Abstract
Satellite cells are skeletal muscle stem cells with a principal role in postnatal skeletal muscle regeneration. Satellite cells, like many tissue-specific adult stem cells, reside in a quiescent state in an instructive, anatomically defined niche. The satellite cell niche constitutes a distinct membrane-enclosed compartment within the muscle fiber, containing a diversity of biochemical and biophysical signals that influence satellite cell function. A major limitation to the study and clinical utility of satellite cells is that upon removal from the muscle fiber and plating in traditional plastic tissue culture platforms, their muscle stem cell properties are rapidly lost. Clearly, the maintenance of stem cell function is critically dependent on in vivo niche signals, highlighting the need to create novel in vitro microenvironments that allow for the maintenance and propagation of satellite cells while retaining their potential to function as muscle stem cells. Here, we discuss how emerging biomaterials technologies offer great promise for engineering in vitro microenvironments to meet these challenges. In engineered biomaterials, signaling molecules can be presented in a manner that more closely mimics cell-cell and cell-matrix interactions and matrices can be fabricated with diverse rigidities that approximate in vivo tissues. The development of in vitro microenvironments in which niche features can be systematically modulated will be instrumental not only to future insights into muscle stem cell biology and therapeutic approaches to muscle diseases and muscle wasting with aging, but also will provide a paradigm for the analysis of numerous adult tissue-specific stem cells.
Keywords: Skeletal muscle stem cell, self-renewal, hydrogel, tethered ligand, matrix stiffness
Satellite cells as skeletal muscle stem cells
Satellite cells play a critical role in postnatal skeletal muscle homeostasis, growth, repair and regeneration. They comprise ~2–7% of all muscle fiber-associated nuclei (Rudnicki et al., 2008). As first shown by electron microscopy more than four decades ago, satellite cells are mononucleated cells intimately juxtaposed between the sarcolemma of a muscle fiber and the basal lamina that surrounds that fiber (Mauro, 1961). This anatomical compartment serves as an instructive environment, or niche, in which satellite cells can be maintained in a quiescent state or be activated to divide and differentiate in response to extrinsic stimuli associated with muscle growth and repair.
Skeletal muscles are composed of bundles of contractile muscle fibers called myofibers that are large, differentiated, multinucleated cells. Myofibers are postmitotic and are formed by the fusion of committed myogenic progenitors. Under normal physiological conditions, skeletal muscle is characterized by infrequent cell turnover, with only ~1–2% of myofiber nuclei replaced each week (Schmalbruch and Lewis, 2000). Satellite cells within their niche are mitotically quiescent, but are subject to active regulation by extrinsic signals that maintain their lineage identity and function (Charge and Rudnicki, 2004; Dhawan and Rando, 2005). Quiescent satellite cells are defined by (i) the expression of the paired-box transcription factor, Pax7, which is essential for postnatal satellite cell maintenance and self-renewal (Kuang et al., 2006; Seale et al., 2000), and (ii) the absence of expression of the basic helix-loop-helix (bHLH) transcription factors MyoD and myogenin, which are critical to myogenic differentiation (Cornelison and Wold, 1997; Zammit et al., 2004). Remarkably, recent evidence shows that Pax7, though necessary for satellite cell function in young mice, is not essential in adulthood (Lepper et al., 2009). In response to muscle injury, damage, or degeneration, satellite cells become activated, proliferate, and give rise to a population of myogenic progenitor cells known as myoblasts. Myoblasts can undergo transient amplification and then further specialization within the myogenic lineage into mononucleated muscle cells (myocytes) that are capable of fusion with myofibers.
In limb muscles, activation of satellite cells and specialization of their differentiated progeny is associated with changes in expression of myogenic transcription factors. Satellite cell activation is characterized by upregulation of the myogenic regulatory factor Myf5 (Tajbakhsh et al., 1996), which is critical for myogenic progenitor proliferation and specialization (Gayraud-Morel et al., 2007; Tajbakhsh et al., 1997). In diaphragm muscle, Pax3, a different paired-box transcription factor, is constitutively expressed but not essential for satellite cell function (Montarras et al., 2005). In contrast, Pax3 is transiently upregulated during satellite cell activation in hindlimb muscles (Boutet et al., 2007; Conboy and Rando, 2002). Myoblasts retain Pax7 and Myf5 expression and induce the expression of MyoD, which is necessary for their differentiation into fusion-competent cells (Cornelison et al., 2000; Sabourin et al., 1999). Further commitment and fusion into myofibers is associated with a downregulation of Pax3/7 and Myf5 expression and induction of factors essential to myofiber function, including MyoD, myogenin and myosin heavy chain (MHC) (Knapp et al., 2006; Venuti et al., 1995). For further discussion of functional roles of these myogenic transcriptional regulators, readers are referred to other reviews (Charge and Rudnicki, 2004; Dhawan and Rando, 2005; Le Grand and Rudnicki, 2007; McKinnell et al., 2008; Rudnicki et al., 2008).
To satisfy the classic definition of a tissue-specific stem cell, a single skeletal muscle stem cell must possess the ability to differentiate and undergo self-renewal (i.e., generate at least one copy of itself following cell division). Accordingly, upon mitosis, at least one cell progeny of a muscle stem cell must retain the cell’s original stem cell potential. Several lines of experimental evidence demonstrate that satellite cells satisfy these requirements, providing a reservoir of skeletal muscle stem cells in adult mice. Satellite cells isolated either from preparations of enzymatically-digested muscle or as mononucleated single cells that migrate away from intact individual myofibers, are capable of differentiation into multinucleated myotubes in vitro and fusion with existing myofibers when injected in vivo (Cerletti et al., 2008; Collins et al., 2005; Cornelison et al., 2001; Fukada et al., 2004; Konigsberg et al., 1975; Kuang et al., 2007; Montarras et al., 2005; Sacco et al., 2008; Sherwood et al., 2004). Careful transplantation of single genetically-labeled myofibers, with their associated satellite cells, has been performed into either radiation-ablated or regeneration-limited recipient mice (Collins et al., 2005). Strikingly, not only were labeled donor cell-derived muscle fibers detected in recipient mouse muscles, but also labeled mononuclear cells located within the satellite cell niche (Collins et al., 2005). Importantly, cells derived from these single transplanted myofibers contributed to muscle regeneration after injury, providing evidence of self-renewal (Collins et al., 2005). These findings were corroborated and extended by transplantation of enriched populations of mononucleated muscle stem cells prospectively isolated by fluorescence-activated cell sorting (FACS) based on (i) immunoreactivity with combinations of satellite cell-associated surface markers, including α7-integrin (Sherwood et al., 2004; Sacco et al., 2008), β1-integrin (Cerletti et al., 2008; Kuang et al., 2007), CD34 (Beauchamp et al., 2000; Sacco et al., 2008), CXCR4 (Cerletti et al., 2008), syndecan-3/-4 (Cornelison et al., 2004; Tanaka et al., 2009), ABGC2 (Tanaka et al., 2009), and the antigen for the SM/C-2.6 monoclonal antibody (Fukada et al., 2004); (ii) expression of satellite cell-associated transgenic reporters such as Pax3-GFP (Montarras et al., 2005); or (iii) side-population Hoechst-efflux characteristics (Gussoni et al., 1999; Tanaka et al., 2009). These FACS-enriched cells are capable of contributing to robust muscle regeneration, showing that they retain muscle stem cell function following isolation and transplantation into recipient mice.
Recently, the first definitive demonstration that adult satellite cells fulfill the definition of a muscle stem cell was obtained by injecting single freshly isolated CD34+ α7-integrin+ FACS-sorted cells from transgenic GFP/luciferase mouse muscles into irradiated limbs of immunodeficient mice (Sacco et al., 2008). Progeny from these single transplanted cells not only fused into myofibers, but also generated more Pax7+ satellite cells that persisted in recipient muscles, thus satisfying the requirements that a stem cell be capable of both differentiation and self-renewal. Critical to the evaluation of muscle stem cell characteristics in these experiments was the injection of a single cell, as when more than one cell is injected, it is not possible to discern whether some cells differentiated and others self-renewed.
The single-cell studies described above were enabled by the use of in vivo bioluminescence imaging (BLI). Using BLI, the proliferative behavior of muscle stem cells could be monitored dynamically, providing insights into the time course and magnitude of stem cell contributions to muscle tissues in a manner not feasible using traditional retrospective histological analyses. This technology should prove useful in direct comparisons of muscle stem cells (i) isolated by different criteria, (ii) delivered to mice subjected to different injury paradigms, (iii) delivered to diverse mouse models of human muscle degenerative diseases, and (iv) maintained and/or expanded in different culture microenvironments.
Together, these results clearly demonstrate that cells endogenous to myofibers (satellite cells) can both contribute to muscle regeneration and have the self-renewal properties that define muscle stem cells. It should be noted that cells from other sources, including mesoangioblasts (Sampaolesi et al., 2006), hematopoietic progenitors (Camargo et al., 2003; Corbel et al., 2003; Doyonnas et al., 2004; Sacco et al., 2005), muscle-derived stem cells (Deasy et al., 2005), pericytes (Dellavalle et al., 2007), CD133+ stem cells (Benchaouir et al., 2007) and PW1+ muscle interstitial cells (Moresi et al., 2008), are capable of contributing to muscle regeneration. In some cases, these cells are also capable of accessing muscle tissues following dissemination through the circulation, which may be advantageous to cellular therapies for certain skeletal muscle diseases (Blau, 2008).
Satellite cell heterogeneity
Cells found in the muscle satellite cell niche are heterogeneous with respect to their expression of myogenic transcription factors, characteristic cell surface markers, and, importantly, their potential to differentiate and self-renew. Although a diverse number of surface markers have been identified that prospectively enrich for satellite cells, including α7-integrin, β1-integrin, CD34, CXCR4, M-cadherin, and syndecan-3/-4, these markers are not uniformly expressed on myofiber-associated satellite cells (Beauchamp et al., 2000), and, moreover, do not effectively distinguish between quiescent and activated satellite cells (Kuang and Rudnicki, 2008). Immunostaining and multiplexed RT-PCR analyses of single satellite cells isolated from myofibers or through enrichment by CD34+ α7-integrin+ FACS-sorting have shown that, whereas satellite cells homogeneously express Pax7, they heterogeneously express the commitment markers Pax3 and MyoD (Cornelison and Wold, 1997; Sacco et al., 2008). Similarly, two strains of Myf5-reporter transgenic mice, Myf5-nLacZ and Myf5-Cre/ROSA26-YFP, both contain subpopulations of Pax7+Myf5− (~10%) and Pax7+Myf5+ (~90%) satellite cells (Beauchamp et al., 2000; Kuang et al., 2007). Of these two satellite cell subpopulations, the lower-abundance Pax7+Myf5− cells have greatly enhanced muscle regeneration potential following transplantation into permissive recipient mice and exclusively retain self-renewal potential, compared to the higher-abundance Pax7+Myf5+ cells (Kuang et al., 2007). Likewise, single-cell transplantations of CD34+ α7-integrin+-enriched muscle stem cells demonstrate a relatively low frequency (~4%) of successful engraftment (Sacco et al., 2008). While this low engraftment efficiency is in part due to cell death as a result of the exacting technical challenges of this procedure, which entails enzymatic digestion, column purification, FACS, and syringe injection of a single cell into a solid tissue, this low frequency could nonetheless signify the existence of heterogeneity among muscle stem cells within this prospectively isolated cell population. Taken together, these observations suggest that the satellite cell compartment is comprised of a heterogeneous cell population containing both quiescent muscle stem cells and more committed myogenic progenitors, which may be related through a shared hierarchical lineage (Figure 1). Still, outstanding questions persist concerning the origin and significance of these heterogeneities. Do they arise from asymmetric division of satellite cells within their anatomical niches, due to an asymmetric distribution of niche membrane components? Do diverse niche microenvironments maintain distinct satellite cells with different molecular identities and function?
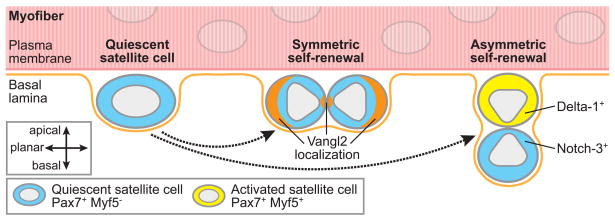
Figure 1. Modes of satellite cell self-renewal.
Symmetric stem cell self-renewal leads to two daughter cells retaining stem cell function, and thus an expansion of the stem cell pool, which is necessary for tissue regeneration following injury or disease. In comparison, asymmetric stem cell self-renewal yields one daughter stem cell and one differentiated cell and therefore is sufficient to maintain the stem cell pool under homeostatic conditions (Morrison and Kimble, 2006). While satellite cells could, in theory, arise from reversion of a differentiated cell back to a stem cell phenotype (Zammit et al., 2004), this schematic illustrates only the modes of quiescent satellite cell self-renewal supported by recent experimental findings using Cre-loxP-mediated cell lineage tracing (Le Grand et al., 2009; Kuang et al., 2007). In Myf5-Cre/ROSA26-YFP transgenic mice, cells that have expressed Myf5, a marker of satellite cell activation which is expressed concomitant with a substantial reduction in potential to contribute to muscle regeneration, express the fluorescent protein YFP. In myofibers isolated from these transgenic mice, quiescent Pax7+Myf5− satellite cells can give rise to either (i) two Pax7+Myf5− quiescent satellite cells (symmetric self-renewal); (ii) one Pax7+Myf5− quiescent satellite cell and one Pax7+Myf5+ activated satellite cell (asymmetric self-renewal); or (iii) two Pax7+Myf5+ activated satellite cells (commitment, not shown in diagram). Moreover, daughter cells from satellite cell divisions are orientated relative to the myofiber plasma membrane and basal lamina in a manner consistent with their commitment status. In the case of symmetric self-renewal, the two daughter quiescent satellite cells are in planar alignment with the myofiber and have been shown to localize components of the planar cell polarity pathway, including Vangl2, in a parallel pattern. In the case of asymmetric self-renewal, the quiescent daughter cell remains in contact with the basal lamina (basal position) and the activated daughter cell is adjacent to the myofiber plasma membrane (apical position), positioned for possible fusion with the myofiber. The apical activated satellite cells express the Notch ligand Delta-1 and basal quiescent satellite cells express the receptor Notch-3, suggesting that the more differentiated progeny activate Notch signaling in adjacent quiescent satellite cells that can contribute to maintenance of their quiescent state. Emerging evidence suggests that these modes of self-renewal are controlled by specific molecular components of the satellite cell niche (see Figure 2).
Modes of satellite cell self-renewal
Although the molecular regulation of satellite cell activation and differentiation has been extensively studied (Charge and Rudnicki, 2004), only recently have the mechanisms by which satellite cells maintain their quiescent muscle stem cell phenotype and self-renewal capacity begun to be elucidated. Stem cells can undergo proliferative self-renewal via two different mechanisms: (i) asymmetric self-renewal whereby each stem cell divides into one stem cell and one differentiated cell, resulting in a constant number of stem cells, or (ii) symmetric self-renewal, or expansion, whereby each stem cell gives rise to two daughter cells, each of which retains full stem cell potential. Although stem cell asymmetric self-renewal is sufficient under steady state conditions, stem cells must be capable of expanding symmetrically to increase in number and restore stem cell pools depleted by injury or disease (Morrison and Kimble, 2006). Here, we focus on several findings that suggest that satellite cells can self-renew through both asymmetric and symmetric divisions (Figure 1).
First, BrdU pulse-chase labeling experiments have demonstrated that a small subset of satellite cells can undertake asymmetric cosegregation of DNA strands during division, leading to one daughter cell retaining the original DNA template (and thus its complete epigenetic signature) and the other daughter cell inheriting the newly synthesized, BrdU-labeled DNA (Conboy et al., 2007; Shinin et al., 2006; Shinin et al., 2009). Critically, this asymmetric cosegregation appears to be associated with adoption by the daughter cells of divergent cell fates. Daughter cells that retain the original DNA template strands express Sca-1 and lack markers of myogenic differentiation. In contrast, daughter cells with the new DNA template strands express desmin but not Sca-1, indicating a more differentiated myogenic phenotype (Conboy et al., 2007). This model requires further investigation to clarify whether Sca-1 expression is truly indicative of a less differentiated myogenic progenitor. Conflicting reports have demonstrated that, in concert with other satellite cell marker selections, either positive or negative Sca-1 selection can enrich for cells with skeletal muscle stem cell properties (Cerletti et al., 2008; Fukada et al., 2004; Gussoni et al., 1999; Kuang et al., 2007; Sacco et al., 2008; Tanaka et al., 2009). Moreover, it has been reported that satellite cells only express Sca-1 upon activation and proliferation (Mitchell et al., 2005). While these findings argue that satellite cells can protect their genomic and epigenetic status through asymmetric division of “immortal” DNA strands (Cairns, 1975), their low frequency raises questions as to whether DNA cosegregation is a fundamental property of satellite cell self-renewal.
Second, satellite cell and myoblast divisions can result in the asymmetric distribution of the cytoplasmic protein Numb, a repressor of Notch signaling (Conboy and Rando, 2002; Shinin et al., 2006). Notch signaling plays a critical role in muscle regeneration through the regulation of satellite cell activation and inhibition of myogenic differentiation (Brack et al., 2008; Conboy and Rando, 2002; Conboy et al., 2003). Thus, asymmetric segregation of Numb suggests that daughter cells might adopt divergent fates, with cells that retain Numb become further differentiated and cells that lose Numb remain less differentiated due to the antagonistic effects of Numb on Notch signaling. Such divergent fates have been documented as (i) Numb+ daughter cells lack Pax3 and express desmin (a marker of myogenic differentiation), indicative of a committed myogenic progenitor, and (ii) Numb− daughter cells express Pax3 (a marker of some activated satellite cells), indicative of a less committed myogenic progenitor (Conboy and Rando, 2002). Conflicting with these interpretations is the observation that higher levels of Numb in daughter cells following asymmetric division are associated with retention of the original DNA template strands (Shinin et al., 2006), which argues that Numb+ progeny have characteristics of less, rather than more differentiated cells. Moreover, prospectively sorted Pax7+Myf5− quiescent satellite cells and Pax7+Myf5+ activated satellite cells express similar levels of Numb (Kuang et al., 2007). Thus, the mechanisms of asymmetric protein segregation and their roles in satellite cell self-renewal may be more complex and heterogeneous than initially suspected and clearly warrant further investigation.
Third, using Cre-loxP (Myf5-Cre/ROSA26-YFP)-mediated lineage tracing, it has been demonstrated that Pax7+Myf5− satellite cells within their myofiber niche can undergo both asymmetric and symmetric self-renewing divisions, dictated by the original cell’s mitotic spindle orientation within the myofiber (Kuang et al., 2007). In this report, asymmetric division of Pax7+Myf5− satellite cells yielded one self-renewed Pax7+Myf5− daughter cell that maintained muscle stem cell potential and one further committed Pax7+Myf5+ daughter cell with a greatly diminished stem cell phenotype. Intriguingly, these daughter cells are aligned in a manner perpendicular to the myofiber. The self-renewed Pax7+Myf5− cell remains in contact with the basal lamina and the more committed Pax7+Myf5+ cell loses contact with the basal lamina and is adjacent to the myofiber plasma membrane, in a position prone to subsequent fusion with the myofiber. Further, in these asymmetric divisions, apical Pax7+Myf5+ committed cells express higher levels of the Notch ligand Delta-1 whereas basal Pax7+Myf5− satellite cells express higher levels of the receptor Notch-3, suggesting that the more differentiated progeny activate Notch signaling in adjacent satellite cells in a juxtacrine manner to maintain their less differentiated state. More frequently observed, symmetric division of Pax7+Myf5− satellite cells can yield either two self-renewed Pax7+Myf5− cells or two committed Pax7+Myf5+ cells, and in both cases the identical daughter cells are in planar alignment with the myofiber plasma membrane and basal lamina (Kuang et al., 2007). Recent findings argue that this symmetric self-renewal is regulated by the niche factor Wnt7a through its activation of the non-canonical Wnt/Frizzled-associated planar cell polarity (PCP) pathway, which controls planar tissue morphogenesis in multiple organisms (Le Grand et al., 2009; Seifert and Mlodzik, 2007). Together, these observations strongly suggest that the mode of satellite cell division and the orientation of satellite cell progeny are governed by molecular signals present in or translocated to the satellite cell niche and their spatial arrangement within the niche (Kuang et al., 2008).
Finally, we note that an additional possible mechanism of muscle stem cell renewal is through cell reversion from a committed state back into a stem cell state. A dynamic clonal analysis of myofiber-derived satellite cells has demonstrated that Pax7+MyoD− satellite cells can become synchronously activated and divide as Pax7+MyoD+ myoblasts (Zammit et al., 2004). While most of these myoblasts then differentiate into Pax7−MyoD+myogenin+ cells, some cells appear to lose MyoD expression and revert back to a satellite cell-like Pax7+MyoD− state. This observation suggests that reversion of commitment of myogenic progenitors could provide a renewable source of muscle stem cells. Further investigation is required to ascertain whether a reversion mechanism definitively contributes to satellite cell renewal.
Microenvironmental components of the satellite cell niche
Tissue-specific adult stem cells reside within instructive, anatomically-defined microenvironments, or “niches”, consisting of unique combinations of cellular (stem and supporting cells), biochemical (soluble, from both local and systemic sources, and extracellular matrix factors), and biophysical (mechanical and structural) components. Initially proposed by Schofield in 1978 (Schofield, 1978), then described for Drosophila melanogaster and C. elegans germline cells (Fuller and Spradling, 2007; Kimble and Crittenden, 2007; Xie and Spradling, 2000), stem cell niches have since been identified in mammalian bone marrow, skin, intestine, brain, testis and skeletal muscle (reviewed in (Fuchs et al., 2004; Jones and Wagers, 2008; Moore and Lemischka, 2006)). In theory, niches dynamically regulate stem cell behavior to maintain a steady state level of slowly dividing stem cells during homeostasis and induce activation and self-renewal of stem cells in response to demands of injury. In practice, the nature of the niche and its components are not well understood in most cases and much remains to be elucidated.
In skeletal muscle, satellite cell niches are located in a compartment between the myofiber plasma membrane and the basal lamina that surrounds the myofiber (Collins et al., 2005; Kuang et al., 2008; Mauro, 1961). Thus, satellite cells are exposed to an asymmetric distribution of niche components, with myofiber signals on their apical surface and basal lamina signals on their basal surface (Figure 2). This “bipolar” arrangement of niche signals is shared by other tissue-specific stem cell niches and is thought to be critical for stem cell polarity and asymmetric self-renewal (Fuchs et al., 2004; Kuang et al., 2008). The myofiber basal lamina is a network of extracellular matrix components, composed of type IV collagen, laminin, fibronectin, entactin, and other proteoglycans and glycoproteins (Sanes, 2003). Accordingly, satellite cells express α7- and β1-integrins on their basal surfaces to enable engagement with laminin and other basal lamina components (Burkin and Kaufman, 1999). The proteoglycan components of this basal lamina can bind an array of secreted growth factors derived from systemic, satellite cell, or myofiber sources. These include basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and the diverse Wnt family of glycoprotein ligands (Brack et al., 2008; DiMario et al., 1989; Golding et al., 2007; Le Grand et al., 2009; Machida and Booth, 2004; Tatsumi et al., 1998). These factors stimulate satellite cell survival, activation, and/or proliferation. The binding soluble factors to proteoglycans in the extracellular matrix or on the satellite cell surface (e.g. syndecan-3/4) can provide a local repository of these signaling molecules, either sequestered in an inactive zymogen form or presented in an active signaling state (Cornelison et al., 2001; Jenniskens et al., 2006; Langsdorf et al., 2007; Olwin and Rapraeger, 1992; Tatsumi et al., 1998).
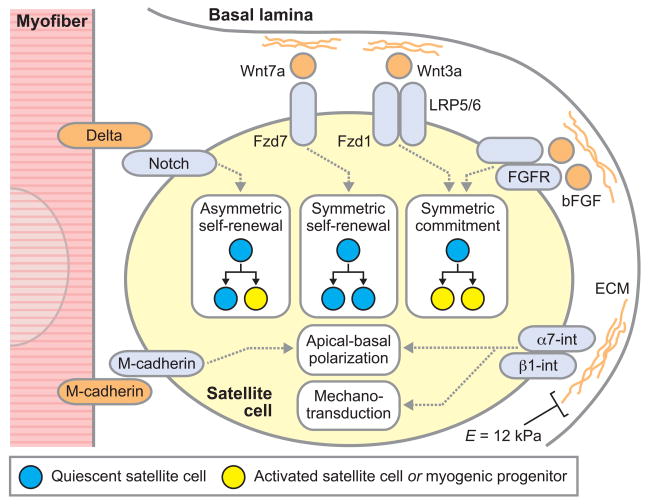
Figure 2. Niche components that regulate satellite cell fate and function.
Satellite cells reside in a niche between the myofiber plasma membrane and basal lamina consisting of a diversity of biochemical and biophysical signals that dictate their fate and function. This diagram illustrates some key niche components, their recognition by satellite cell receptors, and roles in regulating satellite cell self-renewal, proliferation, commitment, and polarity. Satellite cell niche components can be asymmetrically distributed, with factors associated with the myofiber plasma membrane presented to the apical surface of satellite cells and factors associated with the basal lamina presented to the basal surface of satellite cells. The myofiber basal lamina is a network of extracellular matrix components, including type IV collagen, laminin, fibronectin, and proteoglycans. These basal lamina ECM components facilitate satellite cell adhesion via integrin binding and activate integrin-related signaling to establish, in concert with myofiber-associated signals, apical-basal polarity in satellite cells and allow satellite cells to sense the mechanical stiffness of their microenvironment. (Resting healthy skeletal muscle has an elastic modulus (E) of ~12 kPa.) Whether apical-basal polarity or sensing of the microenvironmental mechanical stiffness provide critical determinants of satellite cell fate and function remain to be elucidated. Proteoglycan components of the ECM can bind an array of soluble glycoproteins, including bFGF and Wnt ligands, which are derived from systemic, satellite cell, or myofiber sources, localizing these factors to the satellite cell basal surface. Moreover, cell surface ligands, including the Delta family of ligands for the Notch receptor, are expressed by myofibers and satellite cells themselves and can stimulate satellite cells by engagement with their cognate receptors. Artificial satellite cell niches will allow a systematic analysis of the influences of these specific components on stem cell fate and function.
Similarly, myofibers generate numerous factors that, via secretion or presentation on the myofiber plasma membrane, influence satellite cell behavior. Myofibers secrete SDF-1, which binds the receptor CXCR4 present on satellite cells and activates a migratory response (Ratajczak et al., 2003; Sherwood et al., 2004). Myofibers express M-cadherin on their plasma membrane that facilitates myofiber-satellite cell adhesion and may play a role in their fusion (Irintchev et al., 1994). Additionally, satellite cells themselves express ligands that regulate their own cell fates through autocrine and juxtacrine signaling. These include the ligands for the Notch receptor family, which regulate satellite cell quiescence and self-renewal (Conboy and Rando, 2002; Conboy et al., 2003; Kuang et al., 2007). It should be noted that there are the multiple ligand and receptor members in Notch signaling, and their relative function and interactions remain to be clearly elucidated.
Satellite cells can be influenced by microenvironmental factors arising from sources outside of their immediate myofiber niche, including proximal and distal cells. Soluble factors such as the TGF-β family member myostatin (McCroskery et al., 2003), IGF-1 (Machida and Booth, 2004), and Wnt3a (Brack et al., 2007) arising from the systemic circulation and neighboring interstitial cells can diffuse through the myofiber basal lamina and exert extrinsic control on satellite cell function. The importance of these contributions to the satellite cell microenvironment is highlighted by the finding that a significant majority of satellite cell niches in mice are found in close proximity to both muscle microvasculature (Christov et al., 2007) and neuromuscular junctions (Kelly, 1978).
Biophysical properties of the satellite cell niche
Cells can sense and respond to extracellular biophysical cues through molecular mechanotransduction mechanisms such as integrin-based focal adhesion complex signaling (Geiger et al., 2009; Lopez et al., 2008). The elastic stiffness of the extracellular microenvironment, often assessed by a mechanical property called the elastic modulus (E), can strongly affect proliferation, differentiation, and/or morphogenesis of numerous cell types, including skeletal muscle cells (Guilak et al., 2009; Lopez et al., 2008). Resting healthy skeletal muscle tissue (with contribution from both cells and extracellular matrix) and cultured myotubes both possess a similar elastic stiffness (E = ~12 kPa) (Collinsworth et al., 2002; Engler et al., 2004). The biophysical properties of skeletal muscle are altered in aging, disease, and injury, as evidenced by the stiffer elastic modulus (>18 kPa) observed in muscle from the dystrophin-deficient mdx mouse model of Duchenne muscular dystrophy (Engler et al., 2004; Stedman et al., 1991) and in aged animals (Gao et al., 2008; Rosant et al., 2007). These changes in muscle biophysical properties are likely due to increased extracellular matrix deposition during injury- and regeneration-associated fibrosis regulated by infiltrating fibroblasts.
Recent findings demonstrate that these subtle changes in elastic stiffness can regulate activation, proliferation, and differentiation of satellite cells and myogenic progenitors in vitro. As assessed by myotube formation and striation, myoblasts of the established C2C12 cell line optimally differentiate on collagen-coated polyacrylamide (PA) gels with biophysical properties closely mimicking skeletal muscle elasticity (~12 kPa) and have greatly diminished differentiation on softer (<5 kPa) and stiffer (>20 kPa) PA gels (Engler et al., 2004). Similarly, the proliferation rate of primary myoblasts on PA gels cross-linked with entactin, type IV collagen, and laminin is optimal on ~21 kPa gels and reduced on either softer (~3 kPa) or stiffer (~80 kPa) substrates (Boonen et al., 2009). Furthermore, mesenchymal stem cells (MSCs) cultured on a collagen-coated PA gel substrate with muscle-like elastic modulus (~11 kPa) differentiate into cells with myogenic characteristics, including myotube morphology and expression of Pax7 and myogenin, whereas MSCs cultured on softer (~1 kPa) or stiffer (~35 kPa) gels differentiate into cells with neurogenic and osteogenic characteristics, respectively (Engler et al., 2006). Importantly, these studies have focused on the role of elastic stiffness in modulating myoblast behavior, but not stem cell or progenitor behavior on two-dimensional substrata in vitro. These recent findings highlight the significant influence of biophysical stimuli on cell function, which has largely been neglected in the plastic tissue culture modalities routinely employed.
Satellite cell niches in aging
During aging, the regenerative capacity of skeletal muscle declines (Grounds, 1998). Although this decline is associated with a diminished number of satellite cells in aged muscle (Bockhold et al., 1998), muscle stem cell function does not appear to be impaired. Instead, changes in the microenvironment impede satellite cell behavior. In heterochronic parabiotic pairings (in which young and aged mice shared a common circulatory system), satellite cell-driven muscle regeneration in aged mice was rejuvenated by exposure to the systemic circulation of young mice (Conboy et al., 2005) and, conversely, muscle regeneration in young mice was attenuated upon exposure to an aged circulation (Brack et al., 2007). These remarkable observations indicate that aged satellite cells are not intrinsically defective in their regenerative potential but instead factors extrinsic to satellite cells inhibit muscle regeneration in aged mice. Further experiments have elucidated that the regenerative defect in aged mice stems from decreased levels of the Notch ligand Delta-1 expressed on the myofiber plasma membrane (Conboy et al., 2003) and increased levels of circulating Wnt3a (Brack et al., 2007). Circulating Wnt3a stimulates β-catenin signaling in satellite cells and their myogenic progeny, leading (i) to reduced muscle regeneration by antagonizing Notch-induced satellite cell proliferation and (ii) to increased fibrosis by stimulating premature myogenic differentiation (Brack et al., 2007; Brack et al., 2008). Accordingly, ectopic activation of Notch signaling and inhibition of Wnt3a–β-catenin signaling restore the satellite cell-driven muscle regeneration in aged mice (Brack et al., 2007; Brack et al., 2008; Conboy et al., 2003). Aging has also been associated with changes in the myofiber basal lamina. For example, in aged myofibers, increased ECM deposition and a change in its composition (e.g. increased collagen) are thought to negatively affect satellite cell-myofiber interactions due to increased satellite cell adhesion to the basal lamina within the niche (Snow, 1977). Moreover, these ECM changes decrease ability of the basal lamina to serve as a reservoir for ECM-bound growth factors (Alexakis et al., 2007). These results demonstrate that the microenvironmental composition of the satellite cell niche is altered with aging, which has significant consequences for satellite cell function.
State of the art: current myogenic culture approaches
The muscle stem cell potential of satellite cells has been limited to direct transplantation of myofiber-attached satellite cells or freshly isolated cells that have never been exposed to tissue culture. If maintained in vitro, satellite cells immediately begin to specialize as myoblasts and their potential for self-renewal and contribution to myofibers in muscle tissue diminishes rapidly (Beauchamp et al., 1999; Cao et al., 2005; Fan et al., 1996; Montarras et al., 2005; Rando and Blau, 1994; Relaix et al., 2005; Sacco et al., 2008), demonstrating that the muscle stem cell phenotype of satellite cells is lost soon after removal from their in vivo niche, a feature shared by other adult stem cells such as hematopoietic stem cells (Dykstra et al., 2006; Zhang et al., 2003). These findings explain the disappointing results of clinical trials in which myoblasts derived from extensive propagation in standard tissue culture conditions were transplanted into Duchenne muscular dystrophy patients (reviewed in (Blau, 2008)). While the intrinsic differentiation responses of satellite cells upon culture have provided a useful paradigm to examine the specialization and proliferation of satellite cell-derived myogenic progenitors (myoblasts), there remains a critical need, motivated by both biological and therapeutic concerns, to be able to robustly maintain and propagate satellite in culture. The rapid loss of satellite cell phenotype following removal from the endogenous niche argues that extrinsic signals from the diverse microenvironmental components of the niche are necessary for maintaining skeletal muscle stem cell potential (Kuang et al., 2008). Thus, the development of cell culture technologies that successfully mimic key properties of the complex regulatory microenvironment of the in vivo satellite cell niche is of critical importance.
State of the art: current myogenic culture approaches
Methods using collagen-coated tissue culture plastic are well-established for robustly generating primary myoblast cultures from enzymatically-digested and adhesion-purified skeletal muscle cells (Rando and Blau, 1994). When maintained in appropriate media and at low confluency, myoblasts can be expanded following a crisis period during which most cells die, leading to the preferential selection of highly proliferative cells. Using specific serum compositions, differentiation can be prevented. Although experiments with primary myoblasts can provide some insights into satellite cell fate and function in vitro in response to diverse stimuli, these cells are unable to contribute to muscle regeneration in a self-renewing manner and thus are not muscle stem cells (Montarras et al., 2005; Sacco et al., 2008).
On Matrigel substrata or if maintained in suspension, quiescent satellite cells in freshly isolated single myofibers are activated immediately and can divide within ~48 hr of culture. These cells then migrate away from the niche, cross the myofiber basal lamina, and either differentiate or further proliferate as myoblasts (Kuang et al., 2006; Shefer and Yablonka-Reuveni, 2005). By focusing on the period preceding migration from the myofiber niche, these myofiber cultures have been useful for dissecting aspects of satellite cell self-renewal (Kuang et al., 2007; Le Grand et al., 2009). However, the myofiber culture model is an unsatisfactory satellite cell culture system due to technical limits such as inability to access and perturb cell niche microenvironmental components, short culture duration, and low-throughput (single myofiber isolation is highly labor-intensive). To date, novel bioengineering approaches for controlling the maintenance, proliferation, and differentiation of myogenic progenitors in vitro have almost exclusively used primary myoblasts and are often focused on the generation of functional tissue-engineered skeletal muscle (Eberli et al., 2009; Levenberg et al., 2005; Yan et al., 2007). Culture platforms for modeling satellite cell biology are lacking and their development would greatly advance the muscle stem cell field.
Emerging technologies for engineering in vitro satellite cell niches
The development and definitive evaluation of in vitro approaches for the prolonged maintenance of satellite cells by components of the in vivo satellite cell niche microenvironment will require a fusion of emerging technologies. Recent advances in prospective satellite cell isolation (Cerletti et al., 2008; Sherwood et al., 2004; Sacco et al., 2008; Tanaka et al., 2009) and assessment of muscle stem cell function (including engraftment, regeneration, and self-renewal) through in vivo bioluminescence imaging (Sacco et al., 2008) provide essential tools for these purposes. Further development of engineered cell culture models capable of spatial and temporal control of biochemical and biophysical niche components and novel methods for clonal analysis of satellite cell behavior will be necessary. Promisingly, numerous biomaterial systems, including 2D and 3D hydrogels with tunable elastic moduli and controlled ligand patterning and release, have been recently developed to mimic in vitro stem cell niches and study microenvironmental regulation of stem cell proliferation and fate (Discher et al., 2009; Flaim et al., 2008; Matthias P. Lutolf and Helen M. Blau, 2009; Saha et al., 2007; Sands and Mooney, 2007).
Of particular interest for the in vitro maintenance of satellite cells are the widely-used poly(ethylene glycol) (PEG)-based hydrogels, which have both tunable matrix stiffness and controlled ligand tethering for inducing receptor activation and cell adhesion (Lutolf and Hubbell, 2005). By adjusting synthesis chemistry, PEG hydrogels can attain a wide range of elastic moduli, including the ~10–15 kPa stiffness range of healthy resting muscle that is also optimal for myogenic differentiation in vitro (Boonen et al., 2009; Collinsworth et al., 2002; Engler et al., 2004). Further, a novel PEG hydrogel chemistry has been recently developed to enable photodegradable control of matrix stiffness without toxicity to cells (Kloxin et al., 2009). This ability to temporally alter biophysical properties in a noninvasive manner could be useful for recapitulating the progression of biophysical changes associated with muscle fibrosis or disease (Engler et al., 2004; Stedman et al., 1991) in hydrogel-based satellite cell niches.
PEG hydrogels are also advantageous because they are largely resistant to nonspecific cell adhesion mediated by protein adsorption and allow for experimentally-determined presentation of ligands, through either covalent or non-covalent (e.g. streptavidin-biotin) binding chemistries, to facilitate cell adhesion through specific protein modifications. In addition, this property enables presentation of growth factors in a physiologically relevant “tethered” manner. In these systems, cells are not exposed to soluble growth factors in the medium, which is currently that cell culture norm. Instead, tethering is akin to the physiological sequestration of growth factors within a satellite cell niche’s myofiber plasma membrane and basal lamina (Langsdorf et al., 2007). Surface tethering of growth factor ligands has been reported to provide physical restraints on receptor-ligand internalization and the consequent advantageous properties of sustained receptor surface residency and activation and prolonged maintenance of ligand concentrations (Alberti et al., 2008; Fan et al., 2007; Lutolf et al., 2009; Platt et al., 2009). Tethering can be spatially controlled on the nanoscale to facilitate ligand clustering for increased receptor activation (Irvine et al., 2002). Further, we have recently demonstrated that PEG hydrogels containing “microwell” arrays that are highly suitable for single-stem cell clonal assays, can be formed using conventional PDMS stamping methods (Lutolf et al., 2009). The ability to control microscale features, biophysical properties, ligand presentation modes, and nanoscale patterning confer PEG-based hydrogels with advantageous characteristics for their implementation as engineered in vitro satellite cell niches. Whereas significant challenges remain in engineering PEG hydrogels with asymmetric distributions of biochemical and biophysical components in order to mimic three-dimensional (3D) “bipolar” in vivo satellite cell niches, much can be gleaned from 2D systems. Indeed, a systematic analysis of the role of specific niche components in tethered and soluble form in conjunction with modulation of the biophysical properties such as tissue rigidity is now possible, providing an extremely potent means of rigorously elucidating regulators of muscle stem cell function.
Applications of engineered in vitro satellite cell niches
Many questions regarding satellite cell biology could be addressed using engineered in vitro satellite cell niches. Of great interest is an elucidation of the components of the in vivo satellite cell niche microenvironment that are necessary for maintenance of satellite cell quiescence and stem cell potential over prolonged (one week or more) culture. The in vivo satellite cell niche consists of numerous soluble and extracellular matrix factors and biophysical stimuli (Kuang et al., 2008) that could have a significant degree of interdependency in regulating satellite cells. A molecularly defined in vitro system would be extremely useful in parsing the relative contributions of these microenvironmental features and mimicking specific aspects of cell-cell and cell-ECM interactions without the complexity of co-culturing disparate cell types. The satellite cell fates within these engineered niches could be evaluated through (i) dynamic imaging of muscle stem cell differentiation status using transgenic reporters as readouts, and/or (ii) single-cell analyses in conjunction with time-lapse microscopy. Such real-time studies would greatly extend current retrospective analyses requiring immunostaining for quiescent satellite cell markers.
A second critical application of in vitro satellite cell niches will be to ascertain mechanisms of asymmetric and symmetric satellite cell self-renewal. While emerging evidence indicates that asymmetric satellite cell self-renewal is stimulated by Notch and symmetric self-renewal is stimulated by Wnt7a–planar cell polarity pathway signaling, it is currently unclear what influence other prevalent niche features, such as ECM ligands (e.g. laminin, collagen), matrix stiffness, and other growth factors associated with satellite cell proliferation but not necessarily self-renewal (e.g. bFGF, Wnt3a), have in governing these critical satellite cell division mechanisms. In bioengineered niches, the satellite cell signaling mechanisms stimulated by soluble and tethered growth factor ligands could be compared and dissected in a manner not possible using standard culture platforms.
Due to the polarized nature of satellite cells divisions (asymmetric divisions are largely apical-basal and symmetric divisions are planar) within their in vivo niche, the development of an in vitro 3D “bipolar” niche model may be required. Such a 3D niche model could capture the unique molecular aspects of the myofiber plasma membrane and basal lamina on distinct hydrogel surfaces allowing questions on how niche asymmetry leads to satellite cell polarization to be addressed. Moreover, these experiments could lead to insights on whether heterogeneity in asymmetric and symmetric divisions are due primarily to variations in specific niche factors or are largely cell-autonomous in origin. A clinical goal of such studies would be robust expansion of satellite cells in vitro through directed symmetric self-renewal for experimental and therapeutic purposes.
Lastly, engineered in vitro satellite cell niches will be useful for modeling changes in satellite cell function during aging and muscle disease. For example, in aging, satellite cell niches have an altered balance of Notch and Wnt3a signaling that negatively affects muscle regeneration (Brack et al., 2007; Conboy et al., 2003), but it is likely that additional changes in satellite cell niches arise as a result of aging and disease, including changes in ECM deposition and stiffness. An in vitro satellite cell niche could explore the combined regulation of satellite cell function with changes in these multiple niche components. Finally, these studies could eventually lead to ex vivo expansion of muscle stem cells for cell-based therapies and perturbation of in vivo niches to stimulate endogenous stem cell function for therapeutic purposes.
Conclusions
In recent years, significant progress has been made in understanding how satellite cells can act as tissue-specific adult stem cells in skeletal muscle. Advances in the prospective isolation of satellite cells and the demonstration of their muscle stem cell properties following in vivo transplantation by novel approaches such as non-invasive bioluminescence imaging have provided critical tools for assessing satellite cell function. Of critical importance to the regulation of satellite cell functions in skeletal muscle homeostasis, regeneration, and aging are the diversity of instructive biochemical and biophysical signals present in its myofiber membrane-associated niche microenvironment. Yet, to date, studies of the molecular nature of these niche signals have relied on in vivo models and short-term cultures of isolated myofibers, as current approaches for the in vitro maintenance and characterization of the muscle stem cell functions of satellite cells are lacking. Application of emerging biomaterials technologies offers significant promise for the development of strategies to mimic biochemical and biophysical features of the in vivo niche microenvironment. Notably, engineered biomaterials allow for (i) the inclusion of niche signaling molecules in a manner resembling their in vivo presentation by cells and ECM, and (ii) fabrication of matrix substrates with variable rigidities that can replicate healthy, aged, and diseased skeletal muscle tissues. Development of in vitro satellite cell niche models should provide novel insights into the molecular cues critical for the in vitro maintenance and expansion of muscle stem cells. This will be instrumental not only to advances in satellite cell biology and therapeutic approaches to muscle diseases and aging-related muscle wasting, but also to generate a general paradigm for the study of adult tissue-specific stem cells.
Acknowledgments
We thank Karen Havenstrite, John Ramunas, Adrien de Tonnac, and Nora Leonardi for helpful discussions. B.D.C. and P.M.G. are supported by NIH postdoctoral training grants 5R25CA118681 and CA09151, respectively. A.S. is supported by MDA grant 4063. H.M.B. is supported by NIH grants AG024987 and AG009521, MDA grant 4320, California Institute of Regenerative Medicine grant RT1-01001-1, and the Baxter Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, Bornhauser M, Pompe T, Nagy A, Werner C, Zandstra PW. Functional immobilization of signaling proteins enables control of stem cell fate. Nat Methods. 2008;5:645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchaouir R, Meregalli M, Farini A, D’Antona G, Belicchi M, Goyenvalle A, Battistelli M, Bresolin N, Bottinelli R, Garcia L, Torrente Y. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;1:646–657. doi: 10.1016/j.stem.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Blau HM. Cell therapies for muscular dystrophy. N Engl J Med. 2008;359:1403–1405. doi: 10.1056/NEJMcibr0805708. [DOI] [PubMed] [Google Scholar]
- Bockhold KJ, Rosenblatt JD, Partridge TA. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve. 1998;21:173–183. doi: 10.1002/(sici)1097-4598(199802)21:2<173::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:C1338–1345. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Cao B, Deasy BM, Pollett J, Huard J. Cell therapy for muscle regeneration and repair. Phys Med Rehabil Clin N Am. 2005;16:889–907. viii. doi: 10.1016/j.pmr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Collinsworth AM, Zhang S, Kraus WE, Truskey GA. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol. 2002;283:C1219–1227. doi: 10.1152/ajpcell.00502.2001. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, Blau HM, Rossi FM. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9:1528–1532. doi: 10.1038/nm959. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol. 2000;224:122–137. doi: 10.1006/dbio.2000.9682. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 2004;18:2231–2236. doi: 10.1101/gad.1214204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16:3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989;244:688–690. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyonnas R, LaBarge MA, Sacco A, Charlton C, Blau HM. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci U S A. 2004;101:13507–13512. doi: 10.1073/pnas.0405361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B, Ramunas J, Kent D, McCaffrey L, Szumsky E, Kelly L, Farn K, Blaylock A, Eaves C, Jervis E. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc Natl Acad Sci U S A. 2006;103:8185–8190. doi: 10.1073/pnas.0602548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberli D, Soker S, Atala A, Yoo JJ. Optimization of human skeletal muscle precursor cell culture and myofiber formation in vitro. Methods. 2009;47:98–103. doi: 10.1016/j.ymeth.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol. 2007;312:13–28. doi: 10.1016/j.ydbio.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Golding JP, Calderbank E, Partridge TA, Beauchamp JR. Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp Cell Res. 2007;313:341–356. doi: 10.1016/j.yexcr.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Hue KA, Mayes AM, Griffith LG. Simulations of cell-surface integrin binding to nanoscale-clustered adhesion ligands. Biophysical journal. 2002;82:120–132. doi: 10.1016/S0006-3495(02)75379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- Jenniskens GJ, Veerkamp JH, van Kuppevelt TH. Heparan sulfates in skeletal muscle development and physiology. J Cell Physiol. 2006;206:283–294. doi: 10.1002/jcp.20450. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kelly AM. Perisynaptic satellite cells in the developing and mature rat soleus muscle. Anat Rec. 1978;190:891–903. doi: 10.1002/ar.1091900409. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp JR, Davie JK, Myer A, Meadows E, Olson EN, Klein WH. Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development. 2006;133:601–610. doi: 10.1242/dev.02249. [DOI] [PubMed] [Google Scholar]
- Konigsberg UR, Lipton BH, Konigsberg IR. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol. 1975;45:260–275. doi: 10.1016/0012-1606(75)90065-2. [DOI] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Langsdorf A, Do AT, Kusche-Gullberg M, Emerson CP, Jr, Ai X. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev Biol. 2007;311:464–477. doi: 10.1016/j.ydbio.2007.08.053. [DOI] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Lopez JI, Mouw JK, Weaver VM. Biomechanical regulation of cell orientation and fate. Oncogene. 2008;27:6981–6993. doi: 10.1038/onc.2008.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. Perturbation of single hematopoietic stem cell fates in artificial niches. Integrative Biology. 2009;1:59–69. doi: 10.1039/b815718a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21:1–14. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PO, Mills T, O’Connor RS, Kline ER, Graubert T, Dzierzak E, Pavlath GK. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol. 2005;283:240–252. doi: 10.1016/j.ydbio.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Moresi V, Pristera A, Scicchitano BM, Molinaro M, Teodori L, Sassoon D, Adamo S, Coletti D. Tumor necrosis factor-alpha inhibition of skeletal muscle regeneration is mediated by a caspase-dependent stem cell response. Stem Cells. 2008;26:997–1008. doi: 10.1634/stemcells.2007-0493. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1992;118:631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt MO, Roman AJ, Wells A, Lauffenburger DA, Griffith LG. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi-potent marrow stromal cells. J Cell Physiol. 2009;221:306–317. doi: 10.1002/jcp.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rosant C, Nagel MD, Perot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol. 2007;42:301–308. doi: 10.1016/j.exger.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol. 2008;73:323–331. doi: 10.1101/sqb.2008.73.064. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J Cell Biol. 1999;144:631–643. doi: 10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, LaBarge MA, Hammer MM, Kraft P, Blau HM. IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J Cell Biol. 2005;171:483–492. doi: 10.1083/jcb.200506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotechnol. 2007;18:448–453. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Tajbakhsh S. Template DNA-strand co-segregation and asymmetric cell division in skeletal muscle stem cells. Methods Mol Biol. 2009;482:295–317. doi: 10.1007/978-1-59745-060-7_19. [DOI] [PubMed] [Google Scholar]
- Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 1977;185:399–408. doi: 10.1007/BF00220299. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Bober E, Babinet C, Pournin S, Arnold H, Buckingham M. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev Dyn. 1996;206:291–300. doi: 10.1002/(SICI)1097-0177(199607)206:3<291::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol. 1995;128:563–576. doi: 10.1083/jcb.128.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yan W, George S, Fotadar U, Tyhovych N, Kamer A, Yost MJ, Price RL, Haggart CR, Holmes JW, Terracio L. Tissue engineering of skeletal muscle. Tissue Eng. 2007;13:2781–2790. doi: 10.1089/ten.2006.0408. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]