Abstract
The phenotype of wound macrophages has not been studied by direct examination of these cells, yet macrophages recruited to sites of injury are described as alternatively activated macrophages, requiring IL-4 or IL-13 for phenotypic expression. This study characterized wound macrophage phenotype in the PVA sponge wound model in mice. Eighty-five percent of wound macrophages isolated 1 day after injury expressed Gr-1, but only 20% of those isolated at 7 days expressed this antigen. Macrophages from 1-, 3-, and 7-day wounds expressed markers of alternative activation, including mannose receptor, dectin-1, arginase 1, and Ym1, but did not contain iNOS. Day 1 wound macrophages produced more TNF-α, more IL-6, and less TGF-β than Day 7 wound macrophages. Wound macrophages did not produce IL-10. The cytokines considered necessary for alternative activation of macrophages, IL-4 and IL-13, were not detected in the wound environment and were not produced by wound cells. Wound macrophages did not contain PStat6. Wound fluids inhibited IL-13-dependent phosphorylation of Stat6 and contained IL-13Rα2, a soluble decoy receptor for IL-13. The phenotype of wound macrophages was not altered in mice lacking IL-4Rα, which is required for Stat6-dependent signaling of IL-4 and IL-13. Wound macrophages exhibit a complex phenotype, which includes traits associated with alternative and classical activation and changes as the wound matures. The wound macrophage phenotype does not require IL-4 or IL-13.
Keywords: IL-4, IL-13, IL-4 receptor α, mannose receptor, arginase, Ym1
Introduction
Macrophages are endowed with extraordinary phenotypic plasticity, varying their functional repertoire according to the physiologic or pathologic sites to which they are recruited. Macrophages recruited to wounds and other sites of tissue injury are frequently referred to as “repair macrophages” and are believed to express the alternatively activated phenotype, which is associated with allergic and antiparasite responses and is thought to regulate humoral immunity [1,2,3,4]. The alternatively activated macrophage phenotype is induced by IL-4 or IL-13 and is characterized by expression of mannose receptor, dectin-1, arginase 1, and Ym1 [2, 4,5,6]. In describing the alternative activation of macrophages and their putative role in repair, Siamon Gordon noted, “…studies lack adequate characterization of the macrophage phenotype in situ or after cell isolation” [2]. Addressing this deficiency, experiments reported here examined the phenotype of macrophages immediately after isolation from healing wounds in mice. Data to be shown demonstrate that wound macrophages, although exhibiting some of the characteristics of alternatively activated macrophages, express a more complex phenotype that includes traits associated with classical activation.
It was proposed recently that alternatively activated macrophages be designated wound-healing macrophages and that IL-4 and IL-13 induce expression of the wound-healing macrophage phenotype [7]. The present study examined the role of IL-4 and IL-13 in determining the phenotype of macrophages isolated from wounds. Data to be shown do not support a role for IL-4 or IL-13 in the development of wound macrophage phenotype.
MATERIALS AND METHODS
Animals
B6D2F1 male mice, 8–12 weeks of age, were obtained from Taconic Farms (Germantown, NY, USA). IL-4Rα-deficient mice (BALB/c-Il4ratm1Sz/J) and BALB/cJ mice were obtained from Jackson Labs (Bar Harbor, ME, USA). Animals were housed at the Central Research Facilities at Rhode Island Hospital (Providence, RI, USA) and fed mouse chow and water ad libitum. Mice were certified free of common pathogens by the suppliers and were monitored by Brown University/Rhode Island Hospital veterinary personnel. Animal protocols were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital.
Cells
PVA sponge wound model and wound cell isolation.
Mice were anesthetized with pentobarbital (50 mg/kg i.p.). Six PVA sponges (PVA Unlimited, Warsaw, IN, USA), measuring 1 cm × 1 cm × 0.6 cm, were inserted into individual s.c. pockets through a midline dorsal incision under aseptic conditions, and the skin closed with clips [10]. At specified times, mice were killed by CO2 asphyxiation, and the sponges were removed. Wound cells were isolated by repeated compression of the sponges in a Stomacher (Tekmar, Cincinnati, OH, USA). Cell-free wound fluids were obtained by centrifugation of sponges (400 g for 5 min) in the barrel of a syringe that was seated in a sterile tube, as described previously [10]. Differential cell counts were performed on Hema-3 (Biochemical Sciences, Swedesboro, NJ)-stained cytocentrifuge preparations.
Wound cell yield averaged 1 × 106 cells/animal at 1 day after sponge insertion, 3 × 106 cells/animal at 3 days, and 7 × 106 cells/animal at 7 days, as reported previously [10]. Macrophages comprised ∼15% of Day 1 wound cells, ∼30% of Day 3 cells, and over 50% of Day 7 cells. Because of the low yield of macrophages from each individual animal, cell suspensions pooled from multiple animals were used in these studies.
Peritoneal cells.
Following euthanasia, peritoneal cells were obtained by twice lavaging the peritoneal cavity with 5 mL HBSS (Invitrogen, Grand Island, NY, USA) containing no calcium or magnesium with 1% FBS (Hyclone, Logan, UT, USA), 10 mM HEPES buffer (Invitrogen), and 100 U/mL penicillin and streptomycin (Invitrogen).
Blood leukocytes.
Blood was obtained by cardiac puncture. Blood leukocytes (buffy coat cells) were isolated by centrifugation (800 g) of heparinized blood in Wintrobe tubes for 15 min at room temperature. Residual erythrocytes were eliminated by hypotonic lysis.
Separation of macrophages from the wound cell suspension.
FcRs were blocked with antibody specific for FcRγIII/II (1 μg/106 cells) for 15 min at 4°C, and the wound cell suspension was incubated with PE-conjugated antibodies specific for CD2, CD5, and Ly6G antigens (0.05 μg/106 cells). Cells were washed, incubated with anti-PE beads, washed, and depleted of neutrophils and lymphocytes using the MACS system, according to the manufacturer’s recommendations (Miltenyi Biotec, Auburn, CA, USA). The purity of the macrophage suspensions was determined by a differential count of Hema-3-stained cytocentrifuge preparations and varied from 90% to 95%. In some experiments, total wound cells or wound macrophages were cultured in complete medium [RPMI 1640 (Invitrogen), supplemented with 1% FBS, 10 mM HEPES, 2 mM L-glutamine (Invitrogen), and 100 U/mL penicillin and streptomycin] or in X-vivo 15 (Lonza, Walkersville, MD, USA) with 100 U/mL penicillin and streptomycin. When indicated, cells were cultured with rmIL-4 (BD Biosciences, San Jose, CA, USA; 10 ng/mL) or with rmIFN-γ (Invitrogen; 10 U/mL) and LPS (Escherichia coli serotype 055:B5, Sigma-Aldrich, St. Louis, MO, USA; 0.1 μg/mL).
Cell staining and flow cytometry
Antibodies used for cell staining and flow cytometry are noted in Table 1. When necessary, erythrocytes were removed from cell suspensions by hypotonic lysis prior to staining. To stain for surface antigens, FcRs were blocked with anti-FcγRIII/II or a biotin-free FcR block (Accurate Chemical and Scientific Corp., Westbury, NY, USA), and cells were incubated with predetermined optimal concentrations of fluorochrome-conjugated antibodies or isotype controls and washed twice in staining buffer (PBS containing no calcium or magnesium with 1% FBS and 0.09% sodium azide). If biotin-conjugated antibodies were used, cells were incubated subsequently with fluorochrome-conjugated Streptavidin (BD Biosciences) and washed twice. To stain for intracellular antigens, cells were first stained for surface antigens, then fixed and made permeable in paraformaldehyde with saponin (Cytofix/Cytoperm, BD Biosciences), and then washed twice in Permwash (BD Biosciences), according to the manufacturer’s recommendations. Following FcR blocking, cells were incubated with fluorochrome-conjugated antibodies or isotype controls and washed twice in Permwash.
TABLE 1.
Antibodies Used in This Study
| Antigen detected | Clone | Supplier |
|---|---|---|
| CD2 | RM2-5 | BD Biosciences |
| CD3ε | 145-2C11 | BD Biosciences |
| CD5 | 53-7.3 | BD Biosciences |
| CD28 | 37.51 | BD Biosciences |
| CD16/CD32 (FcγRIII/II) | 2.4G2 | BD Biosciences |
| CD124 (IL-4R-α) | mIL4R-M1 | BD Biosciences |
| Gr-1 (Ly6C and Ly6G) | RB6-8C5 | BD Biosciences |
| iNOS | 6 | BD Biosciences |
| Ly6G | 1A8 | BD Biosciences |
| Stat6 (pY641) | J71-773.58.11 | BD Biosciences |
| TNF-α | MP6-XT22 | BD Biosciences |
| F4/80 | CI:A3-1 | Caltag Laboratories (Burlingame, CA, USA) |
| IL-4 | 11B11 | eBioscience (San Diego, CA, USA) |
| IL-13 | eBio13A | eBioscience |
| IL-13Rα2 | 110815 | R&D Systems (Minneapolis, MN, USA) |
| CD68 (macrosialin) | FA-11 | AbDSerotec (Raleigh, NC, USA) |
| CD206 (mannose receptor) | MR5D3 | AbDSerotec |
| Dectin-1 (β-glucan receptor) | 2A11 | AbDSerotec |
| Arginase 1 | polyclonal | gift of Dr. Sidney Morris, University of Pittsburgh (Pittsburgh, PA, USA) |
| Ym1/2 | polyclonal | gift of Dr. Shioko Kimura, National Cancer Institute, National Institutes of Health (Rockville, MD, USA) |
| Stat6 | polyclonal | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| PStat6 | polyclonal | Santa Cruz Biotechnology |
For intracellular cytokine determinations, wound cells were incubated (106 cells/mL) for 6 h in RPMI with 10% FBS and 100 U/mL penicillin-streptomycin in the presence of Brefeldin A (1 mL/106 cells, GolgiPlug, BD Biosciences) or Monensin (0.7 mL/106 cells, GolgiStop, BD Biosciences) and then stained as described above. Positive controls for IL-4 and IL-13 staining were BALB/c splenocytes preincubated for 3 days in complete medium (with 10% FBS and 2×10−5 M 2-ME) with plate-bound anti-CD3ε and anti-CD28 and soluble rmIL-2 (1 U/mL) and rmIL-4 (10 ng/mL) and then stimulated with PMA (5 ng/mL, LC Laboratories, Woburn, MA, USA) and calcium ionomycin (500 ng/mL, Sigma-Aldrich).
Cells were stained for PStat6 as described by Krutzik et al. [11]. Cells were washed in PBS, fixed immediately in formaldehyde (final concentration 1.6%) for 10 min at room temperature, and resuspended in ice-cold methanol for 30 min at 4°C. Cells were washed twice in staining buffer, FcRs blocked, and cells stained with fluorochrome-conjugated antibodies specific for PStat6 at Y641 or the appropriate isotype controls. When indicated, cells were incubated with rmIL-4 (10 ng/mL) for 15 min at 37°C prior to PStat6 staining.
Cells were analyzed using a Becton Dickinson FACSort with CellQuest (BD Biosciences) or FCS Press software (Copyright 1995–2006, Ray Hicks, University of Cambridge, UK). Macrophages in wound cell suspensions were identified by a combination of forward-scatter/SSC properties, F4/80, and Gr-1 staining [12] or by CD68 staining [10], which differentiated wound macrophages from wound neutrophils at all time-points studied (data not shown). Blood monocytes were identified in buffy-coat suspensions by F4/80 staining.
Western blot
Freshly harvested cells were lysed in RIPA buffer with protease inhibitors and when appropriate, phosphatase inhibitors. Cell lysates or wound fluids were size-fractionated in 7.5% or 15% SDS-PAGE, loaded with equal amounts of protein/lane. Proteins were transferred to a nitrocellulose membrane, incubated in blocking buffer, and probed with the antibody of interest. Appropriate peroxidase-conjugated secondary antibody was used for detection. The positive control for iNOS was cell lysate from a mouse macrophage cell line (J774A.1, American Type Culture Collection, Manassas, VA, USA) treated with rmIFN-γ (10 U/mL) and LPS (0.1 μg/mL); for arginase 1, lysate of whole mouse liver; and for Ym1, lysate of whole mouse spleen.
Cytokines
Cytokines were measured by cytometric bead array (TNF-α, IL-6, IL-10, and IL-12p70, BD Biosciences), ELISA (TNF-α, IL-6, IL-10, BD Biosciences; CCL5, CCL17, IL-4, IL-13, TGF-β, R&D Systems), or by ELISPOT (IL-4, IL-13, eBioscience). IL-4 was also assayed by the growth of CT.4S cells (a generous gift of Dr. William Paul, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA) [13, 14]. Briefly, 1 × 104 CT.4S cells in IL-4-free medium (RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin and streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 2×10−5 M 2-ME) were added to wells containing rmIL-4 standards or wound fluid (50% vol:vol). Cells were then incubated for 48 h, and viable cell number was determined by MTT assay [15]. Wound fluid did not interfere with detection of added rmIL-4 in this bioassay. The IL-13 ELISA detected <10% of rmIL-13 added to wound fluid (to a final concentration of 3 ng/mL) and thus, could not be used to measure the concentration of this cytokine in wound fluids. The recovery of rmIL-13 in cell-culture supernatants was 90%. The IL-4/IL-13 content of wound fluid was assessed by the capacity of wound fluids to induce PStat6 in naïve peritoneal cells or J774A.1 cells. Peritoneal cells (1×106 cells) in PBS were treated with rmIL-4 or wound fluids (50% vol:vol) for 15 min at 37°C and then stained for PStat6, as described above. J774A.1 cells (1×106 cells) in DMEM (Invitrogen; supplemented with 10% FBS, 10 mM HEPES, 2 mM L-glutamine, and 100 U/mL penicillin and streptomycin) were treated with rmIL-13 (0.1–10 ng/mL, Biosource, Camarillo, CA, USA), with or without pooled wound fluids (50% vol:vol) for 30 min, then washed in PBS, lysed in RIPA buffer with protease inhibitors and phosphatase inhibitors, and analyzed by Western blotting, as described above.
Statistical analysis
Data shown are means ± sd. Statistical analysis was performed using Student’s t-test or ANOVA with Newman-Keuls, as appropriate. With the exception of those experiments using IL-4Rα knockout mice, all experiments were performed at least twice.
RESULTS
The phenotype of murine wound macrophages
Wound cells obtained 1, 3, or 7 days after wounding were stained and interrogated for markers of alternatively activated macrophages. Blood monocytes (F4/80+ cells), the immediate precursors of wound macrophages (CD68high cells), were analyzed in parallel. Results from these experiments are shown in Figure 1.
Figure 1.
Gr-1, mannose receptor (MR), and dectin-1 in wound macrophages. PVA sponges were inserted s.c. in B6D2F1 male mice. Wound cells were obtained 1, 3, or 7 days later. Leukocytes were isolated from blood. Cells were stained for Gr-1, mannose receptor, or dectin-1 (open histograms) or appropriate isotype control antibodies (shaded histograms). Additionally, wound cells were stained for CD68 and blood leukocytes for F4/80. Histograms show expression of antigens in gated macrophages (CD68high cells) or gated monocytes (F4/80+ cells). Results are representative of three experiments.
Gr-1 is expressed on monocytes and macrophages recruited early into sites of inflammation or injury [16, 17]. Approximately half of blood monocytes expressed Gr-1 antigen, as reported previously from this and other laboratories [12, 18]. In contrast, 85% of Day 1 wound macrophages expressed Gr-1. The fraction of Gr-1+ macrophages decreased with time so that by 7 days after wounding, only 20% of wound macrophages expressed this antigen.
Wound macrophages isolated 1–7 days after injury expressed mannose receptor, whereas blood monocytes did not. The fraction of wound macrophages expressing mannose receptor increased over time: by 7 days after injury, 80% of the cells stained for this receptor. The glucan receptor dectin-1, an additional marker of alternatively activated macrophages [6], was detected equally in blood monocytes and wound macrophages.
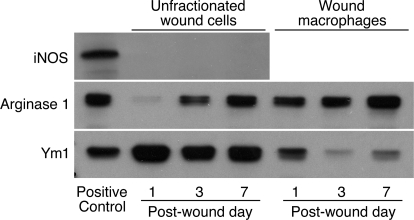
Alternatively activated macrophages contain arginase 1 and Ym1, whereas classically activated macrophages express iNOS [2]. Figure 2 shows results of Western blot analyses for iNOS, arginase 1, and Ym1 in unfractionated wound cells or isolated wound macrophages. Wound cells did not contain detectable iNOS protein at any of the examined time-points. The arginase 1 content of unfractionated wound cells increased from Day 1 to Day 7 after injury. This finding reflects the changing composition of the cellular infiltrate as the wound matures. One day after injury, 85% of the wound cells are neutrophils [10], which in mice are devoid of arginase 1 protein [19]. The increasing arginase content of wound cells over time parallels the increasing fraction of macrophages among total wound cells [10]. Arginase was present in wound macrophages isolated at 1, 3, and 7 days after injury.
Figure 2.
Arginase 1 and Ym1, but not iNOS, are present in wound macrophages. Wound cells were obtained 1, 3, or 7 days after PVA sponge insertion. Macrophages were isolated from the wound cell suspensions by negative selection. Cell lysates from unfractionated wound cells or isolated wound macrophages were size-fractionated and immunoblotted with antibodies for iNOS, arginase 1, and Ym1/2. Results are representative of two experiments.
Ym1 was expressed in the wound cells at all times studied. Comparison of the Ym1 content of total wound cells with that of isolated wound macrophages suggests that nonmacrophage cells, most likely neutrophils, contain Ym1. In this regard, Ym1 is present in neutrophil granules [20]. Wound macrophages isolated 1 day after injury contained more Ym1 than those isolated at 3 and 7 days.
Wound macrophages produce cytokines associated with classical and alternative activation
Wound macrophages were isolated and cultured to determine cytokine production. Data in Table 2 demonstrate that Day 1 wound macrophages produced cytokines associated with classical activation (TNF-α and IL-6), as well as those associated with alternative activation (TGF-β). Day 7 macrophages produced less TNF-α and IL-6 but more TGF-β than Day 1 macrophages.
TABLE 2.
Cytokine Production by Wound Macrophagesa
Macrophages isolated from wounds were cultured in complete medium for 24 h or in serum-free medium for 72 h (for TGF-β determination only). Data are cytokine production/3 × 105 macrophages, mean ± sd of quadruplicate wells, and are representative of two experiments. IL-10 and IL-12p70 were not detected in culture supernatants.
P < 0.05 versus Day 7 macrophages, Student’s t-test. ND, Not detected.
Heterogeneity of wound macrophage populations
To determine whether wound macrophages are a mixture of classically activated and alternatively activated cells or whether they have a phenotype exhibiting some features of each, cells were stained for mannose receptor and TNF-α. Day 1 wound macrophages (F4/80+ cells) expressed mannose receptor and had a range of TNF-α production, as shown in Figure 3. At 7 days, wound macrophages also expressed mannose receptor, but fewer macrophages were high TNF-α producers. Mannose receptor expression did not differentiate TNF-αhigh cells from TNF-αlow or TNF-αneg cells.
Figure 3.
Wound macrophages express mannose receptor and produce TNF-α. Wound cells were isolated 1 and 7 days after PVA sponge insertion, incubated in complete medium with Brefeldin A for 6 h, and stained with fluorochrome-conjugated antibodies for F4/80, TNF-α, and mannose receptor. The figure shows staining for TNF-α and mannose receptor in gated macrophages (F4/80+ cells). Results are representative of two experiments.
Wound macrophages retain phenotypic plasticity
Macrophages isolated from Day 7 wounds were cultured with rmIFN-γ and LPS or with rmIL-4. Data in Table 3 demonstrate that LPS and rmIFN-γ increased the release of TNF-α, IL-6, and CCL5 by wound macrophages, as would be expected with classical activation. Treatment with rmIL-4 increased the production of CCL17, demonstrating the potential for wound macrophages to be polarized by IL-4 to the alternatively activated phenotype.
TABLE 3.
Plasticity of Wound Macrophages: Cytokine and Chemokine Response to IFN-γ/LPS or IL-4a
| TNF-α (pg) | IL-6 (pg) | IL-10 (pg) | CCL5 (pg) | CCL17 (pg) | |
|---|---|---|---|---|---|
| Untreated | 7.5 ± 9.4 | ND | ND | 21.2 ± 1.1 | 0.5 ± 0.0 |
| IFN-γ and LPS | >1000b | 684 ± 54b | 220 ± 13b | 1264 ± 42b | 0.3 ± 0.1 |
| IL-4 | 14.6 ± 8.5 | 2.1 ± 3.2 | ND | 9.2 ± 4.2 | 14.7 ± 0.4b |
Macrophages isolated from Day 7 wound cells were cultured in complete medium for 24 h with or without IL-4 (10 ng/mL) or IFN-γ (10 U/mL) and LPS (0.1 μg/mL). Data are cytokine production/3 × 105 cells, mean ± sd of quadruplicate wells, and are representative of two experiments. IL-12p70 was not detected in culture supernatants.
P < 0.05 versus untreated cells, ANOVA with Newman-Keuls.
IL-4 and IL-13 are not detected in wounds
It has been proposed that IL-4/IL-13 are necessary for the development of the wound-healing macrophage phenotype [7]. However, wound cells isolated 1, 3, or 7 days after injury did not produce IL-4 (Fig. 4) or IL-13 (Fig. 5) in an intracellular cytokine assay. Neither cytokine was detected by ELISA of wound cell culture supernatants or in an ELISPOT assay of total wound cells obtained 1, 3, or 7 days after injury (data not shown).
Figure 4.
Wound cells do not produce IL-4. Wound cells were isolated at 1, 3, and 7 days after PVA sponge insertion, incubated in complete medium with Monensin for 6 h, and stained with fluorochrome-conjugated isotype control (left panels) or IL-4-specific (right panels) antibody. Positive control: BALB/c splenocytes cultured as described in Materials and Methods and stimulated with PMA and calcium ionomycin. Results are representative of two experiments. SSC-H, SSC-height.
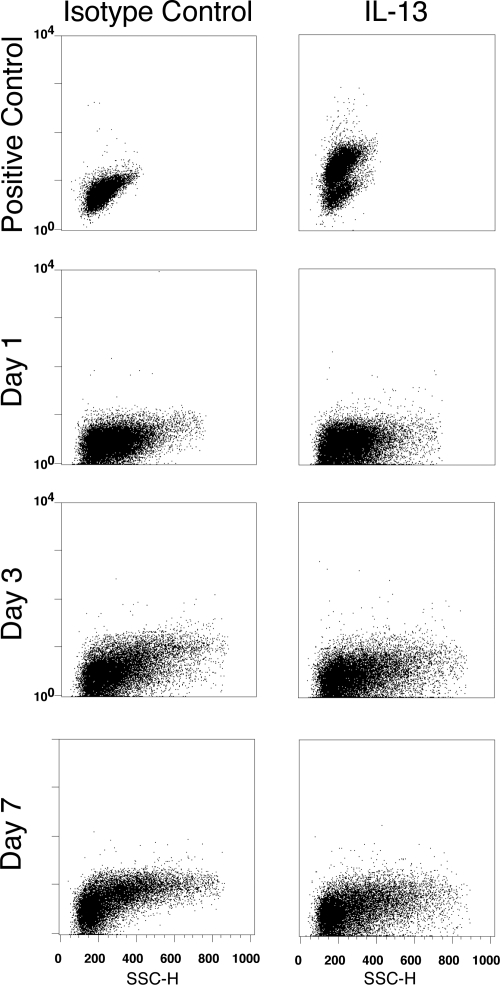
Figure 5.
Wound cells do not produce IL-13. Wound cells were isolated at 1, 3, and 7 days after PVA sponge insertion, incubated in complete medium with Brefeldin A for 6 h, and stained with fluorochrome-conjugated isotype control (left panels) or IL-13-specific (right panels) antibody. Positive control: BALB/c splenocytes cultured as described in Materials and Methods and stimulated with PMA and calcium ionomycin. Results are representative of two experiments.
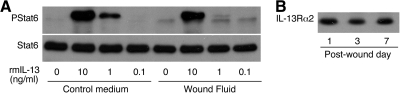
No IL-4 was detected in wound fluids obtained from at least six mice/time-point at 1, 3, or 7 days after wounding, by ELISA or by bioassay using the IL-4-dependent CT.4S cell line (data not shown). The IL-13 content of wound fluids could not be assessed using ELISA, as a result of interference of wound fluid with the detection of added rmIL-13. As IL-4 and IL-13 signal through IL-4Rα to phosphorylate Stat6, PStat6 was used to assess for bioactive IL-4 and IL-13. Freshly isolated wound macrophages expressed IL-4Rα but did not contain PStat6 (Fig. 6), indicating a lack of IL-4 and IL-13 in the wound environment. However, wound macrophages had the capacity to phosphorylate Stat6 in response to rmIL-4 (Fig. 6). Wound fluids were tested for the ability to induce phosphorylation of Stat6 in naïve cells. Wound fluids obtained 1, 3, and 7 days after injury did not induce phosphorylation of Stat6 in resident peritoneal cells (not shown) nor did Day 1 or 7 wound fluids do so in the J774A.1 macrophages. Moreover, Day 1 and Day 7 wound fluids inhibited rmIL-13-induced phosphorylation of Stat6 in J774A.1 cells (Fig. 7A). This finding, together with the limited detection of rmIL-13 by ELISA in the presence of wound fluid, suggested that a component of wound fluid might bind and inactivate IL-13. Supporting this hypothesis, wound fluids obtained at 1, 3, and 7 days contained soluble IL-13Rα2, a high-affinity decoy receptor for IL-13 (Fig. 7B).
Figure 6.

Wound macrophages express IL-4Rα but do not contain PStat6. Wound cells were stained for IL-4Rα (open histograms) or with isotype control antibody (shaded histograms) and for CD68. Histograms show staining for IL-4Rα in gated macrophages (CD68high cells). For PStat6 staining, wound cells were incubated or not with IL-4 (10 ng/mL) for 15 min and then fixed and stained for PStat6 (open histograms) or with isotype control antibody (shaded histograms) and with Gr-1 and F4/80. Histograms show staining for PStat6 in gated macrophages (F4/80+, Gr-1low/int cells). Results are representative of two experiments.
Figure 7.
Wound fluids inhibit rmIL-13-induced phosphorylation of Stat6 and contain IL-13Rα2. (A) J774A.1 cells were treated with rmIL-13, diluted in control media or pooled Day 1 wound fluids. Cells lysates were size-fractionated and immunoblotted with antibodies to PStat6 and Stat6. Identical results were obtained when rmIL-13 was added to Day 7 wound fluids (not shown). (B) Wound fluids obtained at 1, 3, and 7 days after PVA sponge insertion were size-fractionated and immunoblotted with antibodies to IL-13Rα2. Results are representative of two experiments.
The acquisition of the wound macrophage phenotype does not require IL-4Rα
To exclude the possibility that IL-4 and IL-13 were present in the wound at concentrations below the limits of detection of the assays used here, but sufficient to exert a biological effect, additional experiments examined wound macrophages from mice lacking IL-4Rα. As IL-4Rα is a component of Type I and Type II IL-4Rs, mice lacking IL-4Rα are unable to phosphorylate Stat6 in response to IL-4 or IL-13 [21]. Seven day wound macrophages isolated from wild-type or IL-4Rα knockout mice did not differ in mannose receptor, dectin-1 (Fig. 8), arginase 1, Ym1, or iNOS (Fig. 9).
Figure 8.
Mannose receptor and dectin-1 in wound macrophages are independent of IL-4Rα. Wound cells were obtained from wild-type or IL-4Rα knockout (IL-4rα −/−) mice 7 days after PVA sponge insertion. Cells were stained for mannose receptor or dectin-1 (open histograms) or the appropriate isotype control antibodies (shaded histograms) and for CD68. Histograms show expression of the antigens in gated macrophages (CD68high cells).
Figure 9.
iNOS, arginase I, and Ym1 in wound macrophages are independent of IL-4Rα. Wound cells were obtained from wild-type (W.T.) or IL-4Rα knockout mice 7 days after PVA sponge insertion. Macrophages were isolated by negative selection. Cell lysates from unfractionated wound cells or isolated wound macrophages were size-fractionated and immunoblotted with antibodies for iNOS, Arginase 1, and Ym1/2.
The number of wound inflammatory cells at 7 days was greater in IL-4Rα-deficient mice than in wild-type mice (17.5±5.5×106 in the IL-4Rα knockout vs. 10.6±4.3×106 cells/animal in wild-type; P<0.05, Student’s t-test, n≥4 each group). There were no differences in the differential wound cell counts between the groups (40.1±7.4% neutrophils and 58.2±6.5% macrophages in knockouts vs. 37.1±7.4% neutrophils and 60.8±6.6% macrophages in controls).
DISCUSSION
This work examined the phenotype of wound macrophages isolated directly from s.c.-implanted PVA sponges in mice. The PVA sponge model reproduces normal soft-tissue healing in mammals. In this model, an early inflammatory cell infiltrate is followed by ECM deposition and neovascularization, culminating in the encapsulation of the sponge in a collagenous scar [10, 22]. This stereotypical course of events is akin to that seen in skin wounds and in other sites of tissue injury. A specific advantage of the PVA sponge model is that wound cells can be retrieved easily from the sponge and examined directly for phenotypic expression.
Macrophages comprise ∼15% of inflammatory cells recovered from the wound 1 day after sponge insertion. The number of macrophages in the wound increases by ∼40-fold from Days 1 to 7 after injury, and these cells constitute 50% of the cellular infiltrate of the wound by the later day [10]. Macrophage expression of Gr-1 changed over time. Eighty-five percent of Day 1 wound macrophages expressed the Gr-1 antigen, consistent with Gr-1+ blood monocytes being precursors to early inflammatory macrophages [16, 17]. The fraction of macrophages expressing Gr-1 decreased to 20% by Day 7, likely a result of influx of Gr-1neg monocytes, as has been demonstrated in a murine model of myocardial infarction [17], rather than loss of Gr-1 antigen as cells mature. As the total number of macrophages in the wound increases between Days 1 and 7 after injury, the numbers of Gr-1+ and Gr-1neg macrophages increase by approximately tenfold and 200-fold, respectively, in this time interval.
The expression of dectin-1, mannose receptor, and arginase 1 by wound macrophages is consistent with the alternatively activated phenotype [2, 6]. Mannose receptor was acquired by macrophages upon arrival in the wound, whereas dectin-1 was expressed equally in wound macrophages and circulating monocytes. iNOS, a hallmark of classical activation, was not present in wound macrophages. This observation is in agreement with previous reports from this laboratory that iNOS is absent in sterile mouse wounds but is induced in wounds that are infected or colonized with bacteria [23].
The role for alternatively activated macrophages in repair has been predicated almost exclusively on the expression of arginase 1 by these cells. This laboratory first reported arginase in wounds and proposed that a product of the arginase reaction, ornithine, could contribute to the synthesis of collagen through its metabolism to proline [24]. Although this proposal has been echoed in the literature [2, 7], there is no direct evidence that native proline is deficient or rate-limiting for the synthesis of collagen in wounds. Moreover, the lack of arginase 1 expression in human macrophages [19, 25] strongly argues against a necessary role for this enzyme in repair.
Although wound macrophages expressed traits of alternatively activated macrophages, they also produced cytokines (TNF- α, IL-6) that are associated with the classically activated phenotype (Tables 2 and 3). They did not release IL-10 and thus appear to be distinct from IL-10-producing regulatory macrophages [7]. The secretory pattern of macrophages changed as the wound aged. Day 7 macrophages produced less TNF-α and IL-6 and more TGF-β than Day 1 macrophages. The early release of proinflammatory cytokines by wound macrophages, followed by a later increase in the production of the profibrotic cytokine TGF-β, resembles what has been reported in a murine model of myocardial infarction, where early production of TNF-α by monocytes is followed by later production of the proangiogenic cytokine vascular endothelial growth factor [17].
The expression of Ym1 in wound macrophages was lower at Days 3 and 7 than at 1 day after injury, yet arginase 1 and mannose receptor did not decrease with time. Thus, characteristics associated with a particular state of macrophage activation may not be expressed simultaneously in vivo, in agreement with the concept that a single marker cannot be used to define a population of macrophages [7]. Wound macrophages express distinct and heterogeneous phenotypes. The simultaneous expression of mannose receptor and production of TNF-α in wound macrophages, shown in Figure 3, provides evidence that macrophages can share features of classically and alternatively activated cells, as proposed by Mosser and Edwards [7].
It has been proposed that IL-4 and IL-13 are required for development of the wound-healing macrophage phenotype [7]. Although these cytokines have been detected by immunohistochemistry in some models of injury [26, 27], some studies fail to detect IL-4 in murine wounds [28]. The present study found no evidence for IL-4 or IL-13 in wounds. Freshly isolated wound cells did not produce detectable IL-4 or IL-13, as assayed by intracellular cytokine staining, ELISPOT, or ELISA of cell culture medium. IL-4 was not detected in wound fluids at any time. The IL-13 concentration of wound fluid could not be assessed by ELISA; however, wound fluids did not induce phosphorylation of Stat6 in naïve cells and in fact, inhibited IL-13-induced phosphorylation of Stat6. Furthermore, wound cells did not contain PStat6, indicating a lack of IL-4 and IL-13 signaling in the wound environment. Thus, although this study does not exclude completely the presence of IL-13, the data suggest that there is no biologic activity of this cytokine in wounds. However, wound cells retained phenotypic plasticity and were capable of responding to IL-4, as demonstrated in Figure 6 and Table 3.
Experiments in IL-4Rα knockout mice provided further evidence that neither IL-4 nor IL-13 determines wound macrophage phenotype. IL-4Rα is a subunit of the Type I IL-4R (a heterodimer of IL-4Rα and the common γ-chain), which mediates IL-4-dependent activation of Stat6, and the Type II IL-4R (a heterodimer of IL-4Rα and IL-13Rα1), which binds IL-4 and IL-13 and also signals through Stat6 [21, 29]. Wound macrophages from mice deficient in the IL-4Rα did not differ from wild-type mice in expression of mannose receptor, dectin-1, arginase 1, Ym1, or iNOS.
IL-13 can also bind to IL-13Rα2, a high-affinity decoy receptor that does not signal through Stat6 [30, 31]. However, the absence of bioactive IL-13 in the wound environment argues against a role for IL-13Rα2 in development of the wound macrophage phenotype. Further, the presence of soluble IL-13Rα2 in wound fluid suggests a mechanism to limit biological activity of IL-13 in wounds.
Wounds in IL-4Rα knockout mice contained more inflammatory cells than those in wild-type mice. An explanation for these differences is not apparent and may reflect a proinflammatory phenotype in these animals. However, the composition of the cellular infiltrate and the phenotype of wound macrophages did not differ between the wild-type and knockout animals.
This study examined macrophages isolated directly from wounds. Results demonstrate that wound macrophages have a complex phenotype and that IL-4 and IL-13 are not required for the development of wound macrophage phenotype. Thus, findings do not support the concept that wound macrophages are alternatively activated macrophages or that alternatively activated macrophages are wound-healing macrophages.
Present results extend previous reports from this laboratory describing wound macrophage phenotype [32,33,34]. It is likely that multiple factors determine the phenotype of wound macrophages, including macrophage interactions with ECM, other cell types, and soluble factors. The authors are aware that findings in sterile soft-tissue wounds in mice, although reflective of the general pattern of repair in tegumental injuries, may not be applicable to other models of injury, where specialized populations of resident macrophages (i.e., osteoclasts, microglia, Kuppfer cells) may play a role in inflammation and tissue repair. The present study provides a point of departure for further investigations correlating the phenotype of wound macrophages with specific roles in tissue repair.
Acknowledgments
This work was supported by National Institutes of Health grants GM-79227 (to J. M. D.), GM-66194 (to J. S. R.), and GM-42859 (to J. E. A.), the Carter Family Charitable Trust (Armand D. Versaci Research Scholar in Surgical Sciences Award to A. A. T.), and funds allocated to the Department of Surgery by Rhode Island Hospital, a Lifespan partner. S. K. B. was supported by National Institutes of Health grant T32GM-65085. The authors thank William L. Henry Jr., Balduino Mastrofrancesco, Stanley Voigt, and Sara Spangenberger for technical assistance.
Footnotes
Abbreviations: ECM=extracellular matrix, iNOS=inducible NO synthase, m=murine, PStat6=phosphorylated Stat6, PVA=polyvinyl alcohol, RIPA=radioimmunoprecipitation assay, SSC=side-scatter, Y641=tyrosine 641
Authors’ note: Several classifications for macrophage activation have been proposed. These include classical (induced by IFN-γ and LPS) versus alternative (induced by IL-4 or IL-13) [2, 4]; M1 (classical) versus M2 (M2a macrophages are induced by IL-4 or IL-13; M2b by immune complexes in combination with TLR agonists or IL-1β; M2c by IL-10, glucocorticoids, or TGF-β) [8, 9]; and a functional classification (classical/wound-healing/regulatory macrophages) [7]. This manuscript uses the terms “classical activation” and “alternative activation”, as defined by Gordon [2].
References
- Kodelja V, Muller C, Tenorio S, Schebesch C, Orfanos C E, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–493. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor P R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Martinez F O, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Willment J A, Lin H H, Reid D M, Taylor P R, Williams D L, Wong S Y, Gordon S, Brown G D. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- Mosser D M, Edwards J P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martinez F O, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Daley J M, Reichner J S, Mahoney E J, Manfield L, Henry W L, Jr, Mastrofrancesco B, Albina J E. Modulation of macrophage phenotype by soluble product(s) released from neutrophils. J Immunol. 2005;174:2265–2272. doi: 10.4049/jimmunol.174.4.2265. [DOI] [PubMed] [Google Scholar]
- Krutzik P O, Clutter M R, Nolan G P. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
- Daley J M, Thomay A A, Connolly M D, Reichner J S, Albina J E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- Gieni RS, Li Y, HayGlass K T. Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J Immunol Methods. 1995;187:85–93. doi: 10.1016/0022-1759(95)00170-f. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Henderson R B, Hobbs J A, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski F K, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J L, Libby P, Weissleder R, Pittet M J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman D R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes J M, Luckner C, Doschko G, Soler G, Eichmann K, Muller F M, Ho A D, Goerner M, Modolell M. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- Harbord M, Novelli M, Canas B, Power D, Davis C, Godovac-Zimmermann J, Roes J, Segal A W. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J Biol Chem. 2002;277:5468–5475. doi: 10.1074/jbc.M110635200. [DOI] [PubMed] [Google Scholar]
- Ramalingam T R, Pesce J T, Sheikh F, Cheever A W, Mentink-Kane M M, Wilson M S, Stevens S, Valenzuela D M, Murphy A J, Yancopoulos G D, Urban J F, Jr, Donnelly R P, Wynn T A. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomay A A, Daley J M, Sabo E, Worth P J, Shelton L J, Harty M W, Reichner J S, Albina J E. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol. 2009;174:2129–2136. doi: 10.2353/ajpath.2009.080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney E, Reichner J, Bostom L R, Mastrofrancesco B, Henry W, Albina J. Bacterial colonization and the expression of inducible nitric oxide synthase in murine wounds. Am J Pathol. 2002;161:2143–2152. doi: 10.1016/s0002-9440(10)64492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina J E, Mills C D, Barbul A, Thirkill C E, Henry W L, Jr, Mastrofrancesco B, Caldwell M D. Arginine metabolism in wounds. Am J Physiol. 1988;254:E459–E467. doi: 10.1152/ajpendo.1988.254.4.E459. [DOI] [PubMed] [Google Scholar]
- Raes G, Brys L, Dahal B K, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen P J, De Baetselier P, Ghassabeh G H. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, Maquart F X. Implication of interleukin-4 in wound healing. Lab Invest. 2000;80:1337–1343. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- Higgins D M, Basaraba R J, Hohnbaum A C, Lee E J, Grainger D W, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009;175:161–170. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan D, Walker K B, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- LaPorte S L, Juo Z S, Vaclavikova J, Colf L A, Qi X, Heller N M, Keegan A D, Garcia K C. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N, Whitters M J, Jacobson B A, Witek J, Sypek J P, Kasaian M, Eppihimer M J, Unger M, Tanaka T, Goldman S J, Collins M, Donaldson D D, Grusby M J. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α 2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Taguchi J, Murata T, Puri R K. The interleukin-13 receptor α2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- Reichner J S, Fitzpatrick P A, Wakshull E, Albina J E. Receptor-mediated phagocytosis of rat macrophages is regulated differentially for opsonized particles and non-opsonized particles containing β-glucan. Immunology. 2001;104:198–206. doi: 10.1046/j.0019-2805.2001.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessel C C, Henry W L, Jr, Mastrofrancesco B, Reichner J S, Albina J E. Vestigial respiratory burst activity in wound macrophages. Am J Physiol. 1999;276:R1587–R1594. doi: 10.1152/ajpregu.1999.276.6.R1587. [DOI] [PubMed] [Google Scholar]
- Meszaros A J, Reichner J S, Albina J E. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]