Abstract
In human neutrophils, TNF-elicited O2− production requires adherence and integrin activation. How this cooperative signaling between TNFRs and integrins regulates O2− generation has yet to be fully elucidated. Previously, we identified δ-PKC as a critical early regulator of TNF signaling in adherent neutrophils. In this study, we demonstrate that inhibition of δ-PKC with a dominant-negative δ-PKC TAT peptide resulted in a significant delay in the onset time of TNF-elicited O2− generation but had no effect on Vmax, indicating an involvement of δ-PKC in the initiation of O2− production. In contrast, fMLP-elicited O2− production in adherent and nonadherent neutrophils was δ-PKC-independent, suggesting differential regulation of O2− production. An important step in activation of the NADPH oxidase is phosphorylation of the cytosolic p47phox component. In adherent neutrophils, TNF triggered a time-dependent association of δ-PKC with p47phox, which was associated with p47phox phosphorylation, indicating a role for δ-PKC in regulating O2− production at the level of p47phox. Activation of ERK and p38 MAPK is also required for TNF-elicited O2− generation. TNF-mediated ERK but not p38 MAPK recruitment to p47phox was δ-PKC-dependent. δ-PKC activity is controlled through serine/threonine phosphorylation, and phosphorylation of δ-PKC (Ser643) and δ-PKC (Thr505) was increased significantly by TNF in adherent cells via a PI3K-dependent process. Thus, signaling for TNF-elicited O2− generation is regulated by δ-PKC. Adherence-dependent cooperative signaling activates PI3K signaling, δ-PKC phosphorylation, and δ-PKC recruitment to p47phox. δ-PKC activates p47phox by serine phosphorylation or indirectly through control of ERK recruitment to p47phox.
Keywords: superoxide anion generation, p47phox, fMet-Leu-Phe, ERK, PI 3-kinase, phosphorylation
Introduction
Neutrophils are key components of host defense against infection and tissue injury. Neutrophils ingest and kill invading microorganisms through the release of toxic oxygen radicals and proteases. However, dysregulation of neutrophil function contributes to tissue damage, characteristic of the inflammatory process. During inflammatory diseases such as sepsis, inappropriate neutrophil regulation contributes to the pathophysiology of the disease through excessive release of oxygen radicals, proteases, lipid mediators, and cytokines. TNF and other proinflammatory cytokines are important regulators of neutrophil function during the inflammatory response [1,2,3]. Neutrophils possess two TNFRs: a 55- to 60-kDa (TNFR-1) and a 75- to 80-kDa (TNFR-2) receptor; proinflammatory signaling is regulated principally by TNFR-1 [4,5,6,7,8]. Full activation of neutrophils by proinflammatory mediators, such as TNF, requires adherence and ligation of integrins [9,10,11]. Cooperative signaling between integrins and TNF is essential to elicit secretion of O2− and the release of toxic mediators [1, 9, 10, 12,13,14,15].
Previously, we identified δ-PKC and PI3K as critical early regulators of TNFR-1-activated signaling in adherent neutrophils [13, 16,17,18,19]. δ-PKC is a positive regulator of TNF-mediated antiapoptotic signaling and activation of NF-κB [17,18,19,20]. δ-PKC regulates TNF-mediated activation of the MAPK ERK, but not p38 MAPK, indicating selective regulation of TNF signaling in neutrophils [17]. In adherent neutrophils, δ-PKC is recruited to TNFR-1 in response to TNF and is required for assembly of a TNFR-1 signaling complex composed of TNFR-associated death domain, TNFR-associated factor 2, and receptor-interacting protein [18]. The recruitment of δ-PKC to the TNFR-1 complex in adherent neutrophils requires PI3K activity [18, 19]. PI3K is only activated by TNF through cooperative signaling with integrins [13]. Furthermore, PI3K activation is required for antiapoptotic signaling and O2− generation [13, 18, 19].
Production of O2− requires the assembly of an active NADPH oxidase, including the translocation of the cytosolic components p47phox, p67phox, and rac2 to the plasma membrane, where they interact with cytochrome b558 [21,22,23]. Assembly of an active NADPH oxidase for O2− generation requires phosphorylation and translocation of the cytosolic factor p47phox. In cell-free systems, p47phox is phosphorylated by Akt, ERK, and several PKC isotypes, including δ-PKC [24,25,26,27,28].
δ-PKC is a member of the PKC family, a phospholipid-dependent family of serine/threonine kinases, and is present in human neutrophils [29]. δ-PKC activation is a multistep process that regulates its activity and substrate specificity [30,31,32] and includes threonine phosphorylation in the activation loop [δ-PKC (Thr505)], a step mediated by the PI3K/PDK1/Akt signaling pathway [33]. This phosphorylation step then leads to an autophosphorylation step and phosphorylation of δ-PKC at Ser643.
To examine a role for δ-PKC in signaling for TNF-elicited O2− generation, we have used a cell-permeant inhibitory peptide to target δ-PKC selectively. This unique peptide antagonist to δ-PKC is derived from the first unique region (V1) of δ-PKC [34]. Unlike rottlerin, which targets the ATP-binding site of the kinase, the δ-PKC TAT peptide acts as a dominant-negative kinase unique to δ-PKC and does not affect other PKC isotypes such as α-PKC, β-PKC, or ζ-PKC [17, 18, 30, 34]. Coupling of the δ-PKC inhibitory peptide to a membrane-permeant peptide sequence in the HIV TAT gene product allows effective intracellular delivery into target cells [31, 34]. This highly selective inhibitor permits examination of δ-PKC activity directly in human neutrophils, which are end-stage cells, and thus, siRNA studies are not feasible. Our previous studies demonstrated that this δ-PKC TAT peptide is effective in modulating neutrophil function and blocks TNF-mediated antiapoptotic signaling [17, 18]. In this study, using the selective δ-PKC TAT peptide inhibitor, we determined that δ-PKC is a key signaling component in the activation of the NADPH oxidase by TNF in adherent human neutrophils.
MATERIALS AND METHODS
Reagents
Human rTNF was obtained from R&D Systems (Minneapolis, MN, USA). Human plasma FN was purchased from Life Technologies (Gaithersburg, MD, USA). Cytochrome c, cytochalasin B, fMLP, EGTA, Na-orthovanadate, 4-(2-aminoethyl)-benzenesulfonyl fluoride, leupeptin, protease inhibitor cocktail, and phosphatase inhibitor cocktail were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit polyclonal antibodies against ERK1/2, p38 MAPK, Ser241-phosphorylated PDK1, PDK1, Thr505-phosphorylated δ-PKC, and Ser643-phosphorylated δ-PKC were purchased from Cell Signaling Technology (Beverly, MA, USA). A rabbit polyclonal δ-PKC antibody, rabbit polyclonal p47phox antibody, goat polyclonal p47phox antibody, goat anti-rabbit IgG-HRP, and protein A/G PLUS agarose were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA) and BS3 were purchased from Invitrogen. The PI3K inhibitor LY294002 was obtained from Calbiochem (San Diego, CA, USA). The p38 MAPK inhibitor SB203580 and the MEK inhibitors PD098059 and U0126 were obtained from BioMol (Plymouth Meeting, PA, USA). Membrane-blocking solution and a polyclonal antibody to phosphoserine were obtained from Zymed Laboratories (San Francisco, CA, USA). SuperSignal ULTRA chemiluminescence substrate and BCA reagents were obtained from Pierce (Rockford, IL, USA).
δ-PKC inhibitor peptide synthesis
δ-PKC was inhibited selectively using a δV1.1 PKC TAT peptide antagonist that consists of a peptide derived from the first unique region (V1) of δ-PKC (SFNSYELGSL: aa 8–17 of δ-PKC) coupled to a membrane-permeant peptide sequence in the HIV TAT gene product (YGRKKRRQRRR: aa 47–57 of TAT), according to the method of Mochly-Rosen and co-workers [34]. The δ-PKC peptide was cross-linked by an N-terminal Cys-Cys bond to the membrane-permeable TAT peptide [32]. The use of a Cys-Cys disulfide bond between the δ-PKC inhibitory peptide and the TAT peptide transporter permits directed intracellular delivery of the δ-PKC TAT inhibitor. Once the peptide has been taken up by the cells, the disulfide bond is cleaved, thereby trapping the inhibitory peptide intracellularly [31, 35]. A carrier-carrier dimer of the TAT peptide alone was used as a control. The peptides were synthesized by Mimotopes (Melbourne, Australia) by 9-fluorenylmethoxycarbonyl solid-phase chemistry. Peptides were purified to >95% by preparative reverse-phase HPLC [17, 32, 34].
Preparation of human neutrophils
Neutrophils were isolated from heparinized venous blood (10 U/ml), obtained from healthy adult donors, following informed consent, in accordance with Institutional Review Board protocols at the Children’s Hospital of Philadelphia and Temple University (Philadelphia, PA, USA). Donors were healthy adult males and females over the age of 18 who were recruited from the Children’s Hospital of Philadelphia and Temple University community. Standard isolation techniques [36] were used with Ficoll-Hypaque centrifugation, followed by dextran sedimentation and hypotonic lysis to remove residual erythrocytes. Cells were suspended in 10 mM Hepes buffer (pH 7.4). Neutrophil viability was >98%, as determined by trypan blue exclusion. For adherence experiments, neutrophils were incubated on FN-coated or tissue culture-treated plastic plates for 30 min at 37°C. FN-coated wells were prepared according to the method of Nathan et al. [9] using a concentration of 3.4 μg/well.
O2− generation
The generation of O2− was measured as SOD-inhibitable cytochrome c reduction [13, 37]. For studies with nonadherent neutrophils, cells were activated by 1 μM fMLP in the presence of 5 μg/ml cytochalasin B, and the generation of O2− was measured spectrophotometrically over a 10-min time period. For studies with adherent cells, neutrophils were incubated in FN-coated or tissue culture-treated, plastic 96-well plates at a concentration of 1 × 106 cells/well at 37°C for 30 min prior to the addition of TNF or fMLP and measured over a 90- to 120-min time period. For experiments examining the role of δ-PKC in O2− generation, neutrophils were pretreated with buffer, δ-PKC TAT peptide inhibitor (1 μM), or TAT carrier peptide (1 μM), as described previously [17]. For ERK and p38 MAPK inhibitor experiments, neutrophils were pretreated in the absence or presence of the MEK1/2 inhibitors PD098059 (10 μM) or U0126 (10 μM) or the p38 MAPK inhibitor SB203580 (10 μM) for 30 min at 37°C prior to the addition of TNF. The inhibitors were used at concentrations that have been shown to inhibit ERK and p38 MAPK activity effectively in neutrophils [38, 39].
IP of δ-PKC and p47phox
Neutrophils (35×106 cells/condition) were maintained in suspension or plated onto FN-coated wells and incubated for 30 min at 37°C. Samples were then incubated with TNF (25 ng/ml) over a 60-min incubation period. At various time intervals, samples were placed on ice, lysed in IP buffer, and vortexed for 20 min at 4°C. The IP buffer consisted of 10 mM Hepes (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1 mM Na-orthovanadate, 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.2% Nonidet P-40, 5 μg/ml leupeptin, Sigma phosphatase inhibitor cocktail, and Sigma protease inhibitor cocktail. IP of δ-PKC was accomplished by incubation of cell lysates with a rabbit polyclonal anti-δ-PKC overnight at 4°C, followed by incubation with A/G PLUS agarose for 1 h at 4°C. The agarose pellet was collected and washed, and bound proteins were eluted by incubation with 2× SDS-PAGE sample buffer for 5 min at 95°C.
For p47phox IP experiments, quantitating p47phox phosphorylation and recruitment of δ-PKC, p38 MAPK, and ERK, the goat anti-p47phox was cross-linked to Dynabeads Protein G with BS3, according to the manufacturer’s instructions. Briefly, 5 μg goat anti-p47phox was mixed with washed Dynabeads Protein G and incubated for 10 min at room temperature. The beads were washed to remove unbound antibody, resuspended in 20 mM sodium phosphate, 0.15 mM NaCl (pH 7–9), containing 5 mM BS3, and incubated for 30 min at room temperature. The reaction was stopped by adding 1 M Tris HCL (pH 7.5), incubated for 15 min at room temperature, and then washed thoroughly. The cell lysates were precleared by incubating the lysates with 1.0 μg IgG from goat serum together with 20 μl protein A/G PLUS agarose for 30 min at 4°C. The cell lysates were then incubated overnight at 4°C with the cross-linked antibody. The Dynabeads pellets were then washed three times with IP buffer. The samples were eluted by incubation with 2× SDS-PAGE sample buffer for 5 min at 95°C.
IP δ-PKC and p47phox were separated on a 4–12% gradient SDS-PAGE and transferred to nitrocellulose membranes. The phosphorylation state of δ-PKC was determined by Western blot analysis using phospho-specific antibodies, phospho-δ-PKC (Ser643), and phospho-δ-PKC (Thr505), as described previously [40]. Equal loading of δ-PKC was confirmed by reprobing membranes using antibodies that recognize phosphorylated and nonphosphorylated forms of δ-PKC. Serine phosphorylation of p47phox was determined by Western blotting using a phosphoserine antibody. Equal loading of p47phox was confirmed by reprobing the membranes with a rabbit polyclonal anti-p47phox. Coimmunoprecipitation of ERK, p38 MAPK, and δ-PKC was determined by Western blotting.
Measurement of PDK1 phosphorylation
Neutrophils (20×106 cells/well) were incubated in suspension or in FN-coated six-well plates at 37°C. Following incubation with buffer or TNF (25 ng/ml) at 37°C for 5 min, the cells were harvested and cell lysates prepared. The cells were lysed in buffer containing 10 mM Hepes (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1 mM Na-orthovanadate, 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1% Triton X-100, 5 μg/ml leupeptin, Sigma phosphatase inhibitor cocktail, and Sigma protease inhibitor cocktail. Protein concentrations of the cell lysates were determined by the BCA protein assay kit, according to the manufacturer’s instructions (Pierce). Proteins were separated on 4–12% SDS-PAGE gels at a protein concentration of 30 μg/lane. PDK1 activation was determined by immunoblotting of cell lysates using a phospho-specific antibody for PDK1 (Ser241). Equal loading of PDK1 was confirmed by reprobing membranes using an antibody that recognizes phosphorylated and nonphosphorylated forms of PDK1. For experiments examining the role of PI3K in PDK1 activation, neutrophils were incubated with the PI3K inhibitor LY294002 (10 μM) for 20 min prior to the addition of TNF.
Statistical analysis
Results are expressed as mean ± se for number (n) of studies performed. Data were analyzed by Student’s t-test for two group comparisons or ANOVA followed for multiple comparisons. The Tukey-Kramer multiple comparisons post-test was used to evaluate the significance between experimental groups if ANOVA indicated a significant difference; differences were considered significant when P < 0.05.
RESULTS
TNF-mediated O2− generation is δ-PKC-dependent
TNF elicited O2− generation in neutrophils requires adherence and is mediated via the TNFR-1 complex [9, 10, 12, 41]. Adherence of human neutrophils to ECM proteins such as FN produces significant alterations in the kinetics of oxygen radical production in response to soluble mediators. There is a significant delay, lasting from 15 to 60 min, followed by O2− generation, which is enhanced significantly as compared with nonadherent neutrophil responses to the same stimuli [9]. To determine whether δ-PKC is a regulator of TNF-elicited O2− generation in FN-adherent neutrophils, human neutrophils were pretreated with the selective, cell-permeant δ-PKC TAT inhibitory peptide, a TAT carrier control peptide, or buffer alone. Previous studies demonstrated that this dominant-negative δ-PKC TAT peptide inhibits TNF-mediated activation of δ-PKC in neutrophils [17, 18].
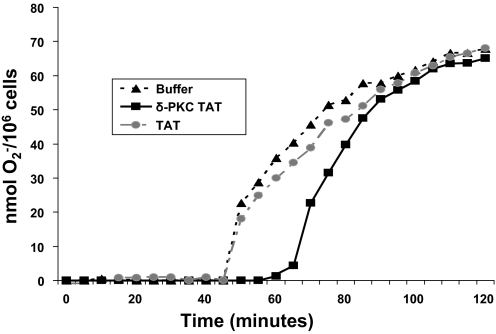
When stimulated with TNF (25 ng/ml), FN-adherent neutrophils produced significant quantities of O2− over a 2-h time period (Fig. 1 and Table 1). In agreement with previous studies, we observed a 30- to 45-min delay in O2− production following TNF stimulation of adherent neutrophils [9, 10, 42, 43]. Most of the O2− was produced within 90 min, following the addition of TNF (Fig. 1 and Table 1). Pretreatment of human neutrophils with the dominant-negative δ-PKC TAT peptide resulted in a significant delay in the onset of TNF-mediated O2− generation but had no effect on the Vmax of the reaction (Fig. 1 and Table 1). The delay in onset of O2− generation in response to TNF produced a 65% decrease of O2− generation at 60 min and a 25% decrease at 90 min (Fig. 1 and Table 1). However, by 120 min, there were no significant differences in the amount of O2− produced (Fig. 1 and Table 1). Conversely, pretreatment with the TAT carrier alone had no significant effect on onset time, Vmax, or total O2− produced (Fig. 1 and Table 1). No significant O2− was generated by neutrophils in the absence of stimuli or by the addition of the TAT carrier or the δ-PKC TAT peptide alone (data not shown). Thus, although inhibition of δ-PKC significantly delayed the onset time of O2− production and the time required to achieve maximal O2−, it did not alter the level of maximal O2− generation in response to TNF. Similar to FN-adherent neutrophils, pretreatment of neutrophils adherent to tissue culture-treated polystyrene with the δ-PKC TAT inhibitory peptide also delayed the onset time of TNF-elicited O2− production (onset time=43±3 min for buffer vs. 63±6 min for δ-PKC TAT peptide-treated neutrophils; n=4 donors in triplicates; P<0.01). These results indicate that the role for δ-PKC in TNF-elicited O2− production is not limited to neutrophils adherent to FN and is part of a more general mechanism. Thus, δ-PKC is a positive regulator of TNF-elicited assembly and activation of the NADPH oxidase for O2− generation in adherent neutrophils.
Figure 1.
TNF-elicited O2− generation in adherent neutrophils is δ-PKC-dependent. TNF-mediated O2− generation was determined in FN-adherent neutrophils pretreated with the specific δ-PKC TAT peptide inhibitor (1 μM), TAT carrier peptide (1 μM), or buffer alone, prior to the addition of TNF (25 ng/ml). O2− generation was measured spectrophotometrically, as SOD-inhibitable reduction of cytochrome c over 120 min (see Materials and Methods). Results are expressed as nmol O2−/106 cells. (Representative graph from four separate donors done in triplicates.)
TABLE 1.
TNF-Elicited O2− Generation Requires δ-PKC
| Buffer | TAT carrier | δ-PKC TAT | |
|---|---|---|---|
| Time of onset (min) | 45 ± 1.9 | 45.5 ± 3.3 | 63 ± 4.5a |
| Vmax (nmol O2−/min) | 3.4 ± 0.2 | 3.5 ± 0.3 | 3.4 ± 0.2 |
| Total nmol O2−/106 cells/60 min | 31 ± 3.5 | 34 ± 4.5 | 11 ± 1.8b |
| Total nmol O2−/106 cells/90 min | 61 ± 3.2 | 68 ± 4.0 | 47 ± 3.1c |
| Total nmol O2−/106 cells/120 min | 71 ± 3.2 | 72 ± 4.0 | 68 ± 3.0 |
Values are mean ± se; n = 4 separate donors run in triplicates.
P < 0.01 δ-PKC TAT versus buffer and TAT carrier;
P < 0.01 δ-PKC TAT versus buffer and TAT carrier;
P < 0.01 δ-PKC TAT versus buffer and TAT carrier.
fMLP-elicited O2− generation is independent of δ-PKC
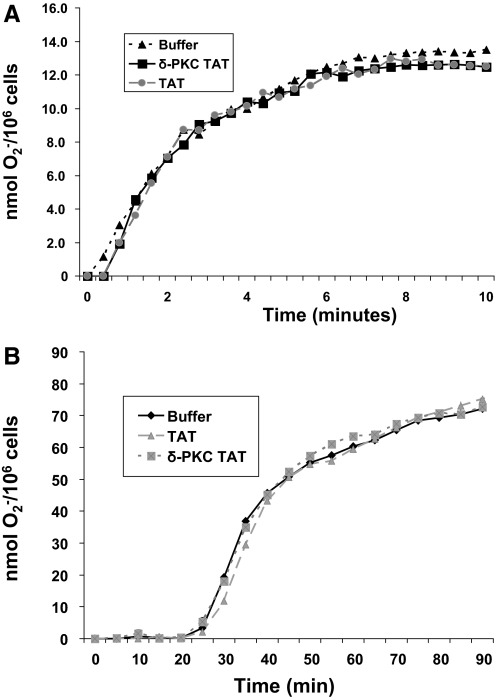
To ascertain whether the regulatory role of δ-PKC in O2− generation was adherence- or ligand-dependent, we determined the role of δ-PKC in O2− generation triggered by the bacterial peptide fMLP in adherent and nonadherent neutrophils. As shown in Figure 2A and Table 2, in nonadherent neutrophils, in the presence of cytochlasin B, fMLP triggered rapid (within 15 s) generation of O2−, which reached a plateau by ∼5 min. In FN-adherent neutrophils, fMLP triggered significant O2− generation, following a lag period of 25 min (Fig. 2B and Table 2). Pretreatment of neutrophils with the dominant-negative δ-PKC TAT peptide had no significant effect on onset time, Vmax, or O2− production triggered by fMLP in nonadherent or FN-adherent neutrophils, indicating that δ-PKC is not essential for activation of O2− generation by fMLP (Fig. 2 and Table 2). Similar findings were found when neutrophils were adherent to tissue culture-treated polystyrene-well plates (data not shown).
Figure 2.
fMLP-triggered O2− generation is δ-PKC-independent in nonadherent and adherent neutrophils. (A) O2− production was measured as described in Figure 1. Neutrophils were pretreated with δ-PKC TAT (1 μM), TAT carrier control peptide (1 μM), or buffer, prior to addition of fMLP (1 μM) + cytochlasin B in nonadherent neutrophils, and O2− generation was measured for 10 min. (Representative graph from four separate donors done in triplicates.) (B) Neutrophils were pretreated with δ-PKC TAT (1 μM), TAT carrier control peptide (1 μM), or buffer, prior to addition of fMLP (1 μM) in FN-adherent neutrophils, and O2− generation was measured for 90 min. (Representative graph from four separate donors done in triplicates.)
TABLE 2.
fMLP-Elicited O2− Generation in Nonadherent and Adherent Neutrophils Is Independent of δ-PKC
| Nonadherent neutrophils | Buffer | TAT carrier | δ-PKC TAT |
|---|---|---|---|
| Time of onset (s) | 11.5 ± 2.4 | 11.0 ± 2.5 | 9.6 ± 2.3 |
| Vmax (nmol O2−/min) | 6.1 ± 0.2 | 5.9 ± 0.3 | 6.3 ± 0.4 |
| Total nmol O2−/106 cells/10 min | 12.9 ± 0.7 | 13.6 ± 0.8 | 13.0 ± 0.6 |
| FN-adherent neutrophils | Buffer | TAT carrier | δ-PKC TAT |
|---|---|---|---|
| Time of onset (min) | 22.7 ± 1.1 | 23.8 ± 3.0 | 21.1 ± 3.4 |
| Vmax (nmol O2−/min) | 3.5 ± 0.2 | 3.2 ± 0.3 | 3.2 ± 04 |
| Total nmol O2−/106 cells/60 min | 67.7 ± 1.5 | 68.1 ± 4.1 | 67.9 ± 2.5 |
Values are mean ± se; n = 4 separate donors run in triplicates.
TNF triggers the association of δ-PKC with p47phox and phosphorylation of p47phox
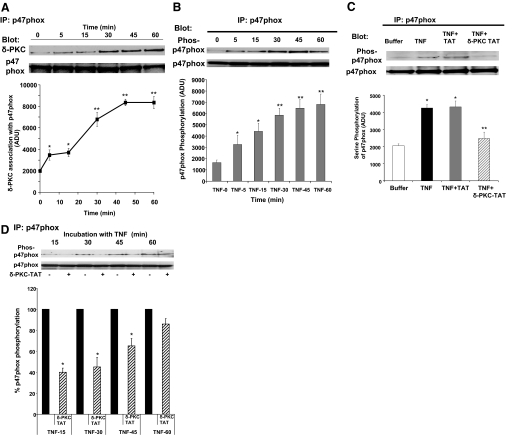
The production of O2− is highly regulated and requires the assembly of an active NADPH oxidase complex. As δ-PKC inhibition altered the lag time in the response to TNF, we focused on early events in the assembly of the NADPH oxidase and generation of O2−. An important step in the activation of the NADPH oxidase is the phosphorylation of the cytosolic p47phox. To determine whether δ-PKC associates with p47phox in TNF-activated neutrophils, p47phox was immunoprecipitated from adherent neutrophils treated with TNF for various time intervals. Coimmunoprecipitation of δ-PKC with p47phox was determined by Western blot analysis. As shown in Figure 3A, cooperative signaling between TNF and integrins resulted in the association of δ-PKC with p47phox. This association was evident by 5 min, reached maximal levels between 30 and 45 min, and was maintained at 60 min.
Figure 3.
TNF triggers the association of δ-PKC with p47phox and phosphorylation of p47phox. (A) TNF-mediated recruitment of δ-PKC to p47phox was determined by coimmunoprecipitation experiments in adherent neutrophils. FN-adherent neutrophils were incubated with TNF (25 ng/ml) for 0–60 min. p47phox was immunoprecipitated, and δ-PKC association was determined by Western blotting. Equal protein loading was determined by Western blot analysis for total p47phox. (Upper) Representative Western blot. (Lower) Densitometry analysis of TNF-mediated association of δ-PKC with p47phox in FN-adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.02, 0 versus 5 min, 0 versus 15 min; **, P < 0.01, 0 versus 30 min, and 60 min, and 15 min versus 30, 45, and 60 min. (B) TNF-mediated serine phosphorylation of p47phox was determined in FN-adherent neutrophils, which were incubated with TNF (25 ng/ml) for 0–60 min, and p47phox was immunoprecipitated. Serine phosphorylation of p47phox was determined by Western blotting using a phosphoserine antibody. Equal protein loading was determined by Western blot analysis for total p47phox. (Upper) Representative Western blot. (Lower) Densitometry analysis of p47phox phosphorylation. Values are mean ± se (n=7 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.05, 0 versus 5 min, 0 versus 15 min; **, P < 0.01, 0 versus 30 min, 45 min, and 60 min, and 5 min versus 30, 45, and 60 min. (C) The role of δ-PKC in p47phox serine phosphorylation was determined by pretreating adherent neutrophils with the specific δ-PKC TAT peptide inhibitor (1 μM), TAT carrier peptide (1 μM), or buffer alone, prior to the addition of TNF (25 ng/ml). p47phox was immunoprecipitated 5 min after TNF addition, and serine phosphorylation of p47phox was determined by Western blotting using a phosphoserine-specific antibody. Equal protein loading was determined by Western blot analysis for total p47phox. (Upper) Representative Western blot. (Lower) Densitometry analysis of TNF-mediated serine phosphorylation of p47phox in FN-adherent neutrophils. Values are mean ± se (n=5 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.01, buffer versus TNF and buffer versus TNF + TAT; **, P < 0.01, TNF versus TNF + δ-PKC TAT and TNF + TAT versus TNF + δ-PKC TAT. (D) The role of δ-PKC in TNF-elicited p47phox serine phosphorylation over a 60-min incubation period. Adherent neutrophils were pretreated with buffer or δ-PKC TAT peptide inhibitor (1 μM), prior to the addition of TNF (25 ng/ml). p47phox was immunoprecipitated following 15, 30, 45, and 60 min incubation with TNF. Serine phosphorylation of p47phox was determined as described in C. Equal protein loading was determined by Western blot analysis for total p47phox. (Upper) Representative Western blot. (Lower) Densitometry analysis of TNF-mediated serine phosphorylation of p47phox following pretreatment with buffer or δ-PKC TAT peptide inhibitor. Results are expressed as the percentage of p47phox phosphorylation with TNF alone at each time-point. Values are mean ± se (n=4 separate neutrophil preparations). *, P < 0.001, TNF versus TNF + δ-PKC TAT at 15, 30, and 45 min.
We next determined whether the interaction of δ-PKC with p47phox was associated with phosphorylation of p47phox. As shown in Figure 3B, there is little phosphorylation of p47phox in unstimulated neutrophils. The addition of TNF to FN-adherent neutrophils triggered serine phosphorylation of p47phox, which was evident at 5 min post-TNF addition. Serine phosphorylation of p47phox in response to TNF increased over time with maximal phosphorylation of p47phox occurring between 30 and 60 min. Thus, the recruitment of δ-PKC, the phosphorylation of p47phox, and the initiation of O2− generation demonstrate similar kinetics (Figs. 1 and 3, A and B).
To demonstrate a causal relationship between p47phox phosphorylation and recruitment of δ-PKC to p47phox, we next determined the effect of δ-PKC inhibition on p47phox phosphorylation in response to TNF in adherent neutrophils. As shown in Figure 3C, pretreatment with the δ-PKC TAT inhibitory peptide decreased TNF-mediated serine phosphorylation of p47phox significantly. In contrast, pretreatment with the TAT carrier alone had no significant effect on p47phox phosphorylation. We found that the pretreatment with the δ-PKC TAT inhibitory peptide decreased TNF-mediated serine phosphorylation of p47phox significantly between 5 and 45 min (Fig. 3, C and D). However, at 60 min post-TNF administration, the δ-PKC TAT peptide inhibitor was not an efficient inhibitor of p47phox serine phosphorylation. It should be noted that this is the time period in which O2− generation commences in neutrophils pretreated with the δ-PKC TAT peptide inhibitor, suggesting that other compensatory signaling pathways may be involved at p47phox phosphorylation at later time-points. Thus, TNF-triggered serine phosphorylation of p47phox at early time-points is dependent on δ-PKC activity.
ERK and p38 MAPK associate with p47phox and are positive regulators of TNF-elicited O2− generation
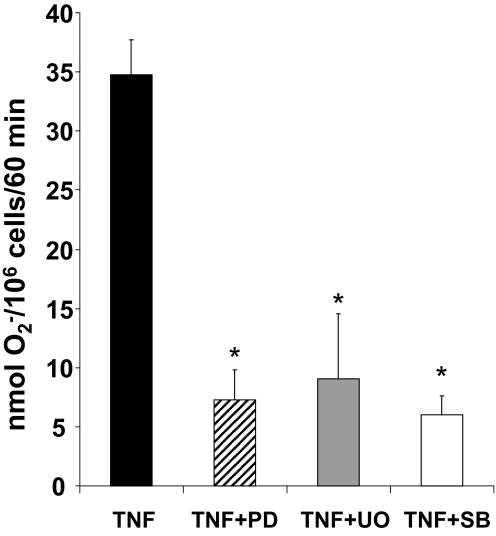
In cell-free studies, p47phox is a substrate for p38 MAPK and ERK [24]. In human neutrophils, TNF can activate multiple signaling pathways, including the MAPKs ERK and p38 MAPK, and these kinases have been implicated as critical regulators of O2− generation [17, 38, 41, 44,45,46]. Whether these kinases are also regulators of TNF-elicited O2− generation in FN-adherent neutrophils is not known. Pretreatment of neutrophils with the MEK1/2 inhibitors PD098059 (10 μM) or U0126 (10 μM) prior to the addition of TNF reduced O2− production significantly, as compared with controls, indicating an important regulatory role for ERK in TNF-mediated O2− generation (Fig. 4). Similarly, pretreatment with the p38 MAPK inhibitor SB203580 (10 μM) also reduced TNF-elicited O2− production significantly in FN-adherent neutrophils (Fig. 4). These MAPK inhibitors, at the concentrations used in our studies, did not trigger O2− production in the absence of TNF (data not shown). Thus, the MAPKs ERK and p38 MAPK are positive regulators of TNF-elicited O2− generation in FN-adherent neutrophils.
Figure 4.
ERK and p38 MAPK are positive regulators of TNF signaling for O2− generation in adherent neutrophils. O2− generation was measured as described in Figure 1. Neutrophils were pretreated with buffer, the p38 MAPK inhibitor SB203580 (SB; 10 μM), or the MEK1/2 inhibitors PD098059 (PD; 10 μM) and U0126 (UO; 10 μM), prior to addition of TNF (25 ng/ml). Results are expressed as nmol O2−/106/60 min and presented as mean ± se (n=3 separate neutrophil preparations done in triplicates). *, P < 0.01, TNF + PD098059 versus TNF; TNF + U0126 versus TNF; TNF + SB203580 versus TNF.
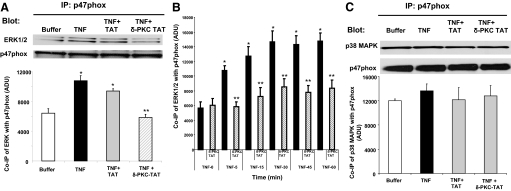
Previous studies demonstrated that δ-PKC regulates TNF-mediated ERK activation but not p38 MAPK activation in human neutrophils [17]. We next determined whether TNF triggers ERK or p38 MAPK association with p47phox and if so, whether it is δ-PKC-dependent. p47phox was immunoprecipitated from adherent neutrophils treated with TNF for 0–60 min. TNF triggered the association of ERK with p47phox in FN-adherent neutrophils as early as 5 min and was maintained over the 60-min incubation period (Fig. 5, A and B). Pretreatment with the δ-PKC TAT inhibitor peptide prior to the addition of TNF decreased the association of ERK with p47phox significantly (Fig. 5, A and B). Pretreatment with the TAT carrier peptide had no significant inhibitory effects on TNF-mediated recruitment of ERK to p47phox (Fig. 5A, and data not shown). In contrast to TNF-stimulated ERK recruitment to p47phox, there was significant association of p38 MAPK with p47phox in adherent neutrophils at Time 0 (Fig. 5C). The addition of TNF did not enhance p38 MAPK association with p47phox significantly (Fig. 5C). Pretreatment with the δ-PKC TAT inhibitor peptide prior to the addition of TNF also had no significant effect on p38 MAPK association with p47phox. Thus, ERK and p38 MAPK are required for TNF-elicited O2− generation, and both kinases associate with p47phox in adherent neutrophils. However, TNF-mediated recruitment of ERK to p47phox is δ-PKC-dependent, and p38 MAPK association with p47phox is δ-PKC-independent.
Figure 5.
Recruitment of ERK and p38MAPK to p47phox in adherent neutrophils. (A) TNF-mediated recruitment of ERK to p47phox is δ-PKC-dependent. Adherent neutrophils were pretreated with buffer, δ-PKC TAT peptide inhibitor (1 μM), or the TAT carrier peptide (1 μM), prior to the addition of TNF (25 ng/ml). p47phox was immunoprecipitated from adherent neutrophils following incubation for 5 min with buffer or TNF. Coimmunoprecipitation of ERK with p47phox was determined by Western blot analysis. Equal protein loading was determined by Western blot analysis for total p47phox (n=4). *, P < 0.01: TNF versus buffer and TNF + TAT versus buffer; **, P < 0.01, TNF + δ-PKC TAT versus TNF and TNF + δ-PKC TAT versus TNF + TAT. (B) The role of δ-PKC in TNF-elicited recruitment of ERK to p47phox over 60 min incubation. TNF-mediated recruitment of ERK to p47phox was determined by coimmunoprecipitation as described in A. FN-adherent neutrophils were incubated with TNF (25 ng/ml) for 0–60 min. p47phox was immunoprecipitated, and ERK association was determined by Western blotting. Equal protein loading was determined by Western blot analysis for total p47phox: densitometry analysis of TNF-mediated association of ERK with p47phox in adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.03, 0 time versus 5, 15, 30, 45, and 60 min; **, P < 0.01, TNF versus TNF + δ-PKC TAT at 5, 15, 30, 45, and 60 min incubation. (C) TNF-mediated recruitment of p38MAPK to p47phox is δ-PKC-independent. Adherent neutrophils were pretreated with buffer, δ-PKC TAT peptide inhibitor (1 μM), or the TAT carrier peptide (1 μM), prior to the addition of TNF (25 ng/ml), as described in A, and coimmunoprecipitation of p38 MAPK with p47phox was determined by Western blot analysis. Equal protein loading was determined by Western blot analysis for total p47phox. Representative Western blot: densitometry analysis of TNF-mediated association of p38MAPK with p47phox in adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU.
Regulation of δ-PKC phosphorylation: activation of the PI3K pathway and PDK1 by TNF in FN-adherent neutrophils
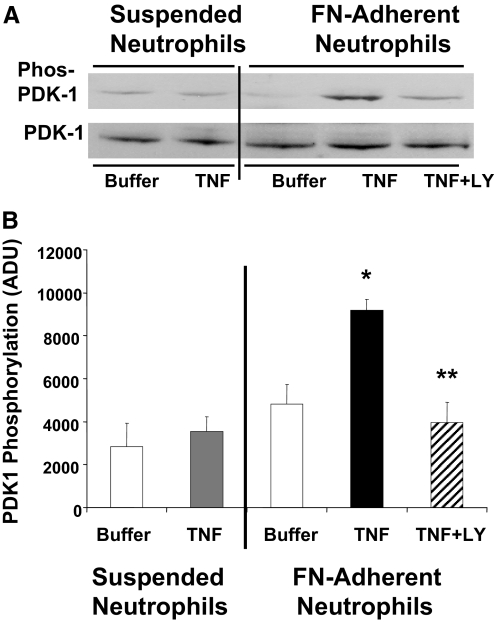
Adherence and thus, cooperative signaling between integrins and TNF could regulate δ-PKC activity through alterations in phosphorylation. PDK1, a member of the PI3K/PDK1/Akt pathway, phosphorylates δ-PKC in the activation loop (Thr505) [33]. Previously, we demonstrated that neutrophil adherence is required for TNF-mediated PI3K activation [13]. To explore the role of this pathway in TNF signaling further, we determined whether TNF could activate PDK1 in human neutrophils and if so, whether activation required cell adherence. PDK1 activation was determined by monitoring phosphorylation of Ser241 in the activation loop of PDK1, a phosphorylation site required for PDK1 activity [47]. In suspended neutrophils, there was little phosphorylation of PDK1 (Ser241) in buffer-treated neutrophils, and the addition of TNF did not enhance Ser241 phosphorylation significantly (Fig. 6). In contrast, TNF elicited significant phosphorylation of PDK1 in FN-adherent neutrophils, whereas FN adherence alone had no significant effect on PDK1 phosphorylation, indicating that TNF and adherence were required for activation of PDK1 by TNF. Phosphorylation of PDK1 was inhibited by pretreatment with LY294002 (10 μM), indicating phosphorylation and activation of PDK1 were PI3K-dependent.
Figure 6.
TNF triggers phosphorylation of PDK1 in adherent neutrophils but not in suspended neutrophils: role of PI3K. TNF-mediated activation of PDK1 was determined in suspended and FN-adherent neutrophils. PDK1 activation was determined by Western blotting using a phospho-specific PDK1 antibody (Ser241) in lysates prepared from adherent and nonadherent neutrophils incubated with TNF (25 ng/ml) or buffer for 5 min at 37°C. The role of PI3K in TNF-mediated activation of PDK1 was determined by pretreating FN-adherent neutrophils with the PI3K inhibitor LY294002 (LY; 10 μM) for 15 min prior to the addition of TNF. Equal protein loading was determined by Western blot analysis for total PDK1. (A) Representative Western blot. (B) Densitometry analysis of TNF-mediated PDK1 activation in suspended and FN-adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.01, TNF-adherent versus buffer-adherent and TNF-adherent versus TNF-suspended; **, P < 0.01, TNF + LY294002-adherent versus TNF-adherent.
Phosphorylation of δ-PKC by TNF: role of PI3K-dependent signaling
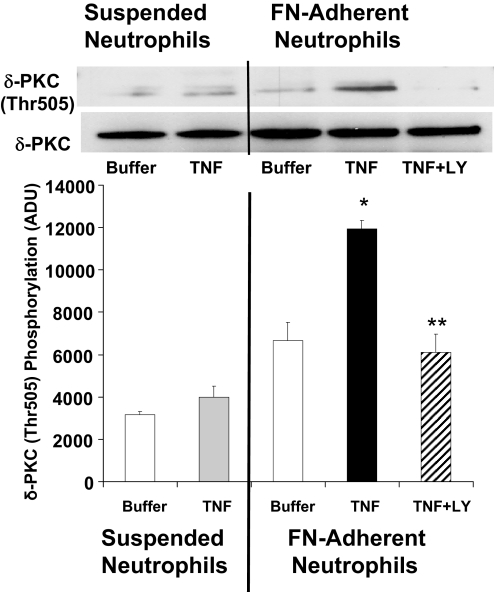
We next examined the phosphorylation pattern of δ-PKC in response to TNF in suspended and adherent neutrophils. As shown in Figure 7, in suspended neutrophils, the addition of TNF had no significant effect on δ-PKC (Thr505) phosphorylation as compared with untreated neutrophils (buffer). The addition of TNF to FN-adherent neutrophils resulted in an almost twofold increase in δ-PKC (Thr505) phosphorylation. Neutrophil adherence alone had no significant effect on δ-PKC (Thr505) phosphorylation. The increases in the extent of phosphorylation [δ-PKC (Thr505)] were inhibited by LY294002, indicating phosphorylation was PI3K-dependent.
Figure 7.
Phosphorylation of δ-PKC (Thr505) by TNF: role of PI3K. Adherent and nonadherent neutrophils were incubated with buffer or TNF (25 ng/ml) for 5 min as described in Materials and Methods. δ-PKC was immunoprecipitated from nonadherent and FN-adherent neutrophils. The role of PI3K in TNF-mediated phosphorylation of δ-PKC was determined by pretreating FN-adherent neutrophils with LY294002 (10 μM) for 15 min prior to the addition of TNF. Thr505 phosphorylation of δ-PKC was determined by Western blot analysis using a phospho-specific δ-PKC antibody (Thr505). Equal protein loading was determined by Western blot analysis for total δ-PKC. (Upper) Representative Western blot. (Lower) Densitometry analysis of TNF-mediated phosphorylation of δ-PKC (Thr505) in suspended and FN-adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.01, TNF-adherent versus buffer-suspended, TNF-adherent versus buffer-adherent, and TNF-adherent versus TNF-suspended; **, P < 0.01, TNF + LY294002-adherent versus TNF-adherent.
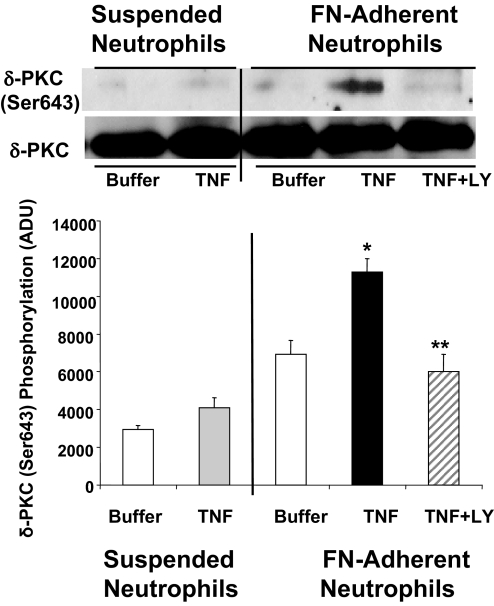
Phosphorylation of δ-PKC on Thr505 leads to an autophosphorylation step and phosphorylation of δ-PKC on Ser643, a site that regulates enzymatic activity and protein:protein interactions [48]. In suspended neutrophils, TNF had little effect on δ-PKC (Ser643) phosphorylation, as compared with neutrophils treated with buffer alone (Fig. 8). Conversely, in FN-adherent neutrophils, there was a significant increase in phosphorylation of δ-PKC (Ser643) in response to TNF. The enhancement in δ-PKC (Ser643) phosphorylation was inhibited by pretreatment with the PI3K inhibitor LY294002. In summary, phosphorylation of δ-PKC (Thr505) and δ-PKC (Ser643) by TNF was adherence-dependent and regulated by PI3K activity. Thus, post-translational modifications of δ-PKC produced by cooperative signaling between TNF and integrins result in activation of δ-PKC and involvement of δ-PKC in the early events of adherence-dependent TNF activation of O2− generation.
Figure 8.
Phosphorylation of δ-PKC (Ser643) by TNF: role of PI3K. Adherent and nonadherent neutrophils were incubated with TNF or buffer as described in Figure 7. δ-PKC was immunoprecipitated, and Ser643 phosphorylation was determined by Western blot analysis using a phospho-specific δ-PKC antibody (Ser645). Equal protein loading was determined by Western blot analysis for total δ-PKC. The role of PI3K in TNF-mediated phosphorylation of δ-PKC was determined by pretreating FN-adherent neutrophils with the PI3K inhibitor LY294002 as described in Figure 7. (Upper) Representative Western blot. (Lower) Densitometry analysis of TNF-mediated phosphorylation of δ-PKC (Ser643) in suspended and FN-adherent neutrophils. Values are mean ± se (n=4 separate neutrophil preparations) and are expressed in ADU. Statistical significance: *, P < 0.05, TNF-adherent versus buffer-adherent, and TNF-adherent versus TNF-suspended; **, P < 0.05, TNF + LY294002-adherent versus TNF-adherent.
DISCUSSION
In human neutrophils, TNF elicits O2− production, an event that requires adherence and integrin activation [9, 10, 13]. How this cooperative signaling regulates O2− generation has yet to be fully elucidated. The results of the present study demonstrate that cooperative signaling between TNF and integrins is required to activate δ-PKC, and this kinase is an important regulator of the early events in TNF-mediated assembly of the NADPH oxidase and O2− generation.
Adherence of neutrophils to ECM, such as FN, results in the activation of β1- and β2-integrins [49]. Similar to previous studies, we found that the cooperative signaling between TNF and these integrins resulted in significant production of O2− following a lag time of 30–45 min [9, 10]. This delay in TNF-elicited O2− generation observed in vitro may represent a protective mechanism that permits neutrophil migration from the circulation to the site of inflammation without injury to host tissue [1, 50]. Inhibition of δ-PKC with the dominant-negative δ-PKC TAT peptide altered TNF-mediated O2− generation in adherent neutrophils significantly. These findings are in agreement with studies in δ-PKC null mice, in which TNF-stimulated O2− generation in neutrophils was also inhibited [51]. Kinetic analysis of O2− production demonstrated that pretreatment with the δ-PKC TAT peptide delayed the activation of the NADPH oxidase significantly. This delay in onset of O2− generation was observed in neutrophils adherent to FN or tissue culture-treated polystyrene, indicating the role for δ-PKC is not matrix-dependent. In contrast to the time of onset, the rate of O2− production was not altered, indicating δ-PKC is involved in the initiation of O2− generation but does not regulate the activity of the NADPH oxidase enzyme complex directly. The finding that δ-PKC inhibition does not block O2− production completely at extended time periods suggests a redundancy, which can be overcome by other signaling elements.
Human neutrophils contain multiple isotypes of PKC, including Ca2+/DG-dependent isotypes α-PKC; alternatively spliced βI-PKC and βII-PKC; Ca2+-independent DG-dependent isotype δ-PKC; and phosphatidylserine-dependent Ca2+/DG-independent ζ-PKC [28, 29, 37, 52,53,54]. In cell-free systems, α-PKC, β-PKC, and δ-PKC are implicated as regulators of the NADPH oxidase and O2− generation [27, 28, 55]. However, in vitro activity does not necessarily predict a role for a particular PKC isotype in the intact neutrophil, where access to substrate and cofactors is critical in controlling signaling specificity. Indeed, in contrast to TNF-elicited O2− generation, δ-PKC activity was not required for fMLP-triggered O2− generation in adherent or nonadherent neutrophils. These findings are consistent with our previous studies in HL-60 cells differentiated to a neutrophillic phenotype (dHL-60) [56]. Depletion of δ-PKC in dHL60 cells by stealth siRNA treatment had no significant effect on O2− generation elicited by fMLP or PMA. Consistent with our results, other studies demonstrated rottlerin had no significant effect on fMLP or PMA-mediated O2− generation [57]. PMA-mediated translocation of p47phox to the cytoskeleton fraction correlated with translocation of α-PKC and βII-PKC but not δ-PKC [58], suggesting that δ-PKC does not have a role in PMA-stimulated activation of p47phox. Thus, in the neutrophil, δ-PKC is not an essential component of all signaling pathways leading to O2− generation and suggests that δ-PKC involvement in O2− generation is ligand-dependent. A role for δ-PKC in regulating O2− generation in other cell types has also been identified. δ-PKC is required for O2− generation in adherent monocytes, transgenic COS-phox cells, and adherent adipocytes [59,60,61,62]. Thus, differing requirements for δ-PKC in different cell systems is not surprising, as specific roles for PKC isotypes and their localization are highly dependent on context [33, 48, 63]. In particular, adherence and engagement of integrins modify PKC activation and localization [64]. Hence, the requirement for δ-PKC in regulating O2− generation in human neutrophils is dependent on the type of ligand and on input from other signaling pathways (i.e., adherence and activation of integrins).
How might δ-PKC activity regulate O2− generation in response to TNF? Our finding that δ-PKC is involved in early events in TNF-mediated O2− production suggests an involvement in the assembly of the NADPH oxidase complex. Assembly of an active NADPH oxidase requires the translocation and association of two cytosolic protein complexes with flavocytochrome b558 [65,66,67]. These protein complexes are comprised of p47phox:p67phox:p40phox and in neutrophils, rac2 coupled to Rho GDP disassociation inhibitor. An important step in the activation of the NADPH oxidase is the phosphorylation of the cytosolic p47phox, which is considered the main organizer of the NADPH oxidase and once activated, is responsible for translocation of the cytosolic complex consisting of p47phox:p67phox:p40phox to form an active NADPH oxidase [65]. Phosphorylation of p47phox induces a conformational change, which releases the binding of p47phox to itself and to p40phox, allowing translocation to the membrane and association with p67phox and Nox2 [68, 69]. p47phox contains multiple serine phosphorylation sites, several of which are putative phosphorylation targets for PKC isotypes, including δ-PKC [23, 27, 55, 68, 70, 71]. In the present study, we demonstrate that δ-PKC is involved in p47phox phosphorylation in response to neutrophil activation through cooperative signaling between TNF and integrins. TNF triggered association of δ-PKC with p47phox in adherent neutrophils, an association that is linked with serine phosphorylation of p47phox. The recruitment of δ-PKC to p47phox and serine phosphorylation of p47phox follows similar kinetics, where maximal recruitment of δ-PKC and maximal phosphorylation of p47phox occur within the same time frame. Maximal association of δ-PKC and serine phosphorylation of p47phox occurred between 30 and 45 min within the same time frame as the initiation of O2− generation. Inhibition of δ-PKC delayed the onset time of TNF-elicited O2− generation significantly in adherent neutrophils. Concomitant with the delay in onset of O2− generation is decreased p47phox phosphorylation, concordant with a role for δ-PKC in phosphorylation of p47phox and activation of the NADPH oxidase in intact cells. Inhibition of p47phox phosphorylation was greater at the initiation of TNF-mediated serine phosphorylation of p47phox, suggesting that other kinases or compensatory signaling pathways may be activated at later time-points.
δ-PKC may phosphorylate specific serine residues directly in p47phox, or alternatively, δ-PKC may regulate MAPK-mediated phosphorylation of p47phox [26, 27, 72]. ERK and p38 MAPK are capable of phosphorylating p47phox [26, 38]. In the present study, inhibitors of ERK and p38 MAPK inhibited TNF-activated O2− generation in adherent neutrophils, indicating that ERK and p38 MAPK are involved in regulating O2− generation. In adherent neutrophils, ERK and p38 MAPK associate with p47phox in response to TNF. However, TNF-mediated recruitment of ERK is δ-PKC-dependent, and p38 MAPK association with p47phox is δ-PKC-independent. These studies suggest that although both MAPKs are required for TNF-elicited O2− generation, there is differential regulation of these kinases in adherent neutrophils. The finding that recruitment of ERK to p47phox in response to TNF was δ-PKC-dependent suggests that δ-PKC acts upstream of ERK, a finding consistent with our previous studies, demonstrating that δ-PKC regulates TNF-mediated ERK activation but not p38 MAPK [17]. Recent studies by Dang et al [26]. have identified p47phox (Ser345) as a phosphorylation site regulated by ERK and p38MAPK. In nonadherent neutrophils, TNF did not activate ERK, and phosphorylation of p47phox (Ser345) was p38 MAPK-dependent [26]. However, association of ERK with p47phox but not p38 MAPK required the addition of TNF in adherent neutrophils. These findings indicate that cooperative signaling between TNF and integrins activates different signaling pathways as compared with TNF in nonadherent cells.
PI3K is a critical regulator of TNF-triggered O2− generation in adherent human neutrophils [13, 73]. The PI3K signaling pathway is only activated by TNF in surface-adherent neutrophils, and activation of p47phox requires PI3K activity [13, 73]. Our results demonstrate that PI3K acts upstream of δ-PKC in adherent neutrophils and that cooperative signaling between integrins and TNF is required for PI3K-dependent phosphorylation of δ-PKC. Activation of δ-PKC is a multistep process that includes threonine phosphorylation in the δ-PKC activation loop, which enhances kinase activity and regulates stability [33, 74]. The phosphorylation of δ-PKC (Thr505) is mediated by PDK1, a member of the PI3K signaling pathway. Enhanced phosphorylation of δ-PKC (Thr505) leads to an autophosphorylation step of δ-PKC (Ser643) at the COOH-terminal turn motif. This site is critical for controlling δ-PKC enzymatic activity and may also be important for regulating protein:protein interactions, as the turn-motif may serve as a docking site. TNF-elicited phosphorylation of δ-PKC (Thr505) and δ-PKC (Ser643) in FN-adherent neutrophils but not in cells in suspension indicates that phosphorylation of δ-PKC requires the integration of signals from TNF binding and integrin activation and the activation of the PI3K/PDK1/Akt pathway. In contrast, δ-PKC was not activated by fMLP [57]. The differential phosphorylation of δ-PKC in adherent neutrophils may regulate TNF-mediated O2− generation by targeting δ-PKC to p47phox and serine phosphorylation of p47phox. In support of this concept is the recent work by Cheng et al. [61], who demonstrated that phosphorylation of δ-PKC (Thr505) is required for δ-PKC-mediated phosphorylation of p47phox and reconstitution of the NADPH oxidase in COS-7 cells expressing Nox2, p22phox, p67phox, and p47phox.
The results of these studies do not rule out other interaction sites for δ-PKC regulation of TNF-elicited O2− generation. In previous studies with adherent neutrophils, time-course experiments established that within 5 min of exposure to TNF, there was physical association of δ-PKC and PI3K with TNFR-1, evidence of δ-PKC and PI3K activation, and significant functional alterations, as determined by TNFR-1 phosphorylation, NF-κB activation, and activation of MAPKs [13, 16,17,18,19]. Another possible target site of δ-PKC is the activation of rac2, which is an important regulator of assembly of the NADPH oxidase, whose activation is delayed by adherence through a PI3K-dependent mechanism [50, 75,76,77]. However, this pathway is involved in fMLP-elicited O2− generation in adherent neutrophils, a cellular event that is δ-PKC-independent. Whether δ-PKC is involved in TNF-mediated rac2 activation through the VAV1 signaling pathway is not known at present.
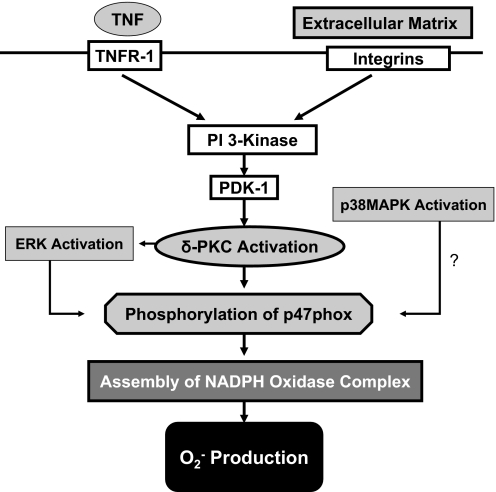
In summary, using a highly selective, cell-permeant δ-PKC TAT inhibitory peptide, we have demonstrated a selective role for δ-PKC in signaling for TNF-elicited but not fMLP O2− generation in adherent neutrophils. Our results indicate involvement of a particular PKC isotype is context-sensitive. In adherent neutrophils, δ-PKC, coimmunoprecipitated with p47phox in response to TNF and δ-PKC inhibition, was associated with decreased phosphorylation of p47phox and decreased recruitment of the MAPK ERK to p47phox, indicating a role for δ-PKC in regulating O2− production at the level of p47phox. Thus, in adherent neutrophils (Fig. 9), cooperative signaling between TNF and integrins leads to the activation of the PI3K/PDK1/Akt signaling pathway. PDK1 is only activated by TNF in adherent neutrophils and is required for phosphorylation of δ-PKC in the activation loop (Thr505), which in turn, triggers autophosphorylation of δ-PKC (Ser643). This response to TNF is not observed in nonadherent neutrophils. These post-translational modifications control δ-PKC activity and substrate specificity. TNF-mediated activation of δ-PKC leads to association with p47phox, recruitment of ERK to p47phox, and δ-PKC-mediated serine phosphorylation of p47phox. The recruitment of δ-PKC to p47phox and serine phosphorylation of p47phox follows similar kinetics, where maximal recruitment of δ-PKC and maximal phosphorylation of p47phox occur within the same time frame. The initiation of O2− generation in response to TNF does not occur until maximal phosphorylation of p47phox is achieved. δ-PKC activation is required for early TNF-mediated phosphorylation of p47phox and recruitment of ERK to p47phox. Thus, δ-PKC is a critical regulator of early events associated with TNF-elicited O2− generation in adherent human neutrophils.
Figure 9.
Model of TNF-elicited O2− generation in adherent neutrophils: role of δ-PKC. In adherent neutrophils, binding of TNF and ligation of integrins lead to cooperative signaling and the activation of PI3K. Activation of the PI3K/PDK1/Akt pathway modifies the phosphorylation pattern of δ-PKC and subsequent δ-PKC activity and substrate specificity. These post-translational modifications of δ-PKC promote recruitment of δ-PKC to p47phox, activation of ERK, and phosphorylation of p47phox. Phosphorylation and activation of p47phox lead to assembly of the NADPH oxidase and generation of O2−.
ACKNOWLEDGMENTS
This work was supported by grants GM 64552 (L. E. K.) and GM39277 (T. C. V.) from the National Institutes of Health (Bethesda, MD, USA). We thank Kayma Freeman for her technical assistance in this study.
Footnotes
Abbreviations: ADU=arbitrary densitometry units, BCA=bicinchoninic acid, BS3=bis(sulfosuccinimidy)suberate, DG=diacylglycerol, ECM=extracellular matrix, FN=fibronectin, IP=immunoprecipitation, O2−=superoxide anion, PDK1=phosphoinositide-dependent protein kinase 1, PKC=protein kinase C, siRNA=small interfering RNA, SOD=superoxide dismutase, Vmax=maximum velocity
References
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Akgul C, Edwards S W. Regulation of neutrophil apoptosis via death receptors. Cell Mol Life Sci. 2003;60:2402–2408. doi: 10.1007/s00018-003-3110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kitagawa S. Regulation of neutrophil functions by proinflammatory cytokines. Int J Hematol. 2006;84:205–209. doi: 10.1532/IJH97.06141. [DOI] [PubMed] [Google Scholar]
- Loetscher H, Pan Y C, Lahm H W, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Schall T J, Lewis M, Koller K J, Lee A, Rice G C, Wong G H, Gatanaga T, Granger G A, Lentz R, Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Smith C A, Davis T, Anderson D, Solam L, Beckmann M P, Jerzy R, Dower S K, Cosman D, Goodwin R G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248:1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- MacEwan D J. TNF receptor subtype signaling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright S D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C F. Respiratory burst in adherent human neutrophils: triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989;73:301–306. [PubMed] [Google Scholar]
- Dewas C, Dang P M, Gougerot-Pocidalo M A, El-Benna J. TNF-α induces phosphorylation of p47(phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-α and granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171:4392–4398. doi: 10.4049/jimmunol.171.8.4392. [DOI] [PubMed] [Google Scholar]
- Korchak H M, Kilpatrick L E. TNFα elicits association of PI 3-kinase with the p60TNFR and activation of PI 3-kinase in adherent neutrophils. Biochem Biophys Res Commun. 2001;281:651–656. doi: 10.1006/bbrc.2001.4406. [DOI] [PubMed] [Google Scholar]
- Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med. 2003;197:63–75. doi: 10.1084/jem.20021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M, Melchior M, Han H, Lyon G J, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L E, Song Y H, Rossi M W, Korchak H M. Serine phosphorylation of p60 tumor necrosis factor receptor by PKC-δ in TNF-α-activated neutrophils. Am J Physiol Cell Physiol. 2000;279:C2011–C2018. doi: 10.1152/ajpcell.2000.279.6.C2011. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L E, Sun S, Mackie D, Baik F, Li H, Korchak H M. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of {δ}-PKC and ERK1/2. J Leukoc Biol. 2006;80:1512–1521. doi: 10.1189/jlb.0406284. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L E, Sun S, Korchak H M. Selective regulation by δ-PKC and PI 3-kinase in the assembly of the antiapoptotic TNFR-1 signaling complex in neutrophils. Am J Physiol Cell Physiol. 2004;287:C633–C642. doi: 10.1152/ajpcell.00486.2003. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L E, Lee J Y, Haines K M, Campbell D E, Sullivan K E, Korchak H M. A role for PKC-δ and PI 3-kinase in TNF-α-mediated antiapoptotic signaling in the human neutrophil. Am J Physiol Cell Physiol. 2002;283:C48–C57. doi: 10.1152/ajpcell.00385.2001. [DOI] [PubMed] [Google Scholar]
- Vancurova I, Miskolci V, Davidson D. NF-κ B activation in tumor necrosis factor α-stimulated neutrophils is mediated by protein kinase Cδ. Correlation to nuclear Iκ Bα. J Biol Chem. 2001;276:19746–19752. doi: 10.1074/jbc.M100234200. [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang P M, Gougerot-Pocidalo M A, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- Babior B M, Lambeth J D, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Sheppard F R, Kelher M R, Moore E E, McLaughlin N J, Banerjee A, Silliman C C. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- El Benna J, Han J, Park J W, Schmid E, Ulevitch R J, Babior B M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- Chen Q, Powell D W, Rane M J, Singh S, Butt W, Klein J B, McLeish K R. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- Dang P M, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen O N, Gougerot-Pocidalo M A, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontayne A, Dang P M, Gougerot-Pocidalo M A, El-Benna J. Phosphorylation of p47phox sites by PKC α, β II, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Kane L H, Rossi M W, Volpp B D, Nauseef W M, Korchak H M. Protein kinase C isotypes and signal-transduction in human neutrophils: selective substrate specificity of calcium-dependent β-PKC and novel calcium-independent nPKC. Biochim Biophys Acta. 1993;1176:276–286. doi: 10.1016/0167-4889(93)90056-u. [DOI] [PubMed] [Google Scholar]
- Kent J D, Sergeant S, Burns D J, McPhail L C. Identification and regulation of protein kinase C-δ in human neutrophils. J Immunol. 1996;157:4641–4647. [PubMed] [Google Scholar]
- Davies S P, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley R, Liron T, Baryza J, Mochly-Rosen D. Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem Biophys Res Commun. 2004;318:949–954. doi: 10.1016/j.bbrc.2004.04.121. [DOI] [PubMed] [Google Scholar]
- Chen L, Wright L R, Chen C H, Oliver S F, Wender P A, Mochly-Rosen D. Molecular transporters for peptides: delivery of a cardioprotective εPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7) Chem Biol. 2001;8:1123–1129. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Parker P J, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- Chen L, Hahn H, Wu G, Chen C H, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn G W, II, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of δ PKC and ε PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Raval A P, Dembner J M, Perez-Pinzon M A, Steinberg G K, Yenari M A, Mochly-Rosen D. Protein kinase C δ mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Korchak H M, Rossi M W, Kilpatrick L E. Selective role for β-protein kinase C in signaling for O-2 generation but not degranulation or adherence in differentiated HL60 cells. J Biol Chem. 1998;273:27292–27299. doi: 10.1074/jbc.273.42.27292. [DOI] [PubMed] [Google Scholar]
- Dewas C, Fay M, Gougerot-Pocidalo M A, El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Zhou M, Meng F, Tybulewicz V L, Lowell C A. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Vary T C, Goodman S, Kilpatrick L E, Lynch C J. Nutrient regulation of PKCε is mediated by leucine, not insulin, in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;289:E684–E694. doi: 10.1152/ajpendo.00613.2004. [DOI] [PubMed] [Google Scholar]
- Brown G E, Stewart M Q, Bissonnette S A, Elia A E, Wilker E, Yaffe M B. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J Biol Chem. 2004;279:27059–27068. doi: 10.1074/jbc.M314258200. [DOI] [PubMed] [Google Scholar]
- Han H, Stessin A, Roberts J, Hess K, Gautam N, Kamenetsky M, Lou O, Hyde E, Nathan N, Muller W A, Buck J, Levin L R, Nathan C. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–361. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein S C, Loike J D. Different Gi-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. [PubMed] [Google Scholar]
- Suzuki K, Hino M, Hato F, Tatsumi N, Kitagawa S. Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-α. Blood. 1999;93:341–349. [PubMed] [Google Scholar]
- McLeish K R, Knall C, Ward R A, Gerwins P, Coxon P Y, Klein J B, Johnson G L. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-α and GM-CSF. J Leukoc Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- Rafiee P, Lee J K, Leung C C, Raffin T A. TNF-α induces tyrosine phosphorylation of mitogen-activated protein kinase in adherent human neutrophils. J Immunol. 1995;154:4785–4792. [PubMed] [Google Scholar]
- Casamayor A, Morrice N A, Alessi D R. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J. 1999;342:287–292. [PMC free article] [PubMed] [Google Scholar]
- Steinberg S F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg J M, Mul F P, Schippers E, Weening J J, Roos D, Kuijpers T W. β1 Integrin activation on human neutrophils promotes β2 integrin-mediated adhesion to fibronectin. Eur J Immunol. 2001;31:276–284. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Zhao T, Benard V, Bohl B P, Bokoch G M. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Clin Invest. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W H, Choi D S, Zhang H, Mu D, McMahon T, Kharazia V N, Lowell C A, Ferriero D M, Messing R O. Neutrophil protein kinase Cδ as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H M, Kilpatrick L E. Roles for β II-protein kinase C and RACK1 in positive and negative signaling for superoxide anion generation in differentiated HL60 cells. J Biol Chem. 2001;276:8910–8917. doi: 10.1074/jbc.M008326200. [DOI] [PubMed] [Google Scholar]
- Sergeant S, McPhail L C. Opsonized zymosan stimulates the redistribution of protein kinase C isoforms in human neutrophils. J Immunol. 1997;159:2877–2885. [PubMed] [Google Scholar]
- Dang P M, Hakim J, Perianin A. Immunochemical identification and translocation of protein kinase C ζ in human neutrophils. FEBS Lett. 1994;349:338–342. doi: 10.1016/0014-5793(94)00700-4. [DOI] [PubMed] [Google Scholar]
- Brown G E, Stewart M Q, Liu H, Ha V-L, Yaffe M B. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC δ in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Korchak H M, Dorsey L B, Li H, Mackie D, Kilpatrick L E. Selective roles for α-PKC in positive signaling for O2− generation and calcium mobilization but not elastase release in differentiated HL60 cells. Biochim Biophys Acta. 2007;1773:440–449. doi: 10.1016/j.bbamcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Pongracz J, Lord J M. Superoxide production in human neutrophils: evidence for signal redundancy and the involvement of more than one PKC isoenzyme class. Biochem Biophys Res Commun. 1998;247:624–629. doi: 10.1006/bbrc.1998.8867. [DOI] [PubMed] [Google Scholar]
- Nixon J B, McPhail L C. Protein kinase C (PKC) isoforms translocate to Triton-insoluble fractions in stimulated human neutrophils: correlation of conventional PKC with activation of NADPH oxidase. J Immunol. 1999;163:4574–4582. [PubMed] [Google Scholar]
- Bey E A, Xu B, Bhattacharjee A, Oldfield C M, Zhao X, Li Q, Subbulakshmi V, Feldman G M, Wientjes F B, Cathcart M K. Protein kinase C δ is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- He R, Nanamori M, Sang H, Yin H, Dinauer M C, Ye R D. Reconstitution of chemotactic peptide-induced nicotinamide adenine dinucleotide phosphate (reduced) oxidase activation in transgenic COS-phox cells. J Immunol. 2004;173:7462–7470. doi: 10.4049/jimmunol.173.12.7462. [DOI] [PubMed] [Google Scholar]
- Cheng N, He R, Tian J, Dinauer M C, Ye R D. A critical role of protein kinase C δ activation loop phosphorylation in formyl-methionyl-leucyl-phenylalanine-induced phosphorylation of p47(phox) and rapid activation of nicotinamide adenine dinucleotide phosphate oxidase. J Immunol. 2007;179:7720–7728. doi: 10.4049/jimmunol.179.11.7720. [DOI] [PubMed] [Google Scholar]
- Talior I, Tennenbaum T, Kuroki T, Eldar-Finkelman H. PKC-δ-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol Endocrinol Metabol. 2005;288:E405–E411. doi: 10.1152/ajpendo.00378.2004. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker P J. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K, Zhang L, Yamada K M, Lafrenie R M. Adhesion of epithelial cells to fibronectin or collagen I induces alterations in gene expression via a protein kinase C-dependent mechanism. J Cell Physiol. 2001;189:79–90. doi: 10.1002/jcp.1142. [DOI] [PubMed] [Google Scholar]
- El-Benna J, Dang P M-C, Gougerot-Pocidalo M A, Marie J C, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Diebold B, Kim J-S, Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2590. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W M. Biological roles for the NOX family NADPH oxidases. J Biol Chem. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B M. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Hata K, Mizuki K, Ito T, Kage Y, Sakaki Y, Fukumaki Y, Nakamura M, Takeshige K. Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J Biol Chem. 1996;271:22152–22158. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- Reeves E P, Dekker L V, Forbes L V, Wientjes F B, Grogan A, Pappin D J, Segal A W. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem J. 1999;344:859–866. [PMC free article] [PubMed] [Google Scholar]
- Faust L R, el Benna J, Babior B M, Chanock S J. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyal C R, Gutierrez A, Young B M, Catz S D, Lin J-H, Tsichlis P N, Babior B M. Modulation of p47phox activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc Natl Acad Sci USA. 2003;100:5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X-P, Zhu X, Fu J, Liu Q, Frey R S, Malik A B. Blockade of class IA phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J Biol Chem. 2007;282:6116–6125. doi: 10.1074/jbc.M610248200. [DOI] [PubMed] [Google Scholar]
- Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Zhao T, Bokoch G M. Critical role of proline-rich tyrosine kinase 2 in reversion of the adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Immunol. 2005;174:8049–8055. doi: 10.4049/jimmunol.174.12.8049. [DOI] [PubMed] [Google Scholar]
- Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- Dib K, Melander F, Axelsson L, Dagher M C, Aspenstrom P, Andersson T. Down-regulation of Rac activity during β 2 integrin-mediated adhesion of human neutrophils. J Biol Chem. 2003;278:24181–24188. doi: 10.1074/jbc.M302300200. [DOI] [PubMed] [Google Scholar]