Abstract
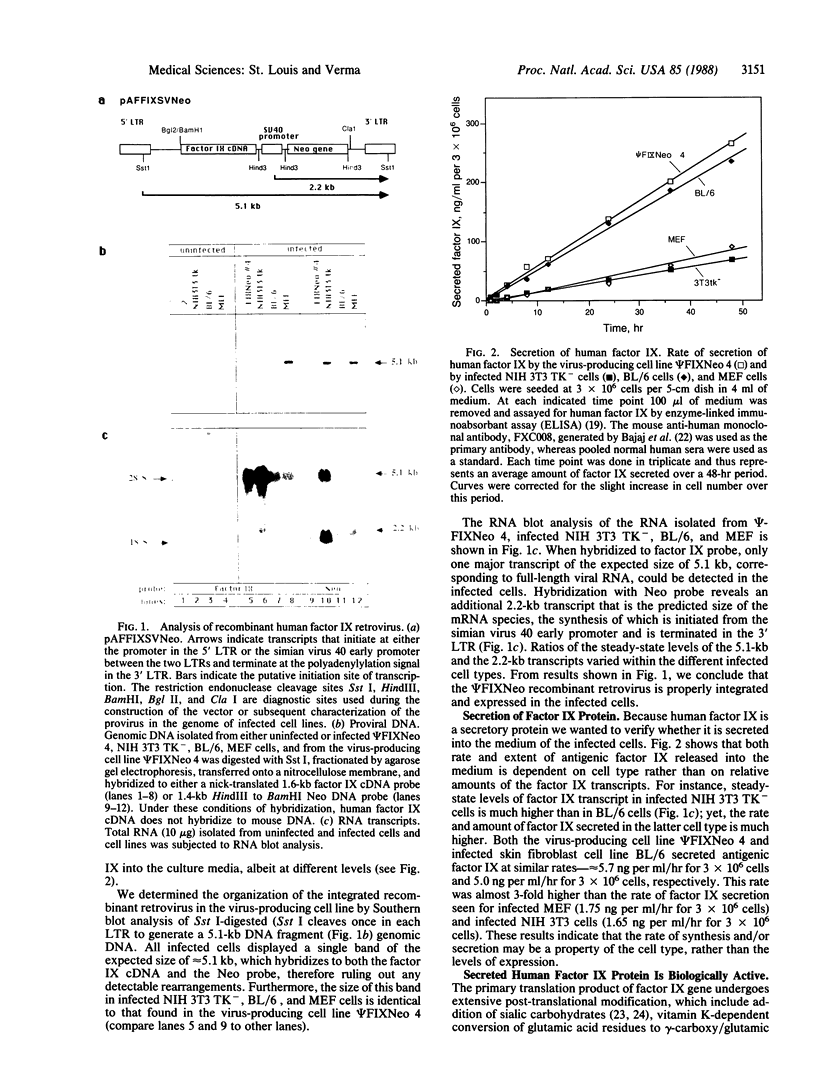
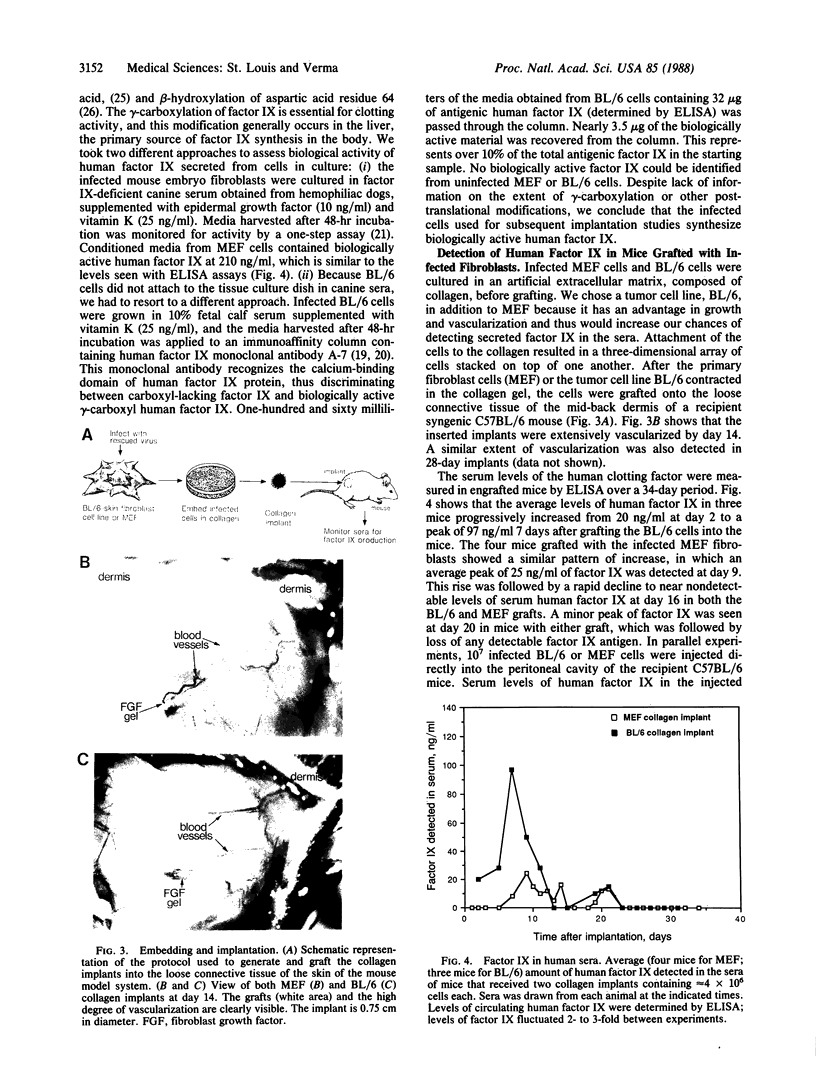
Mouse primary skin fibroblasts were infected with a recombinant retrovirus containing human factor IX cDNA. Bulk infected cells capable of synthesizing and secreting biologically active human factor IX protein were embedded in collagen, and the implant was grafted under the epidermis. Sera from the transplanted mice contain human factor IX protein for at least 10-12 days. Loss of immunoreactive human factor IX protein in the mouse serum is not due to graft rejection. Instead, the mouse serum contains anti-human factor IX antibodies, which react with the protein. We suggest that retroviral-infected primary skin fibroblasts offer an alternative approach to somatic cell gene therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson D. S., Hock R. A., Austen D., Smith K. J., Brownlee G. G., Verma I. M., Miller A. D. Towards gene therapy for hemophilia B. Mol Biol Med. 1987 Feb;4(1):11–20. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Maki S. L. A monoclonal antibody to factor IX that inhibits the factor VIII:Ca potentiation of factor X activation. J Biol Chem. 1985 Sep 25;260(21):11574–11580. [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavin S. I., Weidner S. M. Blood clotting factor IX. Loss of activity after cleavage of sialic acid residues. J Biol Chem. 1984 Mar 25;259(6):3387–3390. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J. Beta-hydroxyaspartic acid in vitamin K-dependent proteins. J Biol Chem. 1983 Oct 25;258(20):12509–12512. [PubMed] [Google Scholar]
- Garver R. I., Jr, Chytil A., Courtney M., Crystal R. G. Clonal gene therapy: transplanted mouse fibroblast clones express human alpha 1-antitrypsin gene in vivo. Science. 1987 Aug 14;237(4816):762–764. doi: 10.1126/science.3497452. [DOI] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chytil A., Karlsson S., Fells G. A., Brantly M. L., Courtney M., Kantoff P. W., Nienhuis A. W., Anderson W. F., Crystal R. G. Production of glycosylated physiologically "normal" human alpha 1-antitrypsin by mouse fibroblasts modified by insertion of a human alpha 1-antitrypsin cDNA using a retroviral vector. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1050–1054. doi: 10.1073/pnas.84.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. Antibody probing of western blots which have been stained with india ink. Anal Biochem. 1986 Aug 1;156(2):315–319. doi: 10.1016/0003-2697(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. C., Chung K. S., Roberts H. R. A simple assay for human factor IX: use of canine hemophilia B plasma as substrate. Thromb Res. 1978 Mar;12(3):497–502. doi: 10.1016/0049-3848(78)90320-1. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C. F., Hardy C., Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984 Sep;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Darlington G. J., Hahn T., Woo S. L. Retroviral gene transfer into primary hepatocytes: implications for genetic therapy of liver-specific functions. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5335–5339. doi: 10.1073/pnas.84.15.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., McGinnis-Shelnutt M., Woo S. L. Retroviral-mediated gene transfer of human phenylalanine hydroxylase into NIH 3T3 and hepatoma cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):409–413. doi: 10.1073/pnas.83.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Morgan J. R., Barrandon Y., Green H., Mulligan R. C. Expression of an exogenous growth hormone gene by transplantable human epidermal cells. Science. 1987 Sep 18;237(4821):1476–1479. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Hock R. A., Osborne W. R., Miller A. D. Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1055–1059. doi: 10.1073/pnas.84.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Skoskiewicz M. J., Howie K. B., Russell P. S., Goodman H. M. Implantation of genetically engineered fibroblasts into mice: implications for gene therapy. Science. 1987 May 8;236(4802):714–718. doi: 10.1126/science.3472348. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Singaraju C., Smith L. F. Factor IX metal ion-dependent antigen assays for measurement of warfarin effect. Am J Clin Pathol. 1987 Mar;87(3):370–376. doi: 10.1093/ajcp/87.3.370. [DOI] [PubMed] [Google Scholar]
- Sorge J., Kuhl W., West C., Beutler E. Complete correction of the enzymatic defect of type I Gaucher disease fibroblasts by retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(4):906–909. doi: 10.1073/pnas.84.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J. W. Mechanism of action of vitamin K: synthesis of gamma-carboxyglutamic acid. CRC Crit Rev Biochem. 1980;8(2):191–223. doi: 10.3109/10409238009105469. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Yee J. K., Skelly H. F., Moores J. C., Respess J. G., Friedmann T., Leffert H. Expression of retrovirally transduced genes in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3344–3348. doi: 10.1073/pnas.84.10.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]









