Abstract
Increased levels of MMP-8 (neutrophil collagenase) have been reported in OB, but the biological role of MMP-8 in OB is not known. MMP-8 is an interstitial collagenase highly expressed by polymorphonuclear leukocytes, which are prominent in early OB. Here, we show that MMP-8 promotes migration of PMNs through the collagen-rich matrix in a mouse heterotopic airway transplant model of OB. Overall, MMP-8−/− mice had significantly fewer PMNs in the airway lumen 2 and 14 days post-transplantation, and the percentage of PMNs traversing the matrix to the lumen was decreased markedly in the MMP-8−/− compared with WT mice at 14 days. There were significantly more PMNs outside of the lumen in the ECM in the MMP-8−/− mice compared with WT mice. In vitro, significantly fewer MMP-8−/− PMNs migrated through 3D cross-linked collagen gels than WT PMNs. MMP inhibitor GM6001 was also able to impede migration of WT PMNs through collagen gels. The decreased migration was likely a result of pericollagenase activity of MMP-8, as WT PMNs expressing MMP-8 were not able to migrate effectively through collagen that was resistant to the collagenase. Protection from OB was seen in the MMP-8−/− mice, as the airway lumen had significantly less obliteration and collagen deposition, suggesting that MMP-8 plays an important role in the pathogenesis of OB.
Keywords: cell trafficking, inflammation, neutrophils
Introduction
MMP-8 is a member of the interstitial collagenase family, which cleaves interstitial collagen at a specific locus in the α-1 chain between Gly 775 and Ile 776. This cleavage generates fragments that are three-fourths and one-fourth the size of the original molecule. The fragments denature spontaneously to gelatins and are processed further by MMP-2, -9, and -8, MT1-MMP, and serine proteases [1, 2]. In addition, MMP-8 cleaves nonmatrix proteins such as serpins, β casein, human α 2-macroglobulin, bradykinin, angiotensin I, and substance P [3]. MMP-8 is known as neutrophil collagenase, as it was described in PMNs, which are the major source of MMP-8 in inflammatory diseases [4, 5]. In the PMN, it is stored in a latent form [pro-MMP-8 (Mr 85 kDa)] within specific granules and is released on activation by inflammatory mediators. The majority of active MMP-8 becomes membrane-bound, and in this form, it is resistant to TIMP-1 and -2. Approximately 92% of the pericellular collagenase activity associated with PMNs in vitro is attributable to membrane-bound MMP-8 [6], yet its function in vivo remains unknown. Other cell types that express MMP-8 are rheumatoid synovial fibroblasts, endothelial cells, activated macrophages, smooth muscle cells, bronchial epithelial cells, mast cells, and chondrocytes [4, 7,8,9,10]. MMP-8 expression and activity have a strong association with chronic inflammatory and fibrotic diseases, such as cystic fibrosis, rheumatoid arthritis, periodontal disease, and chronic skin wounds [11,12,13,14].
Despite a large armamentarium of matrix-degrading proteinases within PMNs, migration through endothelial and matrix barriers has not been thought to be proteinase-dependent. We hypothesized that MMP-8 collagenase activity would be required for PMNs to traverse collagen-rich ECMs in relevant lung diseases, such as OB, which is a manifestation of chronic rejection post-lung transplantation and is characterized by immunological injury, peribronchiolar inflammation, and a fibroproliferative response that begins as subepithelial fibrosis. In association with disruption of the basement membrane and loss of bronchial epithelium, the proliferating fibroblasts invade the airways, deposit collagen, and occlude the airways [15,16,17]. High levels of MMP-8 have been reported in BAL of patients with OB post-lung transplant, but its significance remains unclear [18].
Although the role of adaptive immunity is well established in OB, the role of the innate immune system is being recognized increasingly. PMNs have been shown to cause parenchymal damage and increased rejection in heart transplants [15, 19]. In patients with OB, PMNs are among the first inflammatory cells to be detected in the BAL and lung biopsy specimens [15]. Recent treatment of OB patients with azithromycin showed improvement of lung function in a subset of patients. Detailed analysis of the responder group revealed that only patients with elevated BAL PMN numbers improved with the treatment. PMN counts normalized after treatment, further underlining the importance of PMNs [20].
The high level of type 1 collagen expression in the mouse heterotopic airway model combined with the increase of PMN and MMP-8 in OB makes it an ideal and relevant model to investigate the significance of neutrophil collagenase [18, 21]. To determine the role that MMP-8 plays in PMN migration through ECMs and to study the role of MMP-8 in the pathophysiology of OB, we subjected WT and MMP-8−/− mice to a mouse model of OB.
MATERIALS AND METHODS
Heterotopic airway transplant model
All studies were performed according to institutional guidelines for animal use and care. We used the well-established model of OB involving heterotopic s.c. tracheal transplant with MHC-mismatched combinations of C57BL/6 (H-2b) and BALB/c (H-2d) mice as described previously [16, 22]. Eight- to 12-week-old female MMP-8−/− mice backcrossed 10 generations to a C57BL/6 genetic background, and WT mice on a C57BL/6 and BALB/c genetic background were used. Tracheas from WT BALB/c mice were transplanted s.c. into the backs of WT C57BL/6 (control) and MMP-8−/− C57BL/6 background mice. At 2 days and 14 days, the transplanted mice were killed, and the tracheas were harvested.
Immunohistochemistry
Airways were fixed in formalin-free zinc fixative (BD Biosciences PharMingen, San Diego, CA, USA) cut in half and paraffin-embedded with the cut side down. Cross-sections of tracheal tissues (5 μm) were stained with rat anti-mouse antibodies to granulocyte marker Ly-6C/Ly-6G/Gr-1 (BD Biosciences PharMingen). A standard ABC vector kit (Vector Laboratories, Burlingame, CA, USA) was used. Specific staining was confirmed by using isotype control antibody. All PMNs were counted manually in the entire section (outside and inside of the tracheal lumen) of the allograft under a microscope.
Grading
Sections with H&E stains were graded by two independent observers who were blinded for the groups. A scale from 0 (no luminal obliteration) to 4 (maximal obliteration) was used to grade luminal obliteration.
Image-based analysis for luminal obliteration and collagen
Images were acquired on an Olympus BH-2 with a RT-color (Spot) camera in one session to ensure constant white balance and illumination and were processed with ImageJ (NIH, Bethesda, MD, USA). The luminal area was outlined in three nonconsecutive H&E slides/sample, and the percentage of obliteration was measured using a fixed threshold to exclude nonobliterated areas. For analysis of collagen density, two trichrome-stained (collagen in blue), nonconsecutive slides/sample were processed. The luminal area was outlined, and the color-threshold plug-in was used to gate the nonblue colors before the remaining collagen-positive blue staining was measured as a percentage of the luminal area. Program settings were constant for all slides.
Blood leukocyte analysis
Blood was collected retro-orbitally from WT and MMP-8−/− mice in EDTA tubes (Microvette, Sarstedt, Germany). Total leukocyte count along with a differential count of the leukocyte subset from blood was obtained by the ADVIA® 120 hematology analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). TLC, ANC, AMNC, and AEC in blood were compared between WT and MMP-8−/− mice.
Bone marrow analysis
Bone marrow was obtained from WT and MMP-8−/− mice by flushing femur and tibia. RBCs in the bone marrow sediment were lysed using 0.2 N hypotonic saline and then neutralized with 1.6 N saline. Total numbers of leukocytes from bone marrow of WT and MMP-8−/− mice were counted with a hemacytometer using trypan blue dye exclusion. Cytospins were prepared from the bone marrow cell suspension and stained with Wright-Giemsa for manual differential count.
PMN purification
PMNs from the bone marrow were separated by density gradient centrifugation using 50% and 70% Percoll gradients (GE Healthcare Bio-Sciences AB, Sweden), as per the manufacturer’s suggestions. Bone marrow cells suspended in 1 ml media (DMEM with 10% serum, penicillin, streptomycin, and Fungizone) were placed on top of the Percoll gradient and centrifuged at 2900 rpm at room temperature for 30 min. PMNs in the lower column were aspirated gently, washed, and resuspended in 1 ml DMEM (with penicillin, streptomycin, and Fungizone). The PMNs isolated by this method were >95% viable when assessed by trypan blue staining and >85% pure, as determined by cytospin preparation stained with Wright-Giemsa.
Activation of PMNs
WT and MMP-8−/− PMNs were activated first by priming with PAF (Sigma-Aldrich, St. Louis, MO, USA) at 10−6 M concentration for 15 min, followed by fMLP (Sigma-Aldrich) at 10−6 M concentration at 37°C for 30 min [6]. After stimulation, PMNs were washed and resuspended in 1 ml serum-free DMEM (with penicillin, streptomycin, and Fungizone).
Surface staining of MMP-8 on PMNs by FACS analysis
Stimulated WT and MMP-8−/− mice were stained with rabbit anti-human MMP-8 antibody (1:100; Chemicon, El Segundo, CA, USA) after FcR blockade with anti-mouse CD16/32 antibody (eBioscience, San Diego, CA, USA). Computer analysis for comparing MMP-8 activity between WT and MMP-8−/− PMNs was done using the Summit 4.3 program (Dako-Cytomation, Denmark).
PMN labeling
PMNs were labeled with the fluorescent dye CellTrace CFSE (Invitrogen Corp., Carlsbad, CA, USA), as per the manufacturer’s protocol. The PMNs were resuspended at a final cell concentration of 5 × 105 PMNs/200 μl IMDM (supplemented with 1% FBS, penicillin, streptomycin, and Fungizone).
Inhibition of PMN MMP-8
Activated WT PMNs were treated with a commercially available general MMP inhibitor (GM6001; Millipore Corp., Bedford, MA, USA) at 25 μM concentration and incubated at 37°C for 30 min [23].
Collagenase-resistant collagen
To prepare cleavage-resistant collagen, commercially available EGCG (Sigma-Aldrich) was used to make rat-tail collagen type 1 resistant to MMP-8 digestion [24]. Collagen gels were prepared at a final concentration of 5 mg/ml. Two hundred microliters 0.75 mM EGCG or PBS was added on top of the collagen gels, which were incubated at 37°C for 24 h. Unused EGCG was removed from the gels by washing with PBS for 24 h.
To determine if EGCG-treated collagen was resistant to MMP-8, rat-tail collagen type 1 (pH 4) was dissolved in predetermined volumes of cold, sterile 5 mM Tris (pH 8). Sterile, cold 0.1 N NaOH was gently added to the collagen mixture to neutralize (pH 7.2) without gelling. EGCG (Sigma-Aldrich) was added at different concentrations (0.5 mM, 0.75 mM, and 1 mM) to the collagen mixture, and it was placed on a Nutator at 4°C for 24 h. After 24 h, the EGCG-treated collagen was dialyzed using the Slide-A-Lyzer dialysis cassette (Thermo Scientific Pierce, Waltham, MA, USA) in acetic acid (5 mM) at 4°C for 12–18 h to remove unused EGCG.
MMP-8 activation
Commercially available pro-MMP-8 was dissolved in 50 mM Tris (supplemented with 150 mM NaCl, 20 mM CaCl2, pH 7.4) and activated with 40 mM APMA (prepared in DMSO) at 37°C for 2 h. Activated MMP-8 (400 ng/500 mg collagen) was then added to the untreated type 1 collagen and EGCG-treated collagen and incubated at 37°C for 18 h.
Protein gel electrophoresis
Resistance of EGCG-treated collagen to MMP-8 digestion was assessed by protein gel electrophoresis using 7% Tris acetate gel (Invitrogen Corp.). Samples consisted of collagen and EGCG-treated collagen, with and without MMP-8 treatment. After electrophoresis, the protein gel was washed in distilled water, stained with Imperial protein stain (Thermo Scientific Pierce), and dried.
Collagen gel preparation
Collagen gels were prepared from commercially available rat-tail collagen type 1, as per the company’s protocol (BD Biosciences PharMingen) by mixing rat-tail collagen type 1 with a predetermined volume of cold, sterile 10× PBS; cold, sterile 1 N NaOH; and cold, sterile distilled water. The final concentration of this collagen was 5 mg/ml. Four hundred microliters of this collagen mixture was poured into sterile 12 mm Transwell inserts (Costar, Corning, Corning, NY, USA) with a 0.4-μm polycarbonate membrane. These were incubated for 3–4 h at 37°C for gelling, yielding 3D firm, crossed-linked collagen gels with 4 mm thickness and 113 mm2 surface area.
PMN migration assay across collagen gels
The Transwell inserts with 0.4 μm polycarbonate membranes, which do not allow PMNs to penetrate through the membrane, were used. Collagen gels were placed in the sterile 12-well plates. The lower chamber contained IMDM supplemented with 1% FBS, penicillin, streptomycin, Fungizone, and fMLP (10−6 M concentration). The PMN suspension (200 μl; 5×105 PMNs) in IMDM (with 1% FBS, penicillin, streptomycin, and Fungizone) was placed gently on top of the collagen gels and incubated in a CO2 incubator (5% CO2, 95% humidity) at 37°C. At 4 h and 24 h, the Transwells with the collagen gels were removed and fixed in 4% paraformaldehyde and incubated at 4°C for 48–72 h. To confirm that PMNs did not pass through the 0.4-μm polycarbonate membrane, each lower chamber was examined under a fluorescent microscope for CFSE-labeled PMNs. The polycarbonate membranes from the Transwell inserts were cut carefully at the periphery with a #15 scalpel (BD Bard-Parker™), gently peeled off the collagen gels with forceps, placed on the microscope slide, and covered with a cover slip (Fisherbrand). Care was taken to clean the scalpel and forceps with alcohol between each membrane to avoid contamination. The PMNs that had traveled through the collagen gels onto the membrane were counted under fluorescent microscopy. PMN migration assays across collagen gels were done using PMNs from WT, MMP-8−/− mice, and WT PMNs treated with a MMP inhibitor (GM6001). Similar experiments were done to study migration of WT PMNs across EGCG-treated, cleavage-resistant collagen.
Statistical assay
Student’s t-test (unpaired) and Mann-Whitney tests were done, and a Pvalue of <0.05 was considered significant.
RESULTS
Role of MMP-8 in PMN migration through collagen matrices in vivo
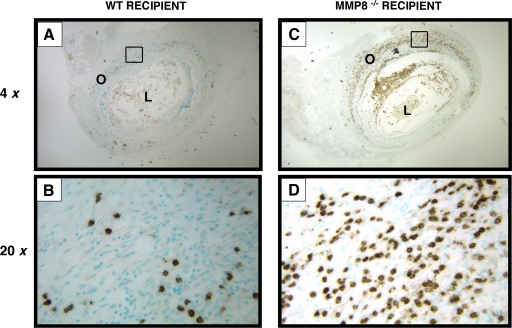
PMNs are one of the earliest inflammatory cells seen in OB, where they are required to migrate through collagen-rich ECMs. We hypothesized that PMN-MMP-8, an interstitial collagenase, plays a role in the migration of PMNs. To test our hypothesis, we used a well-established and reproducible murine model of OB involving heterotrophic s.c. tracheal transplantation [22]. We used MHC-mismatched tracheas from BALB/c mice (H-2d; donor) and transplanted them s.c. into the backs of WT C57BL/6 (H-2b) and MMP-8−/− C57BL/6 recipient mice. Tracheal cross-sections were stained with a specific marker for granulocytes (Ly-6C/Ly-6G/Gr-1). In sections from the tracheas 14 days post-transplant, PMNs were stained with Ly-6C/Ly-6G/Gr-1 in WT (Fig. 1, A and B) and MMP-8−/− recipients (Fig. 1, C and D). There were more PMNs outside of the lumen in MMP-8−/− mice compared with WT recipients and fewer PMNs in the lumen of the MMP-8−/− mice compared with WT recipients.
Figure 1.
MMP-8 is required for PMN migration through collagen matrices in vivo. Tracheal allografts 14 days post-transplant were compared between WT recipients (A and B) and MMP-8−/− recipients (C and D). PMNs were stained with an anti-granulocyte marker (Ly-6C/Ly-6G/Gr-1; brown color). MMP-8−/− recipients have fewer PMNs inside of the tracheal lumen (L) and more PMNs outside (O) of the lumen when compared with WT recipients.
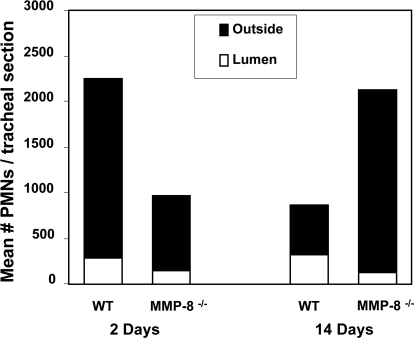
Numbers of PMNs inside of the tracheal lumen and outside of the lumen in each tracheal section were counted manually. Data were expressed as mean ± sem of three independent experiments. PMNs in the tracheal sections (inside of the lumen, outside, and total) from WT recipients were compared with those from MMP-8−/− recipients. Tracheal sections 2 days post-tracheal transplantation showed 1.9-fold fewer PMNs inside of the tracheal lumen of MMP-8−/− recipients when compared with WT recipients (P=0.04). There was a 2.4-fold increase of PMNs outside of the lumen in WT when compared with MMP-8−/− recipient mice (P=0.05). The total number of PMNs (inside of the lumen+outside of the lumen) was higher by 1.7-fold in the WT recipient (P=0.04). Twelve percent of the total cells were present in the lumen in the WT recipients compared with 15% of the total cells in the MMP-8−/− recipient mice.
Analysis of PMNs from tracheal sections 14 days post-transplantation (which correlates with OB) showed 2.7-fold fewer PMNs inside of the tracheal lumen in MMP-8−/− recipients compared with WT recipients (P=0.02). There was a 2.4-fold increase in PMNs outside of the lumen in MMP-8−/− recipients when compared with WT recipients (P=0.01). Overall, the total number of PMNs (inside of the lumen+outside of the lumen) in the entire tracheal section was 2.5-fold greater in the MMP-8−/− recipients when compared with WT recipients (P=0.04). By this time, 38% of the total cells were present in the lumen in the WT recipients compared with only 6% of the total cells in the MMP-8−/− recipient mice (Fig. 2). In our model, at 2 and 14 days post-transplant, there were fewer PMNs in the lumen of the MMP 8−/− mice, suggesting a defect in migration into the lumen.
Figure 2.
Decreased migration of MMP-8−/− PMNs through collagen matrices in vivo. To compare PMN infiltration in the tracheal allograft between WT and MMP-8−/− recipients at 2 days and 14 days post-transplant, PMNs were stained with an anti-granulocyte marker (Ly-6C/Ly-6G/Gr-1) and were counted manually inside and outside of the tracheal lumen. Data are expressed as mean ± sem. Analysis of data 2 days post-transplant (n=5) showed significantly less PMNs inside of the tracheal lumen of MMP-8−/− recipients (147.3±33.2) compared with WT recipients (274.3±39.7; P=0.04). There were significantly more PMNs outside of the lumen in WT recipients (1974±467.9) compared with MMP-8−/− recipients (808.9±176.8; P=0.05). Twelve percent of the total cells were present in the lumen in the WT recipients compared with 15% of the total cells in the MMP-8−/− recipient mice. At 14 days post-transplant (n=5), there were significantly less PMNs inside of the tracheal lumen in the MMP-8−/− (129.8±14.9) recipients when compared with the WT recipients (321.8±66.7; P=0.02), and there were significantly more PMNs outside of the lumen in MMP-8−/− recipients compared with the WT recipients (P=0.01). By this time, 38% of the total cells were present in the lumen in the WT recipients compared with only 6% of the total cells in the MMP-8−/− recipient mice.
Comparison of total leukocytes and different leukocyte subsets from blood and bone marrow between WT and MMP-8−/− mice
To evaluate if excessive PMN accumulation in the tracheal matrix in the MMP-8−/− recipient is related to a difference in myelogensis, we compared the TLC and different leukocyte subsets in the blood and bone marrow between WT and MMP-8−/− mice (Table 1) from three experiments. On comparison, there was no significant difference in TLC, ANC, AMNC, and AEC in blood among the genotypes. In the bone marrow, a small but significant increase in the total cell count was seen in the MMP-8−/− mice compared with the WT mice (P=0.03), and there was a trend toward an increase in PMNs, although it was not significant (P=0.09). Hence, there is no difference in PMN myelogenesis or extravasation of PMNs from the blood vessels between WT and MMP-8−/− mice.
TABLE 1.
Leukocyte Subsets from Blood and Bone Marrow in the WT and MMP-8−/− Mice
| Cell Type | WT | MMP-8−/− | P Value |
|---|---|---|---|
| Blood | |||
| Total Leucocytes (×107) | 3.49 | 3.23 | 0.64 |
| Absolute Neutrophils (×105) | 305.7 | 286.3 | 0.72 |
| Absolute Mononuclear Cells (×105) | 3047.7 | 2521 | 0.35 |
| Absolute Eosinophils (×105) | 62 | 83.8 | 0.3 |
| Bone Marrow | |||
| Total Leucocytes (×107) | 2.08 | 2.63 | 0.03 |
| Absolute Neutrophils (×104) | 162.8 | 205.3 | 0.09 |
| Absolute Mononuclear Cells (×104) | 45.8 | 48.7 | 0.58 |
| Absolute Eosinophils (×104) | 0.48 | 0.16 | 0.27 |
Blood and bone marrow were obtained from WT and MMP-8−/− mice and analyzed for total leukocyte count (TLC), absolute neutrophil count (ANC), absolute mononuclear cell count (AMNC), and absolute eosinophil count (AEC).
Migration of WT and MMP-8−/− PMNs through collagen matrices in vitro
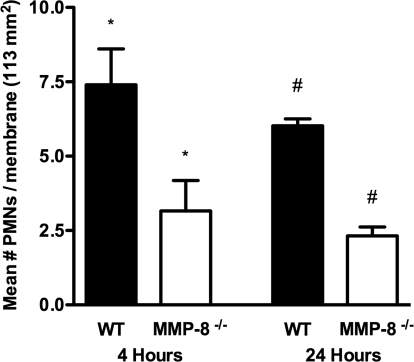
To confirm the requirement of MMP-8 for PMN collagen invasion, we assessed the capacity of WT and MMP-8−/− PMNs to traverse collagen gels in vitro using 3D cross-linked collagen gels prepared from rat-tail collagen type 1. PMNs were stimulated with PAF and fMLP, as WT PMNs stimulated by PAF and fMLP express MMP-8 on their surface, and we confirmed this by FACS analysis (data not shown) [6]. No CFSE-labeled PMNs were seen in the lower chamber containing IMDM, suggesting that PMNs were not able to penetrate through this membrane. PMNs on the 0.4-μm polycarbonate membrane were counted by fluorescent microscopy. Analysis of the PMNs on the membrane in five experiments showed that the WT PMNs were able to penetrate collagen gels 2.3-fold greater than MMP-8−/− PMNs at 4 h (P=0.03) and by 2.6-fold greater at 24 h (P=0.0001) when compared with MMP-8−/− PMNs (Fig. 3). No significant difference was observed in the nonstimulated WT and MMP-8−/− PMNs (data not shown). This suggests that MMP-8 promotes PMN migration through cross-linked collagen.
Figure 3.
MMP-8 is necessary for PMN migration through collagen gels. Migration assays of WT PMNs and MMP-8−/− PMNs across rat-tail collagen type 1 gels in vitro were performed. PMNs that had migrated through the gels onto the 0.4-μm polycarbonate membrane (113 mm2 surface area) were counted under a fluorescent microscope at 4 h and 24 h. Analysis of the data from five experiments (expressed as mean±sem) showed that WT PMNs were able to penetrate collagen gels in significantly greater numbers at 4 h (*, P=0.03) and 24 h (#, P<0.0001) compared with MMP8−/− PMNs.
MMP proteolytic activity is required for migration of PMNs through collagen gels
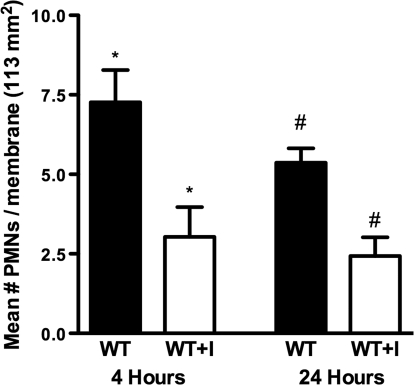
We used a commercially available hydroxamate MMP inhibitor GM6001 (25 μM) to inhibit the activity of MMP-8 in stimulated WT PMNs and studied their migration across collagen gels. As anticipated, WT PMNs treated with the MMP inhibitor demonstrated decreased migration by 2.4-fold at 4 h (P=0.01) and by 2.2-fold at 24 h (P=0.01; Fig. 4) across collagen gels when compared with migration of WT PMNs not treated with the MMP inhibitor. Data are obtained from three experiments. This is similar to results obtained using the MMP-8−/− PMNs, suggesting that the proteolytic activity of MMP-8 is critical for collagen invasion.
Figure 4.
MMP inhibitor decreases migration of PMNs through collagen. Activated PMNs were treated with a MMP inhibitor (GM6001; 25 μM). Migration of WT PMNs treated with the MMP inhibitor (GM6001) and WT PMNs (not treated with the MMP inhibitor) across rat-tail collagen type 1 gels in vitro was compared, as detailed in Materials and Methods. PMNs that migrated through the collagen gels onto the 0.4-μm polycarbonate membrane (113 mm2 surface area) were counted under a fluorescent microscope at 4 h and 24 h. Analysis of the data from three experiments (expressed as mean±sem) showed significantly decreased migration of WT PMNs treated with the MMP inhibitor (WT+I) when compared with the migration of WT PMNs not treated with the MMP inhibitor (WT) at 4 h (*, P=0.01) and 24 h (#, P=0.01).
Specifically, collagenolytic activity is required for PMN migration
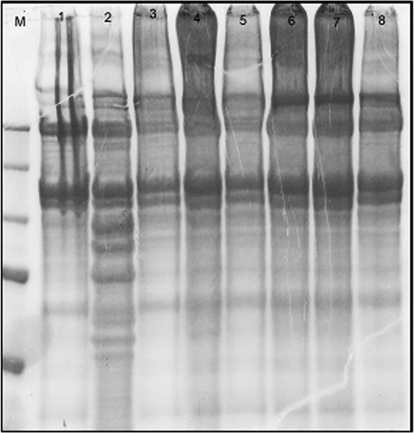
We generated collagenase-resistant collagen using EGCG, a major component of green tea catechins, known to make collagen resistant to digestion by collagenase-1 (MMP-1) by stabilizing the collagen molecule [24]. We treated rat-tail collagen type 1 with EGCG to make it resistant to MMP-8 digestion and then applied APMA-activated MMP-8 to EGCG-treated collagen and non-EGCG-treated collagen. Resistance of EGCG-treated collagen to MMP-8 digestion was evaluated by protein gel electrophoresis. Collagen cleaved by MMP-8 displayed cleavage bands that were not seen with EGCG-treated (0.75 mM and 1 mM) collagen and collagen not treated with MMP-8 (Fig. 5). The above results demonstrate that EGCG-treated collagen shows resistance to cleavage by MMP-8. As collagen treated with lower concentration of EGCG (0.5 mM) showed some degree of cleavage, we used EGCG at 0.75 mM concentration in our experiments to prepare cleavage-resistant collagen gels.
Figure 5.
EGCG-treated collagen is resistant to cleavage by MMP-8. EGCG-treated collagen was analyzed for cleavage resistance to MMP-8 by protein gel electrophoresis. Lane M, Marker; Lane 1, collagen not treated with EGCG and MMP-8; Lane 2, collagen not treated with EGCG but treated with MMP-8; Lane 3, collagen treated with 0.5 mM EGCG but not with MMP-8; Lane 4, collagen treated with 0.5 mM EGCG and MMP-8; Lane 5, collagen treated with 0.75 mM EGCG but not with MMP-8; Lane 6, collagen treated with 0.75 mM EGCG and MMP-8; Lane 7, collagen treated with 1 mM EGCG but not with MMP-8; Lane 8, collagen treated with 1 mM EGCG and MMP-8. Lane 2 shows prominent bands of collagen cleavage. Collagen treated with EGCG (at 0.75 mM and 1 mM; Lanes 6 and 8) showed no bands of cleavage after treatment with MMP-8.
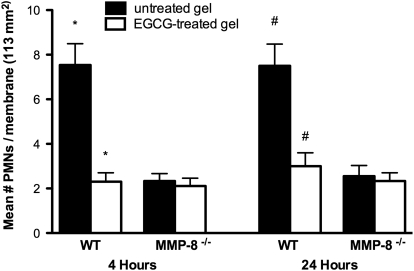
Following the generation of collagenase-resistant collagen, we assessed migration of stimulated WT and MMP-8−/− PMNs across EGCG-treated (0.75 mM), cleavage-resistant collagen and compared their migration across rat-tail collagen type 1 not treated with EGCG. Migration of WT PMNs was decreased by 3.2-fold across EGCG-treated collagen at 4 h (P=0.01) and by 2.4-fold at 24 h (P=0.01) when compared with their migration across collagen gels not treated with EGCG. No significant difference was observed in the migration of MMP-8−/− PMNs, with or without EGCG (Fig. 6). Experiments were repeated three times. This suggests collagenase as the activity mode by which MMP-8 promotes migration of PMNs across collagen barriers.
Figure 6.
Decreased migration of PMNs through EGCG-treated collagen resistant to cleavage by MMP-8. Collagen gels were made resistant to cleavage by MMP-8 by treating them with EGCG. Migration of WT and MMP-8−/− PMNs across EGCG-treated rat-tail collagen type 1 gels was compared with migration of WT and MMP-8−/− PMNs across rat-tail collagen type 1 gels not treated with EGCG. PMNs on the 0.4-μm polycarbonate membrane (113 mm2 surface area) were counted under a fluorescent microscope at 4 h and 24 h. Analysis of the data from three experiments (expressed as mean±sem) showed significantly decreased migration of WT PMNs across EGCG-treated collagen gels (open bars) when compared with their migration across collagen gels not treated with EGCG (solid bars), both at 4 h (*, P=0.01) and 24 h (#, P=0.01). No significant difference was noted in the MMP-8−/− PMNs.
Luminal obliteration
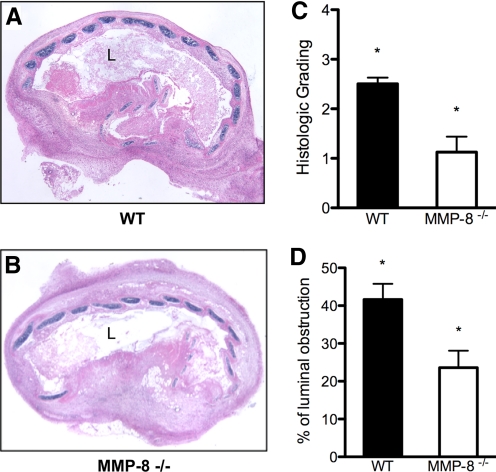
The histology grading of the H&E-stained sections revealed a significant difference between the WT (Fig. 7A) and MMP8−/− recipients (Fig. 7B) in luminal obstruction. Grafts from WT recipients had a median grading of 2.5 compared with MMP-8−/− recipients, where median obliteration severity was 1.25 (P=0.028) on a scale from 0 to 4 (Fig. 7C). The luminal obstruction was also assessed by image analysis in three nonconsecutive slides for each sample to exclude cutting artifacts from two independent experiments. The mean percentage of obstruction in the MMP8−/− recipients was significantly lower compared with the WT mice (32.4±5.2% vs. 52.8±5.8%; P=0.026; Fig. 7D).
Figure 7.
MMP-8−/− recipients show less luminal obliteration. Grafts were explanted 14 days post-transplantation (n=6). Comparison of WT and MMP-8−/− recipients stained with H&E at 40× original magnification shows a higher percentage of luminal obliteration in WT recipients (A) compared with MMP-8−/− recipients (B). Histopathologic grading of H&E-stained samples demonstrates median obliteration was significantly lower in MMP-8−/− animals (1.25 vs. 3.5; P=0.028; C). Quantitative measurement of the area of luminal obstruction by image analysis demonstrated significantly less obstruction in MMP-8−/− recipients (52.8%±5.8 vs. 32.4%±5.2; *, P=0.026; D).
Measurement of fibrosis in the lumen
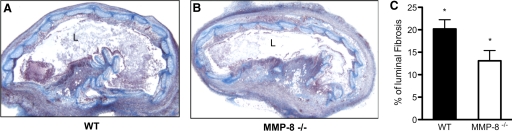
We wanted to evaluate if there was a difference in the fibrosis in the tracheal lumens, as we observed decreased luminal obliteration in the MMP-8−/− mice. This was done by color-threshold image analysis in the trichrome-stained section by delineating the lumen in WT recipients (Fig. 8A) and MMP-8−/− recipients (Fig. 8B). Significantly less fibrosis was seen in the MMP-8−/− mice compared with the WT mice 14 days post-transplantation (20.2±2.05% vs. 13.1±2.3%; P=0.044; Fig. 8C). Data were obtained from two experiments.
Figure 8.
MMP-8−/− mice show protection from luminal fibrosis. Grafts were explanted 14 days post-transplantation and stained for collagen with Masson’s trichrome (n=6). WT recipients demonstrate more luminal fibrosis compared with MMP-8−/− recipients, as seen by the higher amount of collagen (blue; A and B). Image-based quantification of luminal collagen showed that MMP-8−/− mice developed significantly less collagen deposition and fibrosis in the lumen compared with WT recipients at 14 days (13.1±2.3% vs. 20.2±2%; *, P=0.044; C).
DISCUSSION
Mechanisms of PMN extravasation from blood vessels during inflammation are well-defined and do not require proteolytic activity. We wondered whether proteolytic activity would be required for PMN migration through collagen-rich extravascular matrices to reach sites of inflammation. To investigate this, we used a murine heterotrophic tracheal transplant model of chronic rejection known as OB, which is characterized by inflammation and a fibroproliferative response around the airways that extends into the lumen and causes progressive obliteration of the lumen of small airways, leading to respiratory failure and death [15,16,17]. Although it is known that PMNs are among the first inflammatory cells to be detected in the BAL and lung biopsy specimens of patients with OB [15, 20, 25], mechanisms used by PMNs to migrate through this dense collagen matrix need to be determined. MMP-8 is the only interstitial collagenase expressed by PMNs, and it can process collagen I and II more efficiently than MMP-1 and MMP-13 [26]. High levels of MMP-8 have been reported in BAL of patients with OB post-lung transplant, but its significance remains unclear [18].
We have shown that PMNs are decreased significantly in the lumen of the airway in the MMP 8−/− mice compared with WT mice at 2 days and 14 days post-transplantation in the murine OB model. However, a significantly increased number of PMNs are seen in the MMP-8−/− mice outside of the lumen compared with WT mice at 14 days post-transplantation. There was no significant difference in the number of PMNs in the blood or bone marrow in our study, suggesting that MMP-8 does not influence myelogenesis or extravasation from the blood vessels. There was a significant but small increase (1.3-fold) in the total leukocytes in the bone marrow between the WT and the MMP-8−/− mice. The 2.4-fold greater accumulation of MMP-8−/− PMNs in the ECMs outside of the lumen over time may be a result of the ability of MMP-8−/− PMNs to exit blood vessels but not be able to traverse through the matrix. A sustained increase of PMNs in the matrix has also been reported in MMP 8−/− as compared with WT mice in a skin tumor model from 7 to 28 days [27] and also in a wound-healing model [28]. Their study also demonstrated an increase in MMP-9 expression in MMP-8−/− mice. In an OB model using MMP-9−/− recipients, less OB was noted in the MMP-9−/− mice [29]; hence, if there were increased levels of MMP-9−/− in the MMP-8−/− mice in the OB model, it would not explain the reduced luminal obliteration in the MMP-8−/− mice.
We have also confirmed in our in vitro studies that MMP-8−/− PMNs were not able to migrate through the 3D cross-linked rat-tail collagen type 1 gels as efficiently as the WT PMNs. In addition, a small molecular weight MMP inhibitor was also tested in WT-activated PMNs to confirm our results and show that MMP-8-mediated proteolysis allows PMNs to traverse the 3D cross-linked type 1 rat-tail collagen gels more efficiently. This may be mediated by the membrane-bound MMP-8 molecule in PMNs, which is TIMP-resistant and has been shown to be responsible for 90% of the pericellular collagenase activity in vitro [6]. In contrast to PMNs, fibroblasts, which secrete but do not have membrane-bound MMP-8 [7], use membrane-bound MT1-MMP to invade collagen matrices [30]. Other cell types, such as tumor cells, have been shown to use cell membrane-associated MMPs to invade collagen matrices mediated by integrins and adhesion molecules [31, 32]. Furthermore, tumor cells may be able to switch between the proteolytic type of migration and amoeboid movements [33]. It is possible that PMNs, which also express β-1 integrins, use both mechanisms, using the proteolytic mode when they migrate through dense matrices and the nonproteolytic amoeboid mode when they migrate through collagen poor matrices. We have seen a 2.2- to 2.4-fold less migration of PMNs when treated with the MMP inhibitor in in vitro collagen gels (Fig. 4). In fact, concentration of collagen is several-fold higher in vivo than the collagen used in in vitro studies, making it likely that PMNs use proteolysis for migration in collagen-rich matrices [31].
To confirm that cleavage of collagen was important for MMP-8-mediated PMN migration, we made collagenase-resistant collagen gels by treating the collagen with EGCG, which is a green tea compound that renders collagen resistant to cleavage by collagenases, as it increases stability of the triple helical structure of collagen [24]. Indeed, PMNs were not able to migrate effectively through the collagenase-resistant collagen gels. The cleavage may also be important, as the recently described tri-peptide collagen fragments proline-glycine-proline, generated from collagen by MMP-8 and -9 and prolyl endopeptidase, have been shown to be elevated in OB and are chemotactic for PMNs [34, 35].
In addition to its importance in PMN invasion, MMP-8 appears to have important but complex effects on the content of PMNs in vivo. In the acute phase, we have observed fewer neutrophils in the MMP-8−/− mice, outside and inside of the lumen. As in our model, shortly after wounds [28], skin tumor injection [27], lethal hepatitis [36], and LPS [37], there were fewer invading neutrophils in MMP-8−/− mice as compared with WT mice. This might be a result of the capacity of MMP-8 to cleave the N-terminal region of the murine PMN chemokine LPS-inducible CXC chemokine, thus activating the chemokine [27, 37]. MMP-8 has also been shown to enhance the activity of the human CXCL8/IL-8 and CXCL5/epithelial-derived neutrophil-activating factor-78 [37, 38]. In a wound-healing study, there was delay in expression of keratinocyte-derived chemokine and MIP-2 in the MMP-8−/− wounds [28].
The overall increase of PMNs over time in our studies in the MMP-8−/− mice is similar to other studies such as wound healing [28], tumor progression [27], and LPS (S. D. Shapiro, unpublished). This may be a result of the processing and inactivation of chemokines by MMP-8, a well-known phenomena for MMPs, and by modulating apoptosis [1, 28, 38]. MMP-8 has been shown to play this anti-inflammatory role by processing CCL2 and generating truncated forms that are shown to function as inflammatory antagonists [38]. This may also explain the small increase of leukocytes in the bone marrow of the MMP-8−/− mice. However, the tracheal transplant model allows us to study the contribution of MMP-8 in trafficking PMNs through collagen, as we would not have seen the distribution pattern of a significantly fewer number of PMNs inside of the lumen and more outside of the lumen in the ECM if the mechanism were only through processing of chemokines. Also, the in vitro data support a role for MMP-8 for PMNs trafficking through collagen.
In addition to the known effects of MMP-8 on PMN chemokines [1, 27, 28, 36,37,38], in this study, we have shown that MMP-8 is not only responsible for the presence of PMNs in sites of inflammation and injury but also controls the ability of PMNs to penetrate collagen-rich barriers to reach its targets. The MMP-8 blockade also protected against luminal obliteration and collagen deposition inside of the lumen in OB. As OB is the major cause of mortality and morbidity post-lung transplantation as a result of obliteration of airway lumens, this study will provide insights into the MMP-8 blockade as a therapeutic target. The study will also advance our understanding of diseases, where PMNs have to traverse collagen-rich matrices, such as cystic fibrosis, chronic wound healing, and tumor progression. The inhibition of MMP-8 may need to be modulated to balance the positive effects versus potential negative effects that have been noted, such as impaired wound healing and tumor development and progression in response to carcinogens [27, 28].
AUTHORSHIP
S. D. S. has made significant contributions as a coauthor, and M. S. is the principal investigator.
ACKNOWLEDGMENTS
This work was supported by the NIH grant 1 KO8 HL084242-01 (M. S.), Harry Shwachman Cystic Fibrosis Clinical Investigator Award SUBRAM06Q0 (M. S.), NIH R01 HL54853, R01 (S. D. S.), and NIH HL082541 (S. D. S.). We thank Drs. Craig and Norma Gerard for their advice concerning the experiments with resistant collagen.
Footnotes
Abbreviations: 3D=three-dimensional, AEC=absolute eosinophil count, AMNC=absolute mononuclear cell count, ANC=absolute neutrophil count, APMA=4-aminophenylmercuric, BAL=bronchoalveolar lavage, ECM= extracellular matrix, EGCG=(–)-epigallocatechin-3-gallate, MMP-8=matrix metalloproteinase-8, MMP–/–=MMP-8-deficient, MT1=membrane type 1, NIH=National Institutes of Health, OB=obliterative bronchiolitis, PAF= platelet-activating factor, PMN=polymorphonuclear cell, TIMP-1=tissue inhibitor of metalloproteinase 1, TLC=total leukocyte count, WT=wild-type
References
- Parks W C, Shapiro S D. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W C, Wilson C L, Lopez-Boado Y S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Diekmann O, Tschesche H. Degradation of kinins, angiotensins and substance P by polymorphonuclear matrix metalloproteinases MMP 8 and MMP 9. Braz J Med Biol Res. 1994;27:1865–1876. [PubMed] [Google Scholar]
- Prikk K, Maisi P, Pirila E, Sepper R, Salo T, Wahlgren J, Sorsa T. In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. J Pathol. 2001;194:232–238. doi: 10.1002/path.849. [DOI] [PubMed] [Google Scholar]
- Prikk K, Maisi P, Pirila E, Reintam M A, Salo T, Sorsa T, Sepper R. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab Invest. 2002;82:1535–1545. doi: 10.1097/01.lab.0000035023.53893.b6. [DOI] [PubMed] [Google Scholar]
- Owen C A, Hu Z, Lopez-Otin C, Shapiro S D. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004;172:7791–7803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Sorsa T, Konttinen Y T, Ding Y, Sutinen M, Visser H, van Hinsbergh V W, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-α and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- Herman M P, Sukhova G K, Libby P, Gerdes N, Tang N, Horton D B, Kilbride M, Breitbart R E, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- Naesse E P, Schreurs O, Helgeland K, Schenck K, Steinsvoll S. Matrix metalloproteinases and their inhibitors in gingival mast cells in persons with and without human immunodeficiency virus infection. J Periodontal Res. 2003;38:575–582. doi: 10.1034/j.1600-0765.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- Cole A A, Chubinskaya S, Schumacher B, Huch K, Szabo G, Yao J, Mikecz K, Hasty K A, Kuettner K E. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996;271:11023–11026. doi: 10.1074/jbc.271.18.11023. [DOI] [PubMed] [Google Scholar]
- Ratjen F, Hartog C M, Paul K, Wermelt J, Braun J. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase α. Thorax. 2002;57:930–934. doi: 10.1136/thorax.57.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki H, Fujimoto N, Iwata K, Knauper V, Okada Y, Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 8 (neutrophil collagenase) using monoclonal antibodies. Clin Chim Acta. 1996;244:129–143. doi: 10.1016/0009-8981(95)06197-5. [DOI] [PubMed] [Google Scholar]
- Allport J R, Lim Y C, Shipley J M, Senior R M, Shapiro S D, Matsuyoshi N, Vestweber D, Luscinskas F W. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukoc Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- Nwomeh B C, Liang H X, Cohen I K, Yager D R. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–195. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- McDyer J F. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio J A, Weigt S S, Fishbein M C, Lynch J P., III Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- Taghavi S, Krenn K, Jaksch P, Klepetko W, Aharinejad S. Broncho-alveolar lavage matrix metalloproteases as a sensitive measure of bronchiolitis obliterans. Am J Transplant. 2005;5:1548–1552. doi: 10.1111/j.1600-6143.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- Miura M, El-Sawy T, Fairchild R L. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-γ. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaudenaerde B M, Meyts I, Vos R, Geudens N, De Wever W, Verbeken E K, Van Raemdonck D E, Dupont L J, Verleden G M. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. Eur Respir J. 2008;32:832–843. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]
- Sato M, Liu M, Anraku M, Ogura T, D'Cruz G, Alman B A, Waddell T K, Kim E, Zhang L, Keshavjee S. Allograft airway fibrosis in the pulmonary milieu: a disorder of tissue remodeling. Am J Transplant. 2008;8:517–528. doi: 10.1111/j.1600-6143.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- Hertz M I, Jessurun J, King M B, Savik S K, Murray J J. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- Galardy R E, Cassabonne M E, Giese C, Gilbert J H, Lapierre F, Lopez H, Schaefer M E, Stack R, Sullivan M, Summers B. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- Goo H C, Hwang Y S, Choi Y R, Cho H N, Suh H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials. 2003;24:5099–5113. doi: 10.1016/s0142-9612(03)00431-9. [DOI] [PubMed] [Google Scholar]
- Neurohr C, Huppmann P, Samweber B, Leuschner S, Zimmermann G, Leuchte H, Baumgartner R, Hatz R, Frey L, Ueberfuhr P, Bittmann I, Behr J. Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28:468–474. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Hasty K A, Jeffrey J J, Hibbs M S, Welgus H G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987;262:10048–10052. [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Tester A M, Pendas A M, Pitiot A S, Astudillo A, Overall C M, Shapiro S D, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot A S, Astudillo A, Hirose K, Hirata M, Shapiro S D, Noel A, Werb Z, Krane S M, Lopez-Otin C, Puente X S. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21:2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F G, McKane B, Marshbank S, Patterson G A, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. J Heart Lung Transplant. 2005;24:S232–S238. doi: 10.1016/j.healun.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss S J. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Yamada K M. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Lindbom L, Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian U H, Deryugina E I, Strongin A Y, Brocker E B, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Jackson P L, Noerager B D, O'Reilly P J, McQuaid D B, Rowe S M, Clancy J P, Blalock J E. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison M T, Galin F S, Calderon C E, Djekic U V, Parker S B, Wille K M, Jackson P L, Oster R A, Young K R, Blalock J E, Gaggar A. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182:4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Wielockx B, Puimege L, Noel A, Lopez-Otin C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol. 2005;175:7642–7649. doi: 10.4049/jimmunol.175.11.7642. [DOI] [PubMed] [Google Scholar]
- Tester A M, Cox J H, Connor A R, Starr A E, Dean R A, Puente X S, Lopez-Otin C, Overall C M. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE. 2007;2:e312. doi: 10.1371/journal.pone.0000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]