Abstract
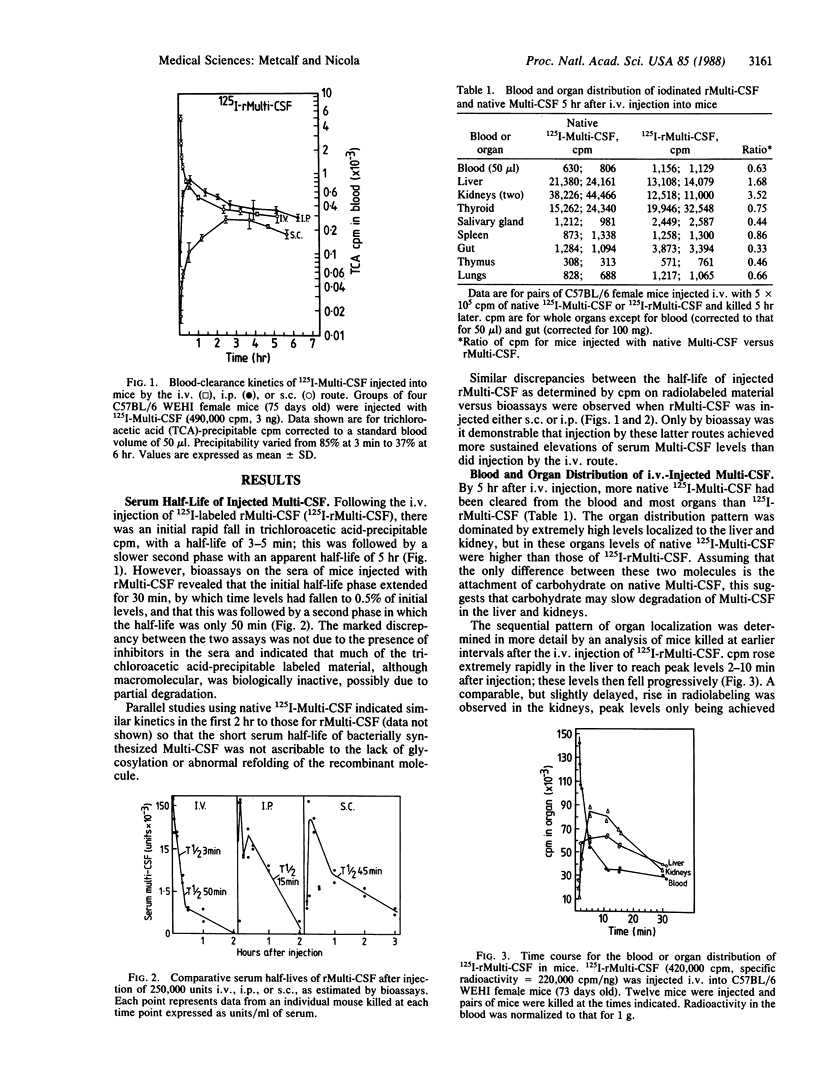
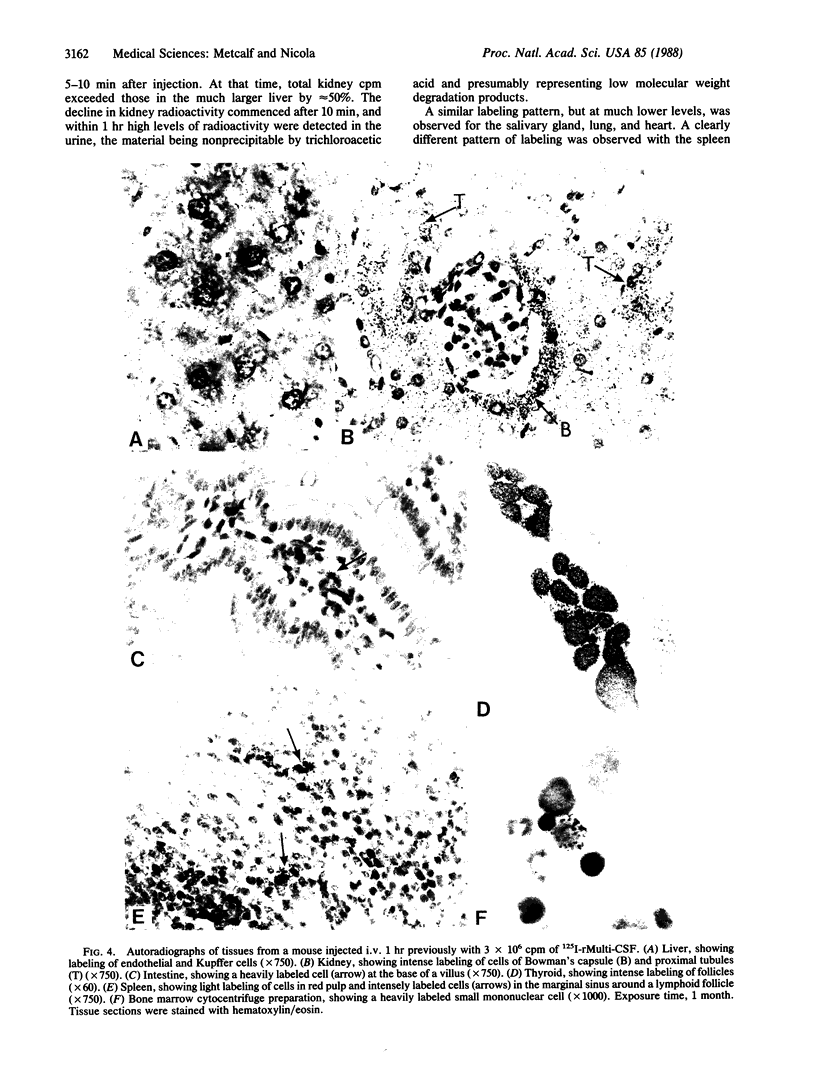
The hemopoietic regulator multipotential colony-stimulating factor [Multi-CSF (interleukin 3)] has proliferative effects on a wide range of hemopoietic cells in vitro and in vivo. Native or recombinant Multi-CSF injected intravenously into adult mice had an initial half-life of 3-5 min and a second phase of 50 min. Clear labeling of hemopoietic cells was observed in the bone marrow and spleen of mice injected intravenously with recombinant 125I-labeled Multi-CSF showing that injected Multi-CSF can obtain access to such cells in situ. A high proportion of injected 125I-labeled Multi-CSF of both types became localized in the liver and in the kidney (in cells of the Bowman's capsule and proximal renal tubules). The kidney appeared to be an active site of degradation of Multi-CSF with the early appearance of low molecular weight labeled material in the urine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever M. A., Thompson J. A., Kern D. E., Greenberg P. D. Interleukin 2 (IL 2) administered in vivo: influence of IL 2 route and timing on T cell growth. J Immunol. 1985 Jun;134(6):3895–3900. [PubMed] [Google Scholar]
- Crapper R. M., Clark-Lewis I., Schrader J. W. The in vivo functions and properties of persisting cell-stimulating factor. Immunology. 1984 Sep;53(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Cutler R. L., Metcalf D., Nicola N. A., Johnson G. R. Purification of a multipotential colony-stimulating factor from pokeweed mitogen-stimulated mouse spleen cell conditioned medium. J Biol Chem. 1985 Jun 10;260(11):6579–6587. [PubMed] [Google Scholar]
- Fung M. C., Hapel A. J., Ymer S., Cohen D. R., Johnson R. M., Campbell H. D., Young I. G. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984 Jan 19;307(5948):233–237. doi: 10.1038/307233a0. [DOI] [PubMed] [Google Scholar]
- Garland J. M., Aldridge A., Wagstaffe J., Dexter T. M. Studies on the in vivo production of a lymphokine activity, interleukin 3 (IL-3) elaborated by lymphocytes and a myeloid leukaemic line in vitro and the fate of IL-3 dependent cell lines. Br J Cancer. 1983 Aug;48(2):247–259. doi: 10.1038/bjc.1983.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Humphries R. K., Messner H., Reid D. M., Sakakeeny M. A. Molecularly cloned and expressed murine T-cell gene product is biologically similar to interleukin-3. Exp Hematol. 1985 May;13(4):249–260. [PubMed] [Google Scholar]
- Hapel A. J., Fung M. C., Johnson R. M., Young I. G., Johnson G., Metcalf D. Biologic properties of molecularly cloned and expressed murine interleukin-3. Blood. 1985 Jun;65(6):1453–1459. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Nicola N. A., Johnson G. R. Quantitative responsiveness of murine hemopoietic populations in vitro and in vivo to recombinant multi-CSF (IL-3). Exp Hematol. 1987 Mar;15(3):288–295. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of iodinated multipotential colony-stimulating factor (interleukin-3) to murine bone marrow cells. J Cell Physiol. 1986 Aug;128(2):180–188. doi: 10.1002/jcp.1041280207. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Porcellini A., Rizzoli V., Pigoli G. Physiologic distribution of colony-stimulating factor in vivo. Blood. 1979 Oct;54(4):894–905. [PubMed] [Google Scholar]




