Abstract
Bimatoprost is the only representative of a novel class of prostaglandin ethanolamide (prostamide) compounds used therapeutically as an efficacious treatment for glaucoma. The pathways through which bimatoprost works to improve uveoscleral outflow to relieve elevated intraocular pressure are similar to those of the conventional prostaglandins used in glaucoma therapy, with some evidence of a preferential action at the trabecular meshwork. The pharmacology of bimatoprost is however, unclear. Pharmacological evidence supports a specific and distinct receptor-mediated agonist activity of bimatoprost at ‘prostamide’ receptors, which is selective to the prostamides as a class. However, other studies have reported either activity of bimatoprost at additional prostanoid and nonprostanoid receptors, or a conversion of bimatoprost to metabolites with agonist activity at prostaglandin FP receptors in the human eye. The formation of endogenous prostamides has been demonstrated in vivo, by a novel pathway involving the cyclooxygenase-2-mediated conversion of endogenous cannabinoid (endocannabinoid) substrates. Irrespective of the pharmacology of bimatoprost and the prostamides in general, further studies are needed to determine the biological role and biochemical pathology of prostamides in the human eye, particularly in glaucoma. Such studies may improve our understanding of uveoscleral flow and may offer new treatments for controlling intraocular pressure.
Keywords: bimatoprost, endocannabinoid, glaucoma, prostamides, trabecular meshwork
Glaucoma is a chronic disease of the optic nerve, caused at least in part by a sustained, elevated intraocular pressure. Mechanisms of optic deterioration include direct axonal damage, structural failure, and altered microvascular supply.1 Intraocular pressure is normally maintained at a steady state and in health eyes assumes a relatively narrow range. The intraocular pressure in any given eye is determined by the rate of fluid (aqueous) production within the eye by the ciliary body and the drainage of aqueous humor from the eye through the trabecular meshwork, aqueous flow, and uveoscleral outflow. In glaucomatous eyes, the increased resistance to aqueous humor outflow is due in part to an increase in extracellular matrix deposited in the trabecular meshwork, but also in other outflow structures such as Schlemm’s canal,2 with the amount of extracellular matrix correlated with the loss of axons in the optic nerve.3 One study found that inflammatory genes were upregulated in trabecular meshwork from primary open-angle glaucomatous eyes under conditions of explant culture, compared to nonglaucomatous eyes.4 However, many genes in the trabecular meshwork underwent altered expression under culture conditions, so the pathological relevance of these changes is unclear. While a host of other cellular and interstitial changes also occur in the outflow facility during glaucoma that lead to the loss of normal drainage via this tissue,5 the pathogenic factors underlying glaucoma are still uncertain. This is partly a consequence of the complex physiological determinants of resistance within the outflow facility.6 In addition, there are difficulties in mimicking primary angle open glaucoma in animal models, and the limited translational value of in vitro approaches such as whole organ, anterior segment and cell culture studies (such as studies in trabecular meshwork cells), ensures that the pathogenesis of glaucoma to date remains enigmatic.4,7
In order to decrease intraocular pressure, most glaucoma drug treatments alter either the rate of aqueous humor production (eg, beta blockers, carbonic anhydrase inhibitors) or the outflow pathway (eg, prostanoids).8 This review will limit itself to a focus on the pharmacology and biochemical pathways of prostanoid-based therapies, in particular the prototypical therapeutic prostaglandin ethanolamide, bimatoprost. Irrespective of the antiglaucoma drug class, many patients on intraocular pressure-lowering drugs experience limitations in either efficacy or compliance, or display adverse side effects with long term use.9 Some patients will continue to progress to blindness.10
Prostanoids, cannabinoids, and prostamide pharmacology in the eye
Many clinically useful intraocular pressure-lowering agents act at prostaglandin FP receptors, responsive to the endogenous prostaglandin, prostaglandin F2α (PGF2α). These include such drugs as latanoprost and travoprost. Prostanoids play an important role in the control of intraocular pressure, primarily by increasing uveoscleral outflow via remodeling of the ciliary body.11 Therapy with prostaglandin analogues has been shown to effectively lower intraocular pressure over the long term,12 is often superior to other glaucoma therapies and shows fewer side effects.13 While the prostanoid-based treatments have been found to possess similar profiles of efficacy of surveyed drugs within this class,14 other studies have demonstrated that bimatoprost is more efficacious.15,16 While other prostaglandin analogues acting at TP, EP, and DP receptors have been investigated in animal models of raised intraocular pressure, none have advanced clinically so far.17
An increase in trabecular meshwork outflow with prostanoid therapy has also been reported.17 The shape and area of the intertrabecular spaces of the trabecular meshwork normally determines the rate of aqueous outflow through this tissue (and hence intraocular pressure). Traditionally, the size of the pores within the trabecular meshwork was thought to be influenced by the tone of the adjacent ciliary muscle (CM), a smooth muscle component of the ciliary body with tendinous connections to the trabecular meshwork. However, studies have shown that trabecular meshwork has in itself contractile properties similar to smooth muscle.18–20 It has been suggested that this allows the trabecular meshwork to actively change the intertrabecular spaces by an autoregulatory mechanism.21 It is believed that trabecular meshwork contraction decreases aqueous outflow, while relaxation increases outflow.20 The relative contribution of the mesh-work component to the overall reduction in intraocular pressure with prostanoid treatments is not known, although the weak FP receptor agonist docosanoid unoprostone has been demonstrated to have a preferential action on the trabecular meshwork,17 as is also suggested for bimatoprost.22 Unoprostone may affect outflow indirectly or directly via additional cellular mechanisms, including the alteration of ion channel activation in the trabecular meshwork.23,24
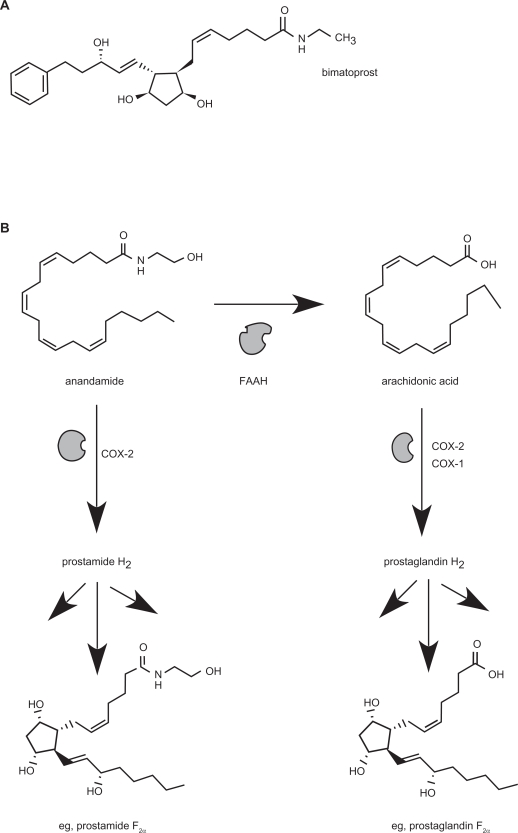
Within this class of prostanoid-based drugs is bimatoprost, which is structurally and chemically similar to the PGF2α analogues used in the treatment of glaucoma (Figure 1a). However, replacement of the carboxylate moiety with an ethanolamide functional group appears to confer to bimatoprost a substantially different pharmacology from the other representative PGF2α agonists.25 It is the first drug of its chemical class, termed the prostaglandin ethanolamides (prostamides), to have a therapeutic application.
Figure 1.
A) Chemical structure of bimatoprost B) Pathway for the production of prostaglandin ethanolamides (prostamides) via COX-2 mediated conversion of the major endocannabinoid, anandamide. Only Prostamide F2α is shown for brevity. Comparison is made alongside conventional prostaglandin production via COX enzymes, showing PGF2α as an example.
Abbreviation: FAAH, fatty acid amide hydrolase.
Bimatoprost has demonstrated efficacy comparable to other prostanoids in the reduction of intraocular pressure.25,26 While bimatoprost elicits a similar and clinically significant increase in uveoscleral outflow and possibly an increase in trabecular outflow facility as for the prostaglandins,17,22 whether the mechanism(s) of its intraocular pressure-lowering action are distinctive from that of the prostaglandins is still not known with certainty. It has been suggested that bimatoprost acts solely at the trabecular meshwork to increase aqueous outflow.27 Certainly, long-term changes in vascularization, inflammatory cell infiltrate, and trabecular meshwork morphology are noted both with prostaglandin agonists and bimatoprost.28,29 Bimatoprost therapy is also associated with similar changes to extracellular matrix markers as with conventional prostaglandin treatments,24 indicating that longer term structural changes follow a similar pathway to the prostaglandins. Studies comparing bimatoprost with latanoprost found that the greater efficacy of intraocular pressure reductions with bimatoprost was offset by a higher incidence of conjunctival hyperemia,15,30 although replacing existing latanoprost therapy with bimatoprost was associated with lower rates of hyperemia.31,32 The reasons for enhanced conjunctival hyperemia with bimatoprost therapy are not known, but it is believed that part of the effect of bimatoprost is manifest as vasodilatation occurring independently of inflammation, generated through endothelial-derived nitric oxide formation.33 Hyperemia with long term prostanoid use has been attributed, either wholly or in part, to the benzalkonium preservative in topical prostanoid ophthalmologic preparations.34,35 However, this most likely does not account for the hyperemia associated with bimatoprost formulations, where the comparative benzalkonium concentrations are relatively low.
Other changes noted with prostanoid therapy generally include elongation and darkening of eyelashes, induced iris darkening, and periocular skin pigmentation, which are mostly similarly evidenced with bimatoprost treatment.36 Topical ocular prostanoids evoke increased immune marker expression in the eye, which infers that the prostanoids can evoke a mild inflammatory reaction.37 This altered immune marker expression is a feature that was also shared with bimatoprost therapy.37
Bimatoprost is a stable chemical entity representative of the endogenous prostamides, which themselves are only relatively recent discoveries. Evidence has demonstrated that a major pathway for the production of endogenous prostamides is via the conversion of endogenous cannabinoid molecules, such as anandamide, via the action of cyclooxygenase-2 (COX-2)38 (Figure 1b). Prostamides were subsequently shown to be produced in vivo utilizing knock-out mice for the normal endocannabinoid metabolising enzyme, fatty acid amide hydrolase (FAAH).39 Another major endocannabinoid molecule, 2-arachidonoyl glycerol is also a substrate for COX-2, producing prostaglandin glycerol esters. A recent study also suggested other pathways for prostamide production exist and are yet to be fully characterized.40 While the constitutive COX-1 does not display an affinity for the endocannabinoids as an enzyme substrate, it is also recognized that the endocannabinoids are substrates for other oxidation pathways aside from COX-2, such as via lipoxygenases and cytochrome P450 enzymes.41
The prostamides have been shown to influence intraocular pressure in a similar fashion to conventional prostaglandin PGF2α agonists.42 However, they are believed to act at receptors distinct from conventional prostaglandins, namely via a separate class of ‘prostamide’ receptors.26,43 Indeed, bimatoprost is believed to act at distinct ‘prostamide’ receptors to mediate intraocular pressure reductions in glaucoma.43,44 The precise biological role of the prostamides compared to prostaglandins generally is not known, either in ocular physiology or in other systems.45 Certainly there appear to be functional pharmacological differences between the two prostanoid classes. Studies utilizing in vitro bioassays such as trabecular meshwork, ciliary and iridial muscle preparations, and cell culture expression systems ostensibly preclude a conventional FP receptor component to bimatoprost’s activity.25,44,46
The pharmacological selectivity of the endogenous prostamides has been well characterized. Prostamides D2, E2, and F2α are only weakly active at human prostaglandin DP, EP, FP, IP, or TP receptors.47 The distinctive pharmacology of the prostamides is supported by the recent development of prostamide-selective antagonists.48 These have been demonstrated to block contractile responses in feline iris to prostamides (including bimatoprost), but not the corresponding prostaglandins.48 Prostamide metabolites were generally not believed to be responsible for activity as assessed in vitro using either recombinant cell lines or functional bioassays.47 However, another in vitro study in human eye tissues showed that bimatoprost was rapidly hydrolyzed in cornea, iris, sclera, and ciliary muscle to its corresponding 17-phenyl-PGF2α metabolite, known to be active at FP receptors.49
Other studies that question the unique pharmacological selectivity of bimatoprost include one demonstrating an activity at cannabinoid receptors in ciliary muscle,50 which contrasts to another study that demonstrated a negligible activity of prostamides at cannabinoid receptors.42 Cannabinoid receptor agonists lower intraocular pressure,51 potentially via an increased outflow facility through an action at the trabecular meshwork.52 An action of the cannabinoid CB1 receptor antagonist SR141716A at ‘prostamide-sensitive’ receptors should be excluded to possibly clarify these anomalous findings.
The contractile effect of bimatoprost was also partly attributed to an agonist activity at the FP receptor, as attested to by the sensitivity of bimatoprost’s actions to the FP receptor antagonist AL8810.50 More recently, prostaglandin FP receptor variants forming heterodimers in cell expression systems accounted for the selectivity of bimatoprost, which was reversible with prostamide-selective antagonists such as AGN211335.53 The molecular identification of the prostamide receptor(s) remains elusive, but speculation of an FP receptor splice variant accounting for the pharmacological variation has been proposed.25 The selectivity based purely on pharmacological actions will continue to raise an ambiguity as to the mechanism of bimatoprost’s actions.
It should also be reiterated that the major endocannabinoid molecules act at cannabinoid receptors expressed in the human eye, mainly in the retina54 but also in the trabecular meshwork.55 Exogenous cannabinoids and endocannabinoids exert functional influences in the eye, including the modulation of aqueous humor production and outflow. Δ9-THC, the active psychotropic component of Cannabis Sativa, has been shown to increase aqueous outflow.56 2-Arachidonoyl glycerol infusion in anterior eye segments increases aqueous humor outflow and alters actin deposition, possibly via an action at the trabecular meshwork.57 Anandamide infusion has also been shown to increase the aqueous humor outflow facility.58 That both endocannabinoid molecules and their COX-2 metabolites, the prostamides, can alter aqueous and uveoscleral outflow independently makes the contribution of their interaction to intraocular pressure difficult to discern in vivo. That both systems are expressed in the eye may be important in eye pathologies, such as glaucoma, where the expression of each system may be altered, with a subsequent modulation of both endocannabinoid and prostamide expression. The functional sequelae of such changes would be intriguing, but has yet to be extensively investigated.
Biochemical pathology in relation to prostamides
There are only a limited number of studies that have separately investigated the expression of the endocannabinoid system and COX-2 in glaucoma, either in animal models or in human glaucoma. None have yet attempted to directly measure prostamide levels in the eye at either normal or elevated intraocular pressure, so potential changes in prostamide generation here are inferred from altered systems involved in their formation.
It is well established that COX-2 expression is inducible under inflammatory conditions; therefore it is attractive to consider that at the tissue level, marked changes in COX-2 activity could dramatically alter the local fate of endocannabinoids. Under such conditions, endocannabinoids may be diverted into the production or prostamides and prostaglandin glycerol esters, which may possess a diverse suite of biological effects that are as yet to be defined.
Elements of both the tissue endocannabinoid system and COX-2 are expressed in the human eye, and perturbations in both endocannabinoid and COX-2 expression seem to follow similar patterns in human glaucoma. Endocannabinoids such as 2-AG are expressed in the human uveoscleral region in reduced levels in human glaucomatous eyes.59 While there is a degree of constitutive COX-2 expression in the human eye, COX-2 expression is significantly reduced in human primary open angle glaucoma.60 Thus, a possible scenario in glaucoma is one of a combination of both reduced COX-2 expression and reduced endocannabinoid substrates for COX-2, impinging upon a potentially important regulatory process for controlling aqueous outflow. The interaction between COX-2 and the cannabinoids is strengthened by evidence that methanandamide, a stable mimic of the endogenous cannabinoid anandamide, directly stimulates COX-2 expression in human nonpigmented ciliary epithelial cells,61 in addition to independently lowering intraocular pressure through an effect on outflow via conventional cannabinioid receptors. The induction of COX-2 in ciliary epithelial cells is a feature also shared by exposure to prostaglandins such as prostaglandin E2 (PGE2).62
In animal studies of the ocular endocannabinoid system, the focus has been on changes in retinal tissue markers as opposed to outflow structures. Nonetheless, as a result of acute elevations in intraocular pressure, rat retina shows enhanced fatty acid amide hydrolase (FAAH) expression and reduced anandamide levels, together with reduced cannabinoid CB1 receptor expression.63 Implications for retinal protection were discussed by Nucci and colleagues.63 However, reduced expression or greater turnover of prostamide substrates in outflow structures as a consequence of such changes in endocannabinoid markers could exacerbate intraocular pressure increases, by impinging on aqueous outflow.
Although ocular COX-2 expression is enhanced in animal models of glaucoma,64 focused studies on humans showing reduced expression of ocular COX-2 in primary open-angle glaucoma and steroid-induced glaucoma60 lend support to a more reliable clinical picture of changes in COX-2 versus animal models. Contrary to the view that COX-2 is proinflammatory, COX-2 expression may be reparative, producing enhanced levels of prostaglandins and prostamides in an effort to restore normal aqueous outflow in acute models of elevated intraocular pressure. The production of prostamides versus prostaglandins in this setting is also an interesting question, where COX-2 expression and activity is altered. Under conditions of cytokine incubation, explants and cell cultures can produce significant proportions of prostamides compared to prostaglandins.65 In some studies of glaucoma, where aqueous humor shows significantly reduced PGE2 levels compared to control,60 there may be a differential reduction in prostamides or prostaglandin concentrations that cannot be distinguished using commercial prostaglandin immunoassays, due to the complete immune cross reactivity between the two classes of prostanoid.65 The implication of such a differential change occurring in the eye is not known, given that both prostamides and prostaglandins mediate increases in uveoscleral outflow and reductions in intraocular pressure. It is interesting to note that prostanoids such as latanoprost directly stimulate COX-2 production in human nonpigmented ciliary epithelial cells and that this is a requirement for matrix metalloproteinase expression.66 Taken together with evidence that human aqueous humor shows significantly reduced PGE2 levels in glaucoma,60 it is conceivable that prostanoids permit the restitution of ocular COX-2 expression, which in turn restores the normal structural and functional components of the outflow facility. As this feature is also shared with endocannabinoid analogues, it will be important to determine if a similar property of the prostaglandins is shared with prostamide therapy.
Studies investigating the effects of long-term use of COX-2 inhibitors (‘coxibs’) with intraocular pressure changes would also be of interest, especially given the reports of ocular side effects associated with their use.67 Nonselective, nonsteroidal anti-inflammatory drugs are used in a variety of ophthalmologic conditions, and coxibs have a demonstrated development potential as new treatments for corneal angiogenesis and diabetic retinopathy.68 Interestingly, administration of the COX-2 selective inhibitor nimesulide was found to enhance the intraocular pressure-lowering effect of latanoprost in patients with primary open-angle glaucoma.69 This likely suggests that the overall COX-2 mediated production of a suite of prostanoids, with sometimes contrasting effects, needs to be considered, rather than just focusing on the role of prostamides in the control of intraocular pressure. Monitoring of intraocular pressure may, in any event, be warranted where COX-2 inhibitors are to be used topically in such settings over an extended period.
The role of endocannabinoid system in glaucoma may thus be of importance, not only because of the direct positive effects on outflow of the endocannabinoid molecules, but also for the provision of substrates for prostamide production, in addition to a potential neuroprotective contribution to the optic nerve.70 The reduced endocannabinoid levels demonstrated in outflow pathways in human glaucomatous eyes59 may exert a marked effect on outflow, both directly through reduced cannabinoid-induced outflow and indirectly, via reduced prostamide production. Irrespective of the final mediator (endocannabinoid or prostamide), there is a potential to investigate the inhibition of endogenous cannabinoid metabolism as a potential antiglaucoma therapy. Anandamide and 2-AG are broken down by the FAAH enzyme. Inhibitors of FAAH or FAAH gene knockouts increase tissue endocannabinoid concentrations,39 including in the trabecular meshwork of the eye.57 Studies will firstly need to directly measure the formation of prostamides to verify their place in the control of intraocular pressure and altered expression in the setting of glaucoma.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Mackenzie P, Cioffi G. How does lowering of intraocular pressure protect the optic nerve? Surv Ophthalmol. 2008;53(Suppl 1):S39–S43. doi: 10.1016/j.survophthal.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 4.Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, non-glaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- 5.Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DH. Trabecular meshwork and uveoscleral outflow models. J Glaucoma. 2005;14:308–310. doi: 10.1097/01.ijg.0000169397.32674.5e. [DOI] [PubMed] [Google Scholar]
- 8.Woodward DF, Gil DW. The inflow and outflow of anti-glaucoma drugs. Trends Pharmacol Sci. 2004;25:238–241. doi: 10.1016/j.tips.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl 1):S57–S68. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Sharts-Hopko NC, Glynn-Milley C.Primary open-angle glaucoma Am J Nurs 200910940–47.; quiz 48. [DOI] [PubMed] [Google Scholar]
- 11.Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000;11:112–115. doi: 10.1097/00055735-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Easthope SE, Perry CM. Topical bimatoprost: a review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging. 2002;19:231–248. doi: 10.2165/00002512-200219030-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hodge WG, Lachaine J, Steffensen I, et al. The efficacy and harm of prostaglandin analogues for IOP reduction in glaucoma patients compared to dorzolamide and brimonidine: a systematic review. Br J Ophthalmol. 2008;92:7–12. doi: 10.1136/bjo.2007.123737. [DOI] [PubMed] [Google Scholar]
- 14.Bean GW, Camras CB. Commercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differences. Surv Ophthalmol. 2008;53(Suppl 1):S69–S84. doi: 10.1016/j.survophthal.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JW, Wei RL. Meta-analysis of 13 randomized controlled trials comparing bimatoprost with latanoprost in patients with elevated intraocular pressure. Clin Ther. 2008;30:622–632. doi: 10.1016/j.clinthera.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.van der Valk R, Webers CA, Lumley T, Hendrikse F, Prins MH, Schouten JS. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol. 2009;62:1279–1283. doi: 10.1016/j.jclinepi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53(Suppl 1):S107–S120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stumpff F, Wiederholt M. Regulation of trabecular meshwork contractility. Ophthalmologica. 2000;214:33–53. doi: 10.1159/000027471. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer E. Trabecular meshwork as a new target for the treatment of glaucoma. Drug News Perspect. 2006;19:151–158. doi: 10.1358/dnp.2006.19.3.985929. [DOI] [PubMed] [Google Scholar]
- 20.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 21.Wiederholt M. Direct involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol. 1998;9:46–49. doi: 10.1097/00055735-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Wan Z, Woodward DF, Cornell CL, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48:4107–4115. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thieme H, Steinhausen K, Ottlecz A, et al. Effects of unoprostone and endothelin 1 on L-type channel currents in human trabecular meshwork cells. Ophthalmic Res. 2005;37:293–300. doi: 10.1159/000087724. [DOI] [PubMed] [Google Scholar]
- 24.Ooi YH, Oh DJ, Rhee DJ. Effect of bimatoprost, latanoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human ciliary body smooth muscle cells. Invest Ophthalmol Vis Sci. 2009;50:5259–5265. doi: 10.1167/iovs.08-3356. [DOI] [PubMed] [Google Scholar]
- 25.Woodward DF, Liang Y, Krauss AH. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol. 2008;153:410–419. doi: 10.1038/sj.bjp.0707434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantor LB. Clinical pharmacology of bimatoprost. Expert Opin Drug Metab Toxicol. 2005;1:151–157. doi: 10.1517/17425255.1.1.151. [DOI] [PubMed] [Google Scholar]
- 27.Wan Z, Woodward DF, Stamer WD. Endogenous bioactive lipids and the regulation of conventional outflow facility. Expert Rev Ophthalmol. 2008;3:457–470. doi: 10.1586/17469899.3.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russ HH, Costa VP, Ferreira FM, et al. Conjunctival changes induced by prostaglandin analogues and timolol maleate: a histomorphometric study. Arq Bras Oftalmol. 2007;70:910–916. doi: 10.1590/s0004-27492007000600005. [DOI] [PubMed] [Google Scholar]
- 29.Richter M, Krauss AH, Woodward DF, Lutjen-Drecoll E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44:4419–4426. doi: 10.1167/iovs.02-1281. [DOI] [PubMed] [Google Scholar]
- 30.How AC, Kumar RS, Chen YM, et al. A randomised crossover study comparing bimatoprost and latanoprost in subjects with primary angle closure glaucoma. Br J Ophthalmol. 2009;93:782–786. doi: 10.1136/bjo.2008.144535. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz S, Mann O. Incidence of hyperemia associated with bimatoprost treatment in naive subjects and in subjects previously treated with latanoprost. Eur J Ophthalmol. 2009;19:400–403. doi: 10.1177/112067210901900312. [DOI] [PubMed] [Google Scholar]
- 32.Kammer J, Katzman B, Ackerman S, Hollander D.Efficacy and tolerability of bimatoprost versus travoprost in patients previously on latanoprost: a 3-month, randomized, masked-evaluator, multicenter study Br J Ophthalmol 2009September1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Dinh T, Woodward DF, et al. Bimatoprost: mechanism of ocular surface hyperemia associated with topical therapy. Cardiovasc Drug Rev. 2005;23:231–246. doi: 10.1111/j.1527-3466.2005.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 34.Henry JC, Peace JH, Stewart JA, Stewart WC. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol. 2008;2:613–621. doi: 10.2147/opth.s3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:2444–2450. doi: 10.1167/iovs.04-1331. [DOI] [PubMed] [Google Scholar]
- 36.Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(Suppl 1):S93–S105. doi: 10.1016/j.survophthal.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues Mde L, Felipe Crosta DP, Soares CP, et al. Immunohistochemical expression of HLA-DR in the conjunctiva of patients under topical prostaglandin analogs treatment. J Glaucoma. 2009;18:197–200. doi: 10.1097/IJG.0b013e31818153f4. [DOI] [PubMed] [Google Scholar]
- 38.Kozak KR, Crews BC, Morrow JD, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 39.Weber A, Ni J, Ling KH, et al. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Moriuchi H, Koda N, Okuda-Ashitaka E, et al. Molecular characterization of a novel type of prostamide/prostaglandin F synthase, belonging to the thioredoxin-like superfamily. J Biol Chem. 2008;283:792–801. doi: 10.1074/jbc.M705638200. [DOI] [PubMed] [Google Scholar]
- 41.Patrignani P, Tacconelli S, Sciulli MG, Capone ML. New insights into COX-2 biology and inhibition. Brain Res Brain Res Rev. 2005;48:352–359. doi: 10.1016/j.brainresrev.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Woodward DF, Carling RW, Cornell CL, et al. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol Ther. 2008;120:71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Burk RM, Woodward DF. A historical perspective and recent advances in prostamide research and therapeutics. Curr Opin Drug Discov Devel. 2007;10:413–421. [PubMed] [Google Scholar]
- 44.Woodward DF, Krauss AH, Chen J, et al. The pharmacology of bimatoprost (Lumigan) Surv Ophthalmol. 2001;45(Suppl 4):S337–S345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- 45.Smid SD. Gastrointestinal endocannabinoid system: multifaceted roles in the healthy and inflamed intestine. Clin Exp Pharmacol Physiol. 2008;35(11):1383–1387. doi: 10.1111/j.1440-1681.2008.05016.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Lu RT, Lai R, et al. Bimatoprost-induced calcium signaling in human T-cells does not involve prostanoid FP or TP receptors. Curr Eye Res. 2009;34:184–195. doi: 10.1080/02713680802669781. [DOI] [PubMed] [Google Scholar]
- 47.Matias I, Chen J, De Petrocellis L, et al. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- 48.Woodward DF, Krauss AH, Wang JW, et al. Identification of an antagonist that selectively blocks the activity of prostamides (prostaglandin-ethanolamides) in the feline iris. Br J Pharmacol. 2007;150:342–352. doi: 10.1038/sj.bjp.0706989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies SS, Ju WK, Neufeld AH, Abran D, Chemtob S, Roberts LJ., 2nd Hydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitro. J Ocul Pharmacol Ther. 2003;19:45–54. doi: 10.1089/108076803762718105. [DOI] [PubMed] [Google Scholar]
- 50.Romano MR, Lograno MD. Evidence for the involvement of cannabinoid CB1 receptors in the bimatoprost-induced contractions on the human isolated ciliary muscle. Invest Ophthalmol Vis Sci. 2007;48:3677–3682. doi: 10.1167/iovs.06-0896. [DOI] [PubMed] [Google Scholar]
- 51.Szczesniak AM, Kelly ME, Whynot S, Shek PN, Hung O. Ocular hypotensive effects of an intratracheally delivered liposomal delta9-tetrahydrocannabinol preparation in rats. J Ocul Pharmacol Ther. 2006;22:160–167. doi: 10.1089/jop.2006.22.160. [DOI] [PubMed] [Google Scholar]
- 52.McIntosh BT, Hudson B, Yegorova S, Jollimore CA, Kelly ME. Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br J Pharmacol. 2007;152:1111–1120. doi: 10.1038/sj.bjp.0707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Y, Woodward DF, Guzman VM, et al. Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. Br J Pharmacol. 2008;154:1079–1093. doi: 10.1038/bjp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Wang X, Wang L. Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells. Mol Vis. 2009;15:1243–1251. [PMC free article] [PubMed] [Google Scholar]
- 55.Stamer WD, Golightly SF, Hosohata Y, et al. Cannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol. 2001;431:277–286. doi: 10.1016/s0014-2999(01)01438-8. [DOI] [PubMed] [Google Scholar]
- 56.Crandall J, Matragoon S, Khalifa YM, et al. Neuroprotective and intraocular pressure-lowering effects of (−)Delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007;39:69–75. doi: 10.1159/000099240. [DOI] [PubMed] [Google Scholar]
- 57.Njie YF, He F, Qiao Z, Song ZH. Aqueous humor outflow effects of 2-arachidonylglycerol. Exp Eye Res. 2008;87:106–114. doi: 10.1016/j.exer.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Njie YF, Qiao Z, Xiao Z, Wang W, Song ZH. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2008;49:4528–4534. doi: 10.1167/iovs.07-1537. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Matias I, Dinh T, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330:1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 60.Maihofner C, Schlotzer-Schrehardt U, Guhring H, et al. Expression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyes. Invest Ophthalmol Vis Sci. 2001;42:2616–2624. [PubMed] [Google Scholar]
- 61.Rosch S, Ramer R, Brune K, Hinz B. R(+)-methanandamide and other cannabinoids induce the expression of cyclooxygenase-2 and matrix metalloproteinases in human nonpigmented ciliary epithelial cells. J Pharmacol Exp Ther. 2006;316:1219–1228. doi: 10.1124/jpet.105.092858. [DOI] [PubMed] [Google Scholar]
- 62.Rosch S, Ramer R, Brune K, Hinz B. Prostaglandin E2 induces cyclooxygenase-2 expression in human non-pigmented ciliary epithelial cells through activation of p38 and p42/44 mitogen-activated protein kinases. Biochem Biophys Res Commun. 2005;338:1171–1178. doi: 10.1016/j.bbrc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 63.Nucci C, Gasperi V, Tartaglione R, et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Invest Ophthalmol Vis Sci. 2007;48:2997–3004. doi: 10.1167/iovs.06-1355. [DOI] [PubMed] [Google Scholar]
- 64.Marshall JL, Stanfield KM, Silverman L, Khan KN. Enhanced expression of cyclooxygenase-2 in glaucomatous dog eyes. Vet Ophthalmol. 2004;7:59–62. doi: 10.1111/j.1463-5224.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 65.Glass M, Hong J, Sato TA, Mitchell MD. Misidentification of prostamides as prostaglandins. J Lipid Res. 2005;46:1364–1368. doi: 10.1194/jlr.C500006-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.Hinz B, Rosch S, Ramer R, Tamm ER, Brune K. Latanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanism. FASEB J. 2005;19:1929–1931. doi: 10.1096/fj.04-3626fje. [DOI] [PubMed] [Google Scholar]
- 67.Fraunfelder FW, Solomon J, Mehelas TJ. Ocular adverse effects associated with cyclooxygenase-2 inhibitors. Arch Ophthalmol. 2006;124:277–279. doi: 10.1001/archopht.124.2.277. [DOI] [PubMed] [Google Scholar]
- 68.Radi ZA, Render JA. The pathophysiologic role of cyclo-oxygenases in the eye. J Ocul Pharmacol Ther. 2008;24:141–151. doi: 10.1089/jop.2007.0078. [DOI] [PubMed] [Google Scholar]
- 69.Costagliola C, Parmeggiani F, Caccavale A, Sebastiani A. Nimesulide oral administration increases the intraocular pressure-lowering effect of latanoprost in patients with primary open-angle glaucoma. Am J Ophthalmol. 2006;141:379–381. doi: 10.1016/j.ajo.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 70.Nucci C, Bari M, Spano A, et al. Potential roles of (endo)cannabinoids in the treatment of glaucoma: from intraocular pressure control to neuroprotection. Prog Brain Res. 2008;173:451–464. doi: 10.1016/S0079-6123(08)01131-X. [DOI] [PubMed] [Google Scholar]