Abstract
Background and objectives: Tissue-advanced glycation end products (AGE) are a measure of cumulative metabolic stress. Assessment of tissue AGE by skin autofluoresence (AF) correlates well with cardiovascular outcomes in hemodialysis (HD) patients. This study aimed to measure and compare tissue AGE levels in HD and peritoneal dialysis (PD) patients and to evaluate the impact of systemic PD glucose exposure.
Design, setting, participants, & measurements: Tissue AGE were measured in 115 established dialysis patients (62 HD and 53 PD) using a cutaneous AF device (AGE Reader; DiagnOptics). Values were compared with an age-matched non–chronic kidney disease database. Review of all previous PD solution delivery/prescription data determined PD glucose exposure.
Results: PD patients were similar in age to HD patients but had a shorter dialysis vintage. There were no differences in ischemic heart disease or smoking history, statin or angiotensin-converting enzyme inhibitor (ACEi) use, lipids, biochemistry, or prevalence of diabetes. More than 90% of both groups had met current dialysis adequacy targets. Skin AF values in PD and HD patients were similar and strongly correlated with historical PD glucose exposure. Skin AF correlated with age in both groups but with dialysis vintage only in PD patients
Conclusions: Cumulative metabolic stress and transient hyperglycemia results in grossly elevated levels of tissue AGE in dialysis patients. In PD patients, this high level of AGE deposition is associated with historical glucose exposure. This observation provides a previously unappreciated potential link between PD exposure to glucose and systemic cardiovascular disease.
The prevalence of cardiovascular disease in patients with chronic kidney disease (CKD) remains high (1), and it is increasingly recognized that this is because of combinations of classical and nonclassical risk factors. The role of oxidative stress, changes in arterial function, and their relative contributions to overall cardiovascular morbidity and mortality remains of paramount importance in patients with CKD, and in particular those on renal replacement therapy.
The Maillard reaction is a biochemical process occurring in tissues and in stored glucose solutions (such as those used for peritoneal dialysis [PD]), resulting in the formation of advanced glycation end products (AGE). AGE bound to structural proteins and deposited in tissues alter function, enhance local cytokine production, and serve as a marker of cumulative metabolic stress (2). In addition, the activation of transcription factors through specific receptor-binding sites (e.g. receptor for AGE [RAGE]) may result in a wide variety of biologic actions and further structural changes to the vascular wall (3). Hyperglycemia favors, but is not a requirement for, AGE formation (4). Several factors such as oxidative and carbonyl stress and reduced renal clearance all seem to increase the production and accumulation of AGEs (5–7). AGE have already been implicated in the progression of chronic, age-related diseases such as atherosclerosis, CKD, and diabetes (3,4,6,8–11). AGEs cannot easily be measured in clinical practice because they are difficult to analyze in complex body fluids such as blood, and the assessment of more significant tissue-bound compounds has previously required biopsy. More recently, however, a simple noninvasive method of AGE measurement has been developed that uses the correlation between collagen-linked fluorescence and AGE content observed in skin biopsies. AGE deposition within tissues can therefore be assessed by the use of skin ultraviolet autofluorescence (AF).
We aimed to measure and compare AGE in patients receiving either hemodialysis (HD) or PD and to study the association between chronic glucose exposure caused by glucose-containing peritoneal fluids and systemic tissue AGE accumulation.
Materials and Methods
Study Population
One hundred fifteen established dialysis patients (62 HD and 53 PD) were recruited from a single center in a cross-sectional study. Minimum duration of renal replacement therapy was 30 d. A medical history of diabetes mellitus, ischemic heart disease, and drug treatment was recorded. Hematology and biochemistry results, time-averaged over 6 mo, and dialysis adequacy was also recorded.
Patients receiving chronic hemodialysis were dialyzed thrice weekly for 4 h with low-flux polysulphone dialyzers, either 1.8 or 2.0 m2 (LOPS 18/20; Braun Medical Ltd., Sheffield, UK). For all treatments, dialysate contained 138 mM sodium, 1 mM potassium, 1.25 mM calcium, 0.5 mM magnesium, 32 mM bicarbonate, 1 g/L glucose, and 3 mM acetate. All treatments were of 4-h duration, and anti-coagulation was achieved with unfractionated heparin. Dialysate flow was 500 ml/min, and conductivity was set at 13.6 mS/cm.
PD patients used lactate/bicarbonate-buffered, 1.36 or 3.86% glucose-containing solutions (Physioneal; Baxter), Extraneal (7.5% icodextrin; Baxter), or Nutrineal (1.1% amino acids; Baxter) as prescribed for routine clinical care. PD glucose exposure was generated from historical PD prescription data and review of all historical Baxter home delivery records. Patient characteristics are listed in Table 1. Appropriate approval was obtained from a local ethics committee.
Table 1.
Demographic details including biochemical, hematologic indices, and use of medication
| PD (n = 53) | HD (n = 62) | P Value | |
|---|---|---|---|
| Age (yr) | 62.5 ± 13.1 | 65.3 ± 15.5 | NS |
| Male (%) | 31 (58%) | 34 (55%) | NS |
| White ethnicity | 49 (92%) | 56 (90%) | NS |
| Diabetes mellitus | 7 (13%) | 6 (10%) | NS |
| Smoking | 3 (6%) | 5 (8%) | NS |
| Dialysis vintage (mo) | 38.4 ± 14.3 | 51.7 ± 34.7 | 0.007 |
| Body mass index (kg/m2) | 26 ± 3 | 27 ± 5 | NS |
| Dialysis adequacy (Kt/V)a | 2.4 ± 0.08 | 1.25 ± 0.18 | NA |
| Previous cardiovascular morbidityb | 6 (11%) | 8 (13%) | NS |
| Total cholesterol (mM) | 4.6 ± 1.0 | 4.4 ± 1.0 | NS |
| HDL cholesterol (mM) | 1.4 ± 0.3 | 1.3 ± 0.3 | NS |
| LDL cholesterol (mM) | 2.2 ± 1.1 | 2.0 ± 0.8 | NS |
| Triglycerides (mM) | 2.2 ± 0.8 | 2.4 ± 1.8 | NS |
| Serum phosphate (mM) | 1.52 ± 0.2 | 1.64 ± 0.4 | NS |
| Serum corrected calcium (mM) | 2.51 ± 0.1 | 2.47 ± 0.1 | NS |
| Calcium × phosphate product (mmol2/L2) | 4.03 ± 0.7 | 4.21 ± 1.0 | NS |
| PTH (pg/ml) | 342 ± 301 | 298 ± 313 | NS |
| Albumin (g/L) | 29.7 ± 3.7 | 32.5 ± 3 | NS |
| Hemoglobin (g/dl) | 12.0 ± 1.14 | 11.8 ± 1.56 | NS |
| Lipid-lowering therapy | 29 (55%) | 27 (44%) | NS |
| Use of calcium channel blockers | 5 (9%) | 6 (10%) | NS |
| Use of ACE inhibitors | 19 (36%) | 9 (15%) | 0.006 |
| Use of β blockers | 3 (6%) | 5 (8%) | NS |
| Use of erythropoietin | 46 (87%) | 56 (90%) | NS |
| Skin autofluourescence (AU) | 3.58 ± 0.75 | 3.7 ± 0.88 | NS |
There were no significant differences in patient characteristics, apart from dialysis vintage and use of angiotensin-converting enzyme inhibitors (ACEi).
NS, not significant; NA, not applicable.
Kt/V is weekly in PD and per session in HD.
Defined as any previous description of ischemic heart disease, heart failure, cerebrovascular disease, or peripheral vascular disease.
Skin AF
All study participants had tissue AGE measured using a previously validated cutaneous AF device using an ultraviolet source at a specific range of wavelengths (AGE Reader; DiagnOptics, Groningen, The Netherlands) (12,13). Values were compared with an age-matched non-CKD database contained within the device, generated from a Dutch cohort. The AF reader illuminates a skin surface of ∼1 cm2, guarded against surrounding light, with an excitation light source between 300 and 420 nm (peak excitation, ∼350 nm). Only light from the skin is measured with a spectrometer in the 300- to 600-nm range, using a 200-μm glass fiber (14). All measurements were performed at room temperature in a semidark environment predialysis or during PD clinic consultations. The nondominant forearm rests on the device, and three readings, all taken within 1 cm of each other away from any areas of bruising or obvious pigmentation, were averaged and reported. A connected personal computer analyzed the degree of AF and correlated that to known normal ranges.
Statistical Analysis
Group data are presented as mean ± SD unless otherwise stated. All data were tested for normality. Analysis was performed using SPSS v12.0.1 (SPSS Inc., Chicago, IL). Categorical data were compared using the χ2 test and continuous data using a paired or unpaired t test or one-way ANOVA with Tukey's correction as appropriate.
Results
Patients receiving PD and HD were relatively well matched. The groups were of a similar age, gender, and ethnicity (predominantly white because of the limitations that skin pigmentation brings to the measurement of skin AF). PD patients were of significantly shorter dialysis vintage and were more likely to be receiving an ACEi. There were no significant differences in biochemical indices averaged over the preceding 6 mo, and the groups were broadly comparable in terms of small solute–based dialysis adequacy (data summarized in Table 1).
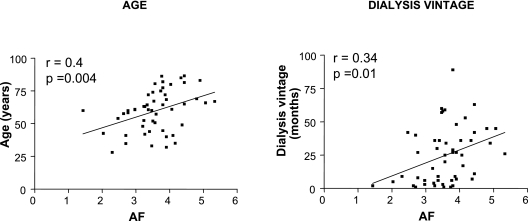
All patients had markedly elevated skin AF values compared with the nonuremic reference values. Despite shorter dialysis vintage, values of AF were similar in PD and HD patients (3.58 ± 0.75 and 3.7 ± 0.88 AU, respectively, P = 0.33). In PD patients, there was a positive correlation between skin AF and patient age, as well as dialysis vintage (r = 0.4, P = 0.004 and r = 0.34, P = 0.01 respectively, Figure 1). Skin AF in HD patients correlated with age but not with dialysis vintage (r = 0.46, P = 0.001 and r = 0.22, P = 0.65, respectively). Because of the small number of factors that correlated with skin AF and the relatively weak associations observed, a multivariate analysis was considered inappropriate.
Figure 1.
Skin AF is associated with age and dialysis vintage (mo) in those receiving PD.
There was no difference in AGEs in those with or without diabetes.
PD Glucose Exposure
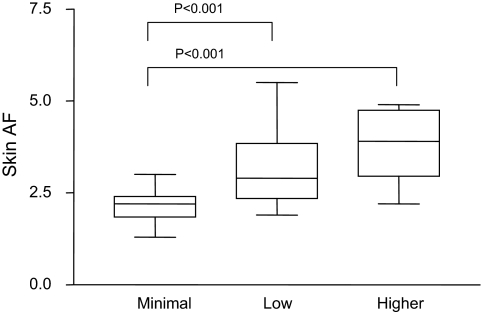
Complete records of home PD solution delivery were obtained for 51/53 patients. Conversion from conventional lactate-based fluids (Dianeal; Baxter) occurred in 2001, and therefore all patients received bicarbonate/lactate biocompatible fluids (Physioneal; Baxter). Patients were classified by their historical glucose exposure during their time on dialysis, and only 5/51 patients had ever been exposed to conventional PD fluids. Nearly one half of the patients (24/51) used icodextrin-based PD fluids (Extraneal; Baxter), and 12/51 patients used or had used amino acid–based fluids (Nutrineal; Baxter) for either nutritional purposes or to maximally avoid PD fluid–based glucose in diabetics. Only 7/51 patients had been exposed to daily high glucose exposure (daily 1 × 3.86% exchanges). This compared with 20/51 who received no more than two 3.86% glucose exchanges per week and 24/51 patients who had never been exposed to any significant increase in dialysate glucose exposure (receiving 1.27% exchanges). This equates to a weekly minimal additional glycemic exposure of 540.4 or 154.4 g/wk, respectively. There were no statistically significant differences in age, dialysis vintage, or other demographic factors between these groups. Skin AF significantly correlated with historical glucose exposure (Figure 2).
Figure 2.
Increasing skin AF is strongly associated with increased glycemic historical exposure in patients receiving PD (minimal = 1.27% exchanges only; low = ≤2 3.86% exchanges/wk; high = daily 3.86% exchanges).
Discussion
We observed that skin AF is increased in CKD patients receiving either HD or PD. In addition, despite shorter dialysis vintage, patients receiving PD with bicarbonate/lactate fluids had similar AF levels to those receiving chronic HD. Furthermore, this is the first study to show clear associations between increased tissue AGE deposition and increased glucose exposure in patients receiving PD.
AGE accumulation is recognized to play an integral role in the pathogenesis of diabetic complications and vascular and renal disease. Because of the complexities of measurement, normal AGE values have not yet been clearly delineated in fully comparative cohorts, but validation data for this technique show a mean skin AF of 0.011 AU in nonuremic control subjects (15). Monami et al. detected an average skin AF value of 2.46 AU in those with diabetes and significantly higher values in patients with microalbuminuria, CKD, diabetic neuropathy, arterial disease, and smoking (16). In our study of HD and PD patients, we observed average skin AF values of 3.64 AU, which was considerably elevated compared with the reference population, although there was no difference between those with and without diabetes. We speculate that the drivers of cumulative metabolic stress may be so overwhelming in the setting of CKD 5 that the presence of diabetes has no additional effect in established dialysis patients.
It is known that AGE are generated in the presence of oxidative stress and additionally accumulate because of diminished renal clearance of AGE precursors in CKD. It is postulated that the deposition of AGE in the basement membrane of the vessel walls results in permanent change and altered cellular responses (3,11,17–19). Endothelial and smooth muscle cells in particular produce cytokines in response to AGE deposition and the upregulation of fibroblasts as a consequence of AGE result in expansion of the extracellular matrix, which is a proinflammatory response important in the development of vascular complications in diabetes (20). However, it is not known whether these observed changes in those receiving dialysis are similar in all patients or whether the process of PD or HD may further influence the natural history of AGE development.
AGE deposition has previously been associated with increased mortality in patients receiving HD (14), and the pathophysiologic mechanisms accounting for these associations require further elucidation. Skin AF has been useful in the assessment of graft dysfunction and outcomes in renal transplant patients (21). Data are scarce on the effect of AGEs and glycemic exposure in PD patients.
Conventional PD fluids rely on glucose as the main osmotic agent, buffered with lactate alone to produce a low pH. During the manufacturing process, conventional fluids result in the production of glucose degradation products (GDPs) (22). GDPs result in local peritoneal toxicity and poorly understood systemic effects. In addition, GDPs also enhance the production of AGE (23). Newer, biocompatible fluids have been developed, such as Physioneal (Baxter), Stay Safe Balance (Fresenius), and Gambrosol Trio (Gambro). Fluids containing an alternative osmotic agent to glucose are also available, using icodextrin or amino acid solutions, with lower or no GDP/AGE components, respectively. Even in patients receiving biocompatible solutions, the use of high glucose concentration solutions in fasted nondiabetic patients results in transient hyperglycemia and prolonged hyperinsulinemia (24), and these metabolic stresses result in short-term hemodynamic changes (25).
Recent work by the Euro Balance group showed that markers of AGE production reflect the composition of PD solutions. In particular, the biocompatible fluids had significantly lower levels of GDPs than conventional solutions (26). Biocompatible PD fluids have already shown beneficial effects on peritoneal mesothelial cells and may preserve vascular function (27). However, our work showed that, even in low GDP-containing fluids, skin AF measurements in PD patients are similar to those receiving HD.
Comparisons of AGEs between PD and HD patients have not previously been performed. Glycemic exposure and GDPs have been implicated in the development of AGEs in those receiving PD, but importantly, HD dialysate contains glucose, reaching concentrations of 200 mg/dl (28). Potentially, therefore, in patients receiving HD, there is significant thrice-weekly additional glycemic exposure in addition to the AGEs generated by metabolic stress during the course of their progressive kidney disease. Further work is needed to study the natural history of AGE development within incident dialysis patients.
Semba et al. (29) recently reported that AGE increase with aging. In addition, the presence of AGE correlated with increased pulse wave velocity, a marker of arterial stiffness. We have previously shown that autonomic dysfunction, arterial stiffness, and vascular calcification are increased in chronic HD patients. Furthermore, the relationship between arterial stiffness and vascular calcification is present in PD patients (30), and markers of oxidative stress correlate with arterial stiffness in those receiving PD (31). Therefore, the AGE deposition may be an additional important factor in the development of structural and functional change within the vasculature.
In summary, AGE are increasingly well recognized to be important in the pathogenesis of cardiovascular morbidity and mortality in patients with CKD and many of the conditions leading to CKD. Increased accumulation of AGE has been observed in patients receiving PD and HD. Furthermore, cutaneous AGE levels measured by skin AF strongly correlates with increased glucose exposure and dialysis vintage.
The relationships between metabolism, vascular structure, and vascular function in CKD remain of paramount importance and may provide a framework to increase our understanding of the interaction between metabolic stress, structural/functional cardiovascular consequences, and associated clinical outcomes.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Parfrey PS: Cardiac disease in dialysis patients: Diagnosis, burden of disease, prognosis, risk factors and management. Nephrol Dial Transplant 15[Suppl 5]: 58–68, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Baynes JW: From life to death–the struggle between chemistry and biology during aging: The Maillard reaction as an amplifier of genomic damage. Biogerontology 1: 235–246, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Aronson D: Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H: Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 325: 836–842, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Baynes JW, Thorpe SR: Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 48: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C: Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant 18: 1272–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Miyata T, Wada Y, Cai Z, Iida Y, Horie K, Yasuda Y, Maeda K, Kurokawa K, van Ypersele de Strihou C: Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with end-stage renal failure. Kidney Int 51: 1170–1181, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR: Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 314: 403–408, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Nicholl ID, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R: Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med 4: 594–601, 1998 [PMC free article] [PubMed] [Google Scholar]
- 11.Yan SF, Ramasamy R, Naka Y, Schmidt AM: Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ Res 93: 1159–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hartog JW, de Vries AP, Lutgers HL, Meerwaldt R, Huisman RM, van Son WJ, de Jong PE, Smit AJ: Accumulation of advanced glycation end products, measured as skin autofluorescence, in renal disease. Ann N Y Acad Sci 1043: 299–307, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gaans RO, Smit AJ: Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetolgia 47: 1324–1330, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ: Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 16: 3687–3693, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Meerwaldt R, Links TP, Graaff R, Hoogenberg K, Lefrandt JD, Baynes JW, Gans RO, Smit AJ: Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia 48: 1637–1644, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Monami M, Lamanna C, Gori F, Bartalucci F, Marchionni N, Mannucci E: Skin autofluorescence in type 2 diabetes: Beyond blood glucose. Diabetes Res Clin Pract 79: 56–60, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H: Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A 91: 9441–9445, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasamy R, Yan SF, Schmidt AM: The RAGE axis and endothelial dysfunction: Maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med 15: 237–243, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM: Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: Insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep 4: 228–237, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Vasan S, Foiles PG, Founds HW: Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin Investig Drugs 10: 1977–1987, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Hartog JW, Smit AJ, van Son WJ, Navis G, Gans RO, Wolffenbuttel BH, de Jong PE: Advanced glycation end products in kidney transplant patients: A putative role in the development of chronic renal transplant dysfunction. Am J Kidney Dis 43: 966–975, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Witowski J, Jorres A, Korybalska K, Ksiazek K, Wisniewska-Elnur J, Bender TO, Passlick-Deetjen J, Breborowicz A: Glucose degradation products in peritoneal dialysis fluids: Do they harm? Kidney Int Suppl 84:S148–S151, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Pischetsrieder M: Chemistry of glucose and biochemical pathways of biological interest. Perit Dial Int 20[Suppl 2]: S26–S30, 2000 [PubMed] [Google Scholar]
- 24.Selby NM, Fialova J, Burton JO, McIntyre CW: The haemodynamic and metabolic effects of hypertonic-glucose and amino-acid-based peritoneal dialysis fluids. Nephrol Dial Transplant 22: 870–879, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Selby NM, Fonseca S, Hulme L, Fluck RJ, Taal MW, McIntyre CW: Hypertonic glucose-based peritoneal dialysate is associated with higher blood pressure and adverse haemodynamics as compared with icodextrin. Nephrol Dial Transplant 20: 1848–1853, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J: The Euro-Balance Trial: The effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 66: 408–418, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Tomo T: Peritoneal dialysis solutions low in glucose degradation products—evidence for clinical benefits. Perit Dial Int 28[Suppl 3]: S123–S127, 2008 [PubMed] [Google Scholar]
- 28.Godfrey AR: Impact of glucose levels on advanced glycation end products in hemodialysis. Hemodial Int 11: 278–285, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L: Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens 22: 74–79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Kocak H, Gumuslu S, Ermis C, Mahsereci E, Sahin E, Gocmen AY, Ersoy F, Suleymanlar G, Yakupoglu G, Tuncer M: Oxidative stress and asymmetric dimethylarginine is independently associated with carotid intima media thickness in peritoneal dialysis patients. Am J Nephrol 28: 91–96, 2008 [DOI] [PubMed] [Google Scholar]