals of Neurology. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kabashi E, Valdmanis PN, Dion P, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genetics. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 57.Van Deerlin VM, Leverenz JB, Bekris LM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurology. 2008;5:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipton AM, White CL, III, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathologica. 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- 59.Leigh PN, Whitwell H, Garofalo O, et al. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain. 1991;114:775–788. doi: 10.1093/brain/114.2.775. [DOI] [PubMed] [Google Scholar]
- 60.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Annals of Neurology. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 61.Tan CF, Eguchi H, Tagawa A, et al. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutations. Acta Neuropathologica. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- 62.Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. American Journal of Pathology. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woulfe J, Kertesz A, Munoz DG. Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathologica. 2001;102:94–102. doi: 10.1007/s004010000346. [DOI] [PubMed] [Google Scholar]
- 64.Neumann M, Kwong LK, Truax AC, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Journal of Neuropathology and Experimental Neurology. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 65.Neumann M, Kwong LK, Sampathu DM, Trojanowski JQ, Lee VM-Y. TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Archives of Neurology. 2007;64:1388–1394. doi: 10.1001/archneur.64.10.1388. [DOI] [PubMed] [Google Scholar]
- 66.Neumann M, Mackenzie IR, Cairns NJ, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. Journal of Neuropathology and Experimental Neurology. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 67.Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. American Journal of Pathology. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackenzie IRA, Rademakers R. The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics. 2007;8:237–248. doi: 10.1007/s10048-007-0102-4. [DOI] [PubMed] [Google Scholar]
- 69.Amador-Ortiz C, Lin W-L, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Annals of Neurology. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathologica (Berlin) 2007;114:221–230. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 71.Hasegawa M, Arai T, Akiyama H, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 72.Holm IE. Ubiquitin-positive inclusions in frontotemporal dementia linked to chromosome 3 (FTD-3) Brain Pathology. 2006;16(Suppl 1):S43. [Google Scholar]
- 73.Davion S, Johnson N, Weintraub S, et al. Clinicopathological correlation in PGRN mutations. Neurology. 2007;69:1113–1121. doi: 10.1212/01.wnl.0000267701.58488.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joeng Y, Cho SS, Park JM, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. Journal of Nuclear Medicine. 2005;46:233–239. [PubMed] [Google Scholar]
- 75.Rabinovici GD, Furst AJ, O’Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 76.Whitwell JL, Clifford RJ, Jr, Baker M, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Archives of Neurology. 2007;64:371–376. doi: 10.1001/archneur.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioral disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Zee J, Rademakers R, Engelborghs S, et al. A Belgian ancestral haplotype harbours a highly prevalent mutation for 17q21-linked tau-negative FTLD. Brain. 2006;129:841–852. doi: 10.1093/brain/awl029. [DOI] [PubMed] [Google Scholar]
- 79.Morris JC, Cole M, Banker BQ, Wright D. Hereditary dysphasic dementia and the Pick-Alzheimer spectrum. Annals of Neurology. 1984;16:455–466. doi: 10.1002/ana.410160407. [DOI] [PubMed] [Google Scholar]
- 80.Lendon CL, Lynch T, Norton J, et al. Hereditary dysphasic disinhibition dementia: a frontotemporal dementia linked to 17q21-22. Neurology. 1998;50:1546–1555. doi: 10.1212/wnl.50.6.1546. [DOI] [PubMed] [Google Scholar]
- 81.Mackenzie IR, Baker M, West G, et al. A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain. 2006;129:853–867. doi: 10.1093/brain/awh724. [DOI] [PubMed] [Google Scholar]
- 82.Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129:3091–3102. doi: 10.1093/brain/awl267. [DOI] [PubMed] [Google Scholar]
- 83.Strong MJ, Lomen-Hoerth C, Caselli RJ, Bigio EH, Yang W. Cognitive impairment, frontotemporal dementia, and the motor neuron diseases. Annals of Neurology. 2003;54(Suppl 5):S20–S23. doi: 10.1002/ana.10574. [DOI] [PubMed] [Google Scholar]
- 84.Ludolph AC, Sperfeld AD. Preclinical trials: an update on translational research in ALS. Neuro-degenerative Diseases. 2005;2:215–219. doi: 10.1159/000089628. [DOI] [PubMed] [Google Scholar]
- 85.DiBernardo AB, Cudkowicz ME. Translating preclinical insights into effective human trials in ALS. Biochimica et Biophysica Acta. 2006;1762:1139–1149. doi: 10.1016/j.bbadis.2006.03.007. [DOI] [PubMed] [Google Scholar]