Abstract
Background and objectives: We assessed the prevalence of obstructive sleep apnea (OSA) and its clinical correlates in a large sample of patients who received a kidney transplant (Tx). We also compared the prevalence of the disorder between dialysis patients who were on the waiting list for a Tx (WL) and Tx patients.
Design, setting, participants, & measurements: This was a cross-sectional study of 100 Tx and 50 WL patients who underwent one-night polysomnography (SLeep disorders Evaluation in Patients after kidney Transplantation [SLEPT] Study). Sociodemographic information and data about medication, comorbidity, and laboratory parameters were collected.
Results: The prevalence of mild (apnea-hypopnea index [AHI] ≥5/h and <15/h), moderate (AHI ≥15/h and <30/h), and severe OSA (AHI ≥30/h) was 18, 11, and 14% in the Tx group and 28, 16, and 10% in the WL group, respectively. The AHI was significantly correlated with age (ρ = 0.34), body mass index (ρ = 0.45), neck circumference (ρ = 0.4), abdominal circumference (ρ = 0.51), and hemoglobin (ρ = 0.24) in the Tx group. The proportion of Tx patients who were treated with three or more antihypertensive drugs was significantly higher in the OSA group (56 versus 31%; P = 0.022). Despite taking significantly more antihypertensive drugs, the average systolic BP was still higher in patients with versus without OSA (147 ± 21 versus 139 ± 18 mmHg; P = 0.059).
Conclusions: The prevalence of OSA is similar in Tx and WL patients and it may contribute to presence of hypertension in patients who receive a Tx.
Obstructive sleep apnea (OSA) is the most clinically important form of sleep-related breathing disorders. The severity of OSA is generally characterized by the apnea-hypopnea index (AHI), which is the number of apneic and hypopneic events per hour of sleep.
The prevalence of moderate and severe OSA syndrome (OSAS; AHI ≥15 and the presence of daytime symptoms of OSA) is 2 to 4% in the general population (1) and is associated with increased cardiovascular morbidity and mortality (2,3). OSAS is reportedly associated with higher risk for stroke, hypertension, diabetes, congestive hearth failure, arrhythmias, and the metabolic syndrome and also with fatal and nonfatal cardiovascular events (4–7)
Previous studies have shown high prevalence of OSA (16 to 54%) in patients with chronic kidney disease (CKD) (8,9). Unruh et al. (10) showed that OSA is more common in hemodialysis patients than in general population.
Although OSA may contribute to the increased cardiovascular risk seen in Tx patients, consistent information about OSA in patients who have received a kidney transplant (Tx) is scarce. Previously, we found that the prevalence of high risk for OSAS is approximately 30% in both WL and Tx patients (11). A case series indicated that AHI did not change after transplantation in 73% of the patients (12). Conversely, Mallamaci et al. (13) recently reported that 22% of renal Tx recipients had a respiratory disturbance index >5, which was similar to results seen in the general population.
We designed this cross-sectional study to determine the prevalence and clinical correlates of OSA in a large, randomly selected sample of Tx patients using polysomnography. On the basis of our previous findings, we hypothesized that the prevalence of OSA would be similarly high in Tx and WL patients. Finally, we expected to find an increased cardio- and cerebrovascular risk in patients with versus without OSA in the Tx population.
Materials and Methods
Sample of Patients and Data Collection
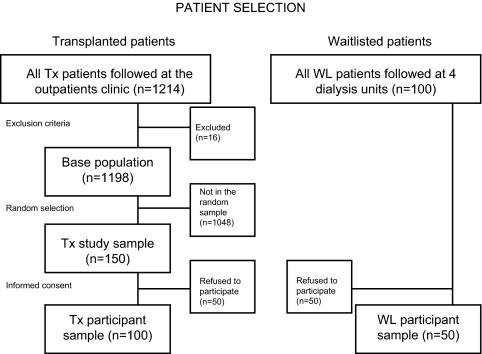
For this study (“SLeep disorders Evaluation in Patients after kidney Transplantation [SLEPT] Study”), potentially eligible patients were selected from all prevalent adult Tx patients (total clinic population; n = 1214) who were regularly followed at a single outpatient transplant center on December 31, 2006. After application of exclusion criteria (previous diagnosis of OSA, recent start (<3 mo) on dialysis or transplantation, active and acute respiratory disorder, acute infection, hospitalization within 1 mo, and surgery within 3 mo), 1198 patients remained (base population; n = 1198). From this base population, we randomly selected and approached 150 patients (Tx study sample) using the simple random sampling strategy offered by SPSS 15.0 (Figure 1).
Figure 1.
Flow chart of the patient selection.
We also asked all (n = 100) eligible dialysis patients who were on the TX waiting list (WL) and were treated at the four largest dialysis centers in Budapest (listed with the previously mentioned transplant center) to participate (WL study sample). Details of medical history, such as age, gender, level of education, tobacco use, and cause and history of CKD, were collected at enrollment.
Laboratory Data
Laboratory data (hemoglobin [Hb], C-reactive protein, serum creatinine, blood urea nitrogen, serum albumin, serum cholesterol, serum triglyceride, and serum HDL and LDL cholesterol) and single-pool Kt/V were extracted from the medical records. Estimated GFR (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) study formula (14).
Comorbidity
Comorbidity was assessed by the modified Charlson Comorbidity Index (CCI) (15,16) completed by the transplant physician who was responsible for the given patient. We also collected data about coronary disease and hypertension from the medical records. Atrial fibrillation was detected by the electrocardiogram recorded during polysomnography.
Polysomnography
Standard, attended overnight polysomnography was performed in our sleep laboratory (SOMNOscreen PSG Tele, SOMNOmedics GmBH, Germany, CE0494). Recordings were manually scored by two somnologists (M.Z.M. and A.S.L.). Sleep stages were determined in 30-s epochs according to Rechtschaffen and Kales (17). Apnea was defined as the absence of airflow for > 10 s; hypopnea was defined as a clearly discernible reduction in airflow for >10 s associated with an arousal and/or reduction in oxygen saturation >3% (18). The AHI was defined as the number of apneas and hypopneas per hour of sleep. Average oxygen saturation was calculated from the oxygen saturation values measured during sleep.
Definition and Classification of OSA
Patients with apnea were separated into three categories of severity: Mild, AHI ≥5 and <15; moderate, AHI ≥15 and <30; and severe, AHI ≥30 (18). Similar to previous publications (19,20), the term “OSA” refers to moderate or severe apnea (AHI ≥15) in this article, unless mentioned otherwise.
Transplantation- and Donor-Related Data and Immunosuppressive Therapy
Transplantation-related information collected included current medications, transplant and dialysis “vintage” (i.e., time elapsed since transplantation or since the initiation of dialysis treatment), time spent on dialysis before transplantation, type of transplantation (deceased-donor or living-donor related), history of cumulative acute rejection, HLA mismatch, titer of pretransplantation panel-reactive antibodies, cold ischemic time, age and gender of donor, and history of delayed graft function. Time elapsed since the initiation of the first treatment for ESRD (cumulative ESRD time) was also calculated. Standard maintenance immunosuppressive therapy generally consisted of prednisolone, either cyclosporin A microemulsion formulation or tacrolimus, combined with mycophenolate mofetil or azathioprine, everolimus, or sirolimus.
Framingham Risk Scores and BP Measurement
The 10-yr coronary heart disease risk was estimated for all Tx patients using the Framingham score (calculated with total cholesterol) (21). Similarly, the 10-yr estimated risk for stroke was calculated according to the modified version of the Framingham Stroke Risk Profile (22).
BP was measured in the clinic three times after 10 min of rest. The average of the three measurements was tabulated. In certain analyses, patients who took three or more antihypertensive drugs were compared with patients who took fewer antihypertensive medications.
Ethical Approval
The study was approved by the Ethics Committee of the Semmelweis University (April 2007). Before enrollment, patients received detailed verbal and written information about the aims and protocol of the study and signed an informed consent.
Statistical Analysis
Statistical analyses were carried out using SPSS 15.0 and STATA 8 software. Results are presented as percentage, mean ± SD, or medians (interquartile range). Continuous variables were compared using t test or the Mann-Whitney U test, and categorical variables were analyzed with χ2 test. Correlation analyses were performed using Pearson or Spearman correlation. Kruskal-Wallis test was used to analyze the relationship between continuous and categorical variables. For multivariate analysis, logistic and negative binomial regressions were applied. Variables were included in the multivariate models on the basis of theoretical considerations and also of the results of the bivariate analyses. Variance influence factors were used to indicate collinearity between independent variables. Only one of those variables showing strong collinearity (e.g., neck and abdominal circumference, body mass index [BMI]) was entered into the multivariate models. In all statistics, two-sided tests were used and the results were considered statistically significant at P < 0.05.
Results
Demographic Data and Baseline Characteristics of the Sample
Of the 250 eligible patients (Tx study sample + WL study sample; see the Materials Methods section); 100 individuals (50 [33%] Tx and 50 [50%] WL) refused to participate. Consequently, the final study population included 100 Tx and 50 WL patients (participants; Figure 1.). Three WL patients were on continuous ambulatory peritoneal dialysis, and 47 WL patients were on hemodialysis. There were no significant differences regarding age and gender between participants and those who refused to participate (data not shown). The basic characteristics (age, gender, eGFR, Hb, and serum albumin) of the 100 participating Tx patients (Tx study sample) were similar to the characteristics of the total clinic population (data not shown). Baseline patient characteristics are shown in Table 1. Most of the variables were similar between the Tx and WL groups (Table 1).
Table 1.
Patient characteristics
| Characteristic | Tx Patients (n = 100) | WL Patients (n = 50) | P |
|---|---|---|---|
| Patients with OSA (%) | 25 | 26 | NS |
| AHI (median [IQR]) | 3.5 (14.3) | 5.6 (14.6) | NS |
| Male (%) | 57 | 54 | NS |
| Age (yr; mean ± SD) | 51 ± 13 | 50 ± 13 | NS |
| Level of education (%) | NS | ||
| primary education or less | 21 | 14 | |
| skilled workers | 15 | 18 | |
| high school or equivalent | 33 | 48 | |
| university diploma | 31 | 20 | |
| Tobacco use (%) | 20 | 20 | NS |
| BMI (kg/m2; mean ± SD) | 27 ± 5 | 25 ± 4 | NS |
| Neck circumference (cm; mean ± SD) | 38 ± 4 | 38 ± 5 | NS |
| Abdominal circumference (cm; mean ± SD) | 98 ± 15 | 95 ± 15 | NS |
| Prevalence of hypertension (%) | 92 | 80 | 0.034 |
| Prevalence of diabetes (%) | 19 | 16 | NS |
| CCI (median [IQR]) | 2 (1) | 3 (3) | 0.001 |
| Hb (g/L; mean ± SD) | 135 ± 17 | 116 ± 15 | <0.001 |
| Serum albumin (g/L; mean ± SD) | 40 ± 3 | 42 ± 4 | 0.011 |
| Serum CRP (mg/L; median [IQR]) | 3.4 (4.5) | 2.2 (7.7) | NS |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 52 ± 19 | N/A | – |
| spKt/V (mean ± SD) | N/A | 1.43 ± 0.24 | – |
| Transplant or dialysis vintage (mo; median [IQR]) | 65.5 (83.0) | 27.0 (42.0) | NA |
| Cumulative ESRD time (mo; median [IQR]) | 101.0 (92.5) | 50.0 (66.0) | <0.001 |
| Hypnotic drugs (%) | 17 | 22 | NS |
| Obstructive apnea index (/h; median [IQR]) | 0.2 (1.4) | 0.6 (1.5) | NS |
| Average oxygen saturation during sleep (%; mean ± SD) | 93.4 ± 2.1 | 93.5 ± 2.6 | NS |
CRP, C-reactive protein; IQR, interquartile range; spKt/V, single-pool Kt/V.
The distribution of the underlying kidney diseases was similar in the two groups, except for the proportion of chronic glomerulonephritis, which was significantly smaller in the Tx group (27 versus 42%; P = 0.048). Eighty-five percent of the Tx patients were taking steroids, 43% were administered cyclosporin A, 71% were on mycophenolate mofetil, 46% patients were administered tacrolimus, and 5% were on azathioprine. Only 1 and 12% of the patients took everolimus and sirolimus, respectively. Six percent of Tx patients had at least one previous transplantation.
Prevalence and Severity of OSA in Tx versus WL Dialysis Patients
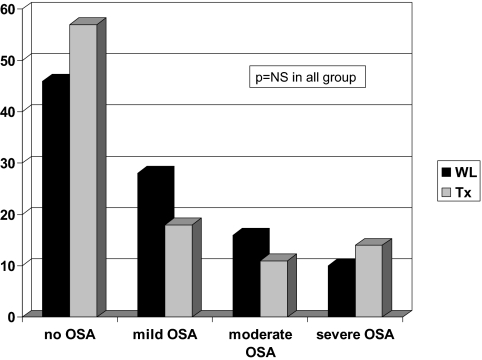
A total of 43% of the Tx and 54% of WL patients had OSA (AHI ≥5; NS). The prevalence of mild, moderate, and severe OSA was similar between the Tx and WL groups: 18, 11, and 14% in the Tx group and 28, 16, and 10% in the WL group, respectively (Figure 2).
Figure 2.
Prevalence and severity of OSA in Tx and WL patients (NS).
Correlates of OSA in the Tx Group
Sociodemographic Characteristics.
The percentage of male patients was significantly higher among patients with versus without OSA in the Tx group. We did not find any association between OSA and the level of education, tobacco use, or age (Table 2). AHI, however, was significantly correlated with age (Table 3).
Table 2.
Characteristics of patients with versus without OSA in the Tx group
| Characteristic | OSA (n = 25) | No OSA (n = 75) | P |
|---|---|---|---|
| Male (%) | 80 | 49 | 0.006 |
| Age (yr; mean ± SD) | 54 ± 12 | 50 ± 13 | NS |
| Level of education (%) | 24 | 20 | NS |
| primary education or less | 24 | 12 | |
| skilled workers | 12 | 40 | |
| high school or equivalent | 40 | 28 | |
| university diploma | |||
| Tobacco use (%) | 20 | 20 | NS |
| BMI (kg/m2; mean ± SD) | 29 ± 5 | 26 ± 5 | 0.005 |
| Neck circumference (cm; mean ± SD) | 40 ± 3 | 37 ± 4 | 0.002 |
| Abdominal circumference (cm; mean ± SD) | 107 ± 12 | 95 ± 15 | 0.001 |
| Prevalence of diabetes (%) | 16 | 20 | NS |
| Prevalence of hypertension (%) | 100 | 89 | 0.091 |
| CCI (median [IQR]) | 2 (0) | 2 (1) | NS |
| Prevalence of coronary heart disease (%) | 8 | 8 | NS |
| Prevalence of congestive heart failure (%) | 8 | 8 | NS |
| Prevalence of peripheral vascular disease (%) | 12 | 12 | NS |
| Prevalence of cerebrovascular disease (%) | 4 | 1 | NS |
| Prevalence of atrial fibrillation (%) | 8 | 1 | NS |
| Hb (g/L; mean ± SD) | 141 ± 17 | 132 ± 16 | 0.018 |
| Serum albumin (g/L; mean ± SD) | 40 ± 4 | 40 ± 3 | NS |
| Serum CRP (mg/L; median [IQR]) | 3.8 (4.3) | 2.8 (4.6) | NS |
| eGFR (ml/min per 1.73 m2; mean ± SD) | 51 ± 18 | 52 ± 19 | NS |
| Transplant vintage (mo; median [IQR]) | 60 (109) | 67 (78) | NS |
| Dialysis vintage (mo; median [IQR]) | 25 (39) | 18 (28) | NS |
| Cumulative ESRD time (mo; median [IQR]) | 117 (147) | 96 (85) | NS |
| Hypnotic drugs (%) | 8 | 20 | NS |
| Average of SBP (mmHg; mean ± SD) | 147 ± 21 | 139 ± 18 | 0.059 |
| Average of DBP (mmHg; mean ± SD) | 85 ± 13 | 83 ± 11 | NS |
| Use of ≥3 antihypertensive drugs (%) | 56 | 31 | 0.022 |
| 10-Yr coronary heart disease risk based on Framingham score, calculation for total cholesterol level (%; median [IQR]) | 14.5 (13.2) | 7.0 (9.0) | 0.008 |
| 10-Yr risk for stroke based on modified version of the Framingham Stroke Risk Profile (%; median [IQR]) | 10.0 (11.2) | 5.0 (5.0) | 0.016 |
| Average oxygen saturation during sleep (%; mean ± SD) | 91.8 ± 1.6 | 94.0 ± 2.0 | <0.001 |
DBP, diastolic BP; SBP, systolic BP.
Table 3.
Correlation between AHI and selected variables in Tx patients
| Parameter | Spearman ρ |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AHI | Abdominal Circumference | Neck Circumference | Age | Hb | BMI | Average Saturation | Stroke Risk | Coronary Heart Disease Risk | |
| AHI | – | ||||||||
| Abdominal circumference | 0.512a | – | |||||||
| Neck circumference | 0.400a | 0.729a | – | ||||||
| Age | 0.338a | 0.388a | 0.173 | – | |||||
| Hb | 0.240b | 0.290a | 0.306a | 0.059 | – | ||||
| BMI | 0.453a | 0.797a | 0.582a | 0.323a | 0.168 | – | |||
| Average saturation | −0.585a | −0.555a | −0.429a | −0.430a | −0.266a | −0.476a | – | ||
| Stroke risk | 0.292a | 0.412a | 0.539a | 0.414a | 0.229b | 0.284a | −0.371a | – | |
| Coronary heart disease risk | 0.404a | 0.640a | 0.540a | 0.667a | 0.205b | 0.467a | −0.449b | 0.728 | – |
P < 0.01.
P < 0.05.
Anthropometric Variables.
Tx patients with versus without OSA had significantly higher BMI and neck and abdominal circumferences (Table 2). The abdominal circumferences in the groups formed by severity of OSA were 92 ± 14 cm in patients without OSA, 103 ± 12 cm in patients with mild OSA, 105 ± 13 cm in patients with moderate OSA, and 108 ± 11 cm in patients with severe OSA (P < 0.001, Kruskal-Wallis test). AHI showed a moderate association with BMI and neck and abdominal circumferences (Table 3).
Comorbidity, Renal Function, and Laboratory Data.
The CCI score was similar in the groups with versus without OSA (Table 2). Tx patients with versus without OSA had similar eGFR (Table 2). Hb was significantly correlated with AHI (Table 3) and was higher in patients with apnea. Serum albumin and serum C-reactive protein levels were similar in the two groups.
Transplantation-Related Data and Medications.
The median transplant vintage, the median dialysis vintage, and cumulative ESRD time all were similar in patients with versus without OSA (Table 2). Donor characteristics (gender, type, and age) and transplantation-related variables (cold ischemic time, cumulative acute rejection rate, panel-reactive antibodies, delayed graft function, and HLA mismatches) were similar in patients with versus without OSA (data not shown). None of the immunosuppressive medications was significantly associated with the presence of OSA (data not shown).
Multivariate Analysis
A negative binomial regression analysis was used to determine the independent associations between AHI and the following variables: Age, gender, albumin, Hb, abdominal circumference, and the use of three or more antihypertensive drugs. In this analysis, age and abdominal circumference were independent predictors of AHI (Table 4).
Table 4.
Negative binomial regression analysis of the predictors of the AHI in Tx patients
| Parameter | IRR | 95% CI | P |
|---|---|---|---|
| Gender | 0.588 | 0.312 to 1.107 | 0.100 |
| Age | 1.031 | 1.009 to 1.054 | 0.006 |
| Albumin | 1.022 | 0.954 to 1.095 | 0.538 |
| Hb | 1.010 | 0.993 to 1.027 | 0.231 |
| Use of ≥3 antihypertensive drugs | 1.094 | 0.622 to 1.925 | 0.755 |
| Abdominal circumference | 1.042 | 1.019 to 1.066 | <0.001 |
CI, confidence interval; IRR, incidence rate ratio.
In a binary logistic regression model (Nagelkerge R2 = 0.231), only abdominal circumference (odds ratio 1.043; 95% confidence interval 1.000 to 1.088; P = 0.05) was independently associated with the presence of moderate to severe OSA after adjustment for the same covariables that were used in the previous multivariable model.
Qualitatively similar results were found when these regression analyses were repeated in the total study population (including both Tx and WL patients). The type of renal replacement therapy (WL versus Tx) was not associated with AHI or with the presence of moderate to severe OSA in these models (data not shown).
Blood Pressure
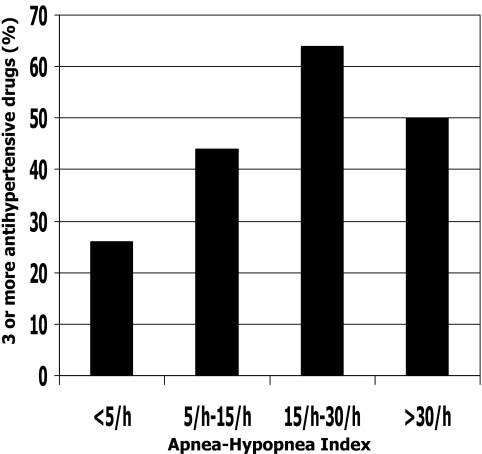
The proportion of Tx patients who were treated with three or more antihypertensive drugs was significantly higher in the OSA group (56 versus 31%; P = 0.022). Moreover, the percentages of patients who were taking three or more antihypertensive drugs were 26, 44, 64, and 50% in the groups with AHI <5/h, AHI ≥5/h and <15/h, AHI ≥15/h and<30/h, and AHI >30/h, respectively (P < 0.05; Figure 3). Despite taking significantly more antihypertensive drugs, the average systolic BP was still higher in patients with versus without OSA (147 ± 21 versus 139 ± 18 mmHg; P = 0.059).
Figure 3.
Association of OSA severity and treatment with three or more antihypertensive drugs (P < 0.05, linear-by-linear association).
Estimated Coronary Heart Disease Risk and Estimated Stroke Risk
In the Tx group, the 10-yr estimated coronary heart disease risk (based on the Framingham score [21]) and the 10-yr estimated stroke risk (based on the modified Framingham stroke risk profile [22]) were twice as high in the patients with versus without OSA (Table 2).
In addition, the average overnight oxygen saturation was inversely associated with both the estimated 10-yr stroke risk (β = −0.196, P = 0.025) and the estimated 10-yr coronary heart disease risk (β = −0.256, P = 0.006) in linear regression models after adjustment for gender, eGFR, and the CCI score.
Discussion
In this study, which enrolling a substantial number of patients, we showed that the prevalence of OSA is similarly high in Tx and WL patients. The condition was very common in the Tx population: One in four patients had moderate or severe OSA.
Previous case studies (23,24) and one recent study (13) suggested that OSA may improve after kidney transplantation. Our previous results (11) and a case series (12), however, suggested that the risk for OSA may not improve substantially after transplantation. We hypothesized, therefore, that the prevalence of OSA would be similar in Tx versus WL patients. The results of this study confirmed our hypothesis.
In this data set, the prevalence of OSA was higher than that published by Mallamaci et al. recently (13). One potential reason for these discordant results is the different method used. Mallamaci et al. report the use of polygraphy and cardiorespiratory recording, whereas we used standard polysomnography with electroencephalogram. Another explanation may be the differences in the study population. Our sample was older and had more patients with diabetes, higher BMI, and somewhat worse mean eGFR.
Several mechanisms may explain the surprisingly high prevalence of OSA in the Tx group. Total body fat (25), abdominal circumference, and BMI may increase after transplantation, which might be associated with increased risk for new-onset OSA. Mallamaci et al. (13) reported the association between respiratory disturbance index and BMI in Tx patients, which is in accordance with our findings. Finally, extracellular water may increase in at least some of the Tx patients (26,27), which may also be associated with increased risk for OSA (28).
Male gender (29), older age (4), several anthropometric parameters (30–32), and also somatic comorbidity (5) are associated with OSA in the general population. We found similar associations in our data set, confirming and also extending the results of Mallamaci et al. (13), except that we did not find any association between the CCI score versus OSA. One potential explanation for this is that the prevalence of comorbid conditions is relatively high in Tx patients compared with the general population. Alternatively, kidney disease– or treatment-specific factors might have modified the association of OSA with these conditions.
Hb was significantly higher in patients with versus without OSA. A potential explanation for this association is the recurrent reduction of oxygen saturation caused by apnea, which stimulates erythropoietin production. Krieger et al. (33) reported a similar association in the general population. Importantly, continuous positive airway pressure treatment decreased the hematocrit level in patients with OSA.
OSA was associated with difficult-to-treat hypertension in our sample. Patients with OSA had higher systolic BP, and the proportion of patients who were prescribed three or more antihypertensive drugs was almost two-fold higher in patients with versus without OSA. The association of OSA and hypertension is well documented in other patient populations (34–36). In fact, a causative relationship between OSA and acute transient increases in nocturnal hypertension has been confirmed in an animal model (37). We propose that the higher BP in Tx patients with OSA may contribute to the high cardiovascular morbidity and mortality of Tx patients.
OSA was also significantly associated with increased estimated risk for cardiovascular and cerebrovascular events. Although the analysis of this problem is confounded by shared risk profiles for OSA and cardiovascular disease (e.g., age, gender, hypertension), these results still might indicate a clinically important aggregation of cardiovascular risk factors. Because OSA is an independent predictor of mortality in the general population (2,3) and overnight hypoxemia is an independent predictor of cardiovascular events in dialysis patients (38), we speculate that the combination of a high Framingham risk score and the presence of OSA might result in an additive increase in cardiovascular risk above and beyond what the shared risk components carry. Prospective trials are needed to confirm the association between OSA and cardiovascular risk in Tx patients and also to reveal whether treatment of OSA reduces cardiovascular morbidity and mortality in this population.
Our study has one of the highest number of Tx patients enrolled in a study in which OSA is diagnosed with polysomnography. We collected a number of important covariables and adjusted for these to make our analysis more reliable. Finally, we enrolled a comparable sample of WL patients and compared the epidemiology of OSA in Tx versus WL patients.
Limitations of this report should also be noted. The cross-sectional design precludes any directional or causal conclusions. Determining sample size was driven mainly by feasibility, and no formal sample size calculations had been done before the study. Post hoc power calculations suggest that this study was powered to detect a difference of 10/h in the AHI between the WL and Tx groups with a power of 90%, but the power is insufficient to detect a smaller difference. Furthermore, the study is not powered to detect a difference in the prevalence of OSA between the two groups, which is <20%.
Patients from a single center were enrolled; therefore, our results are not to be generalized without further considerations. The meaning of the Framingham risk score is confounded by shared risk profiles for OSA and cardiovascular disease. Furthermore, the Framingham risk prediction index might be skewed in patients with CKD. Finally, a substantial proportion of both WL and Tx patients refused to participate. Importantly, we did not find any difference between participants versus nonparticipants; therefore, it is unlikely that refusal introduced a systematic bias that would distort our conclusions significantly. Refusal rate was similar in other studies that used polysomnography in ESRD populations (10,39). We also acknowledge that there is a potentially “unavoidable” selection bias that affects all studies of sleep disorders that are based on polysomnography, such as ours: Only motivated or symptomatic patients accept the stress of polysomnography, whereas “good sleepers” may opt to avoid this test. We cannot exclude the presence of this bias in our study.
Conclusions
This is the first report to present data from a large number of Tx patients to compare the prevalence of OSA between Tx and WL patients. The prevalence of OSA was high in both the Tx and the WL groups. In addition, Tx patients with OSA had higher BP despite taking more antihypertensive medications. We suggest that screening for OSA should be routinely performed, and appropriate treatment should be offered for Tx patients.
Disclosures
None.
Acknowledgments
This study was supported by grants from the National Research Fund (OTKA; TS-049785 and F-68841), the Hungarian Kidney Foundation, and the Foundation for Prevention in Medicine. This article was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (M.N. and M.Z.M.). M.Z.M. was a recipient of the Hungarian Eötvös Scholarship. The research of M.N. has been supported by an unrestricted research grant from Canadian Home Healthcare Inc.
We thank the patients and the staff in the Department of Transplantation and Surgery, Semmelweis University Budapest, and also the patients and the personnel at the four dialysis centers in Budapest for participation and help in this survey. We also thank Csaba P. Kovesdy for critical reading of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM: Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31:1071–1078, 2008 [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall NS, Wong KK, Liu PY, Cullen SRJ, Knuiman MW, Grunstein RR: Sleep Apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep 31:1079–1085, 2008 [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M: Sleep-disordered breathing and neuropsychological deficits: A population-based study. Am J Respir Crit Care Med 156:1813–1819, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Reichmuth KJ, Austin D, Skatrud JB, Young T: Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med 172:1590–1595, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunai A, Keszei AP, Kopp MS, Shapiro CM, Mucsi I, Novak M: Cardiovascular disease and health-care utilization in snorers: A population survey. Sleep 31:411–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V: Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353:2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann U, Becker HF, Birkhahn M, Peter JH, von Wichert P, Schutterle S, Lange H: Sleep-apnea in patients with end-stage renal disease and objective results. Clin Nephrol 53:460–466, 2000 [PubMed] [Google Scholar]
- 9.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S: Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung 184:43–49, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, Sharief I, Punjabi NM, Newman AB: Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol 17:3503–3509, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Molnar MZ, Szentkiralyi A, Lindner A, Czira ME, Szabo A, Mucsi I, Novak M: High prevalence of patients with a high risk for obstructive sleep apnoea syndrome after kidney transplantation: Association with declining renal function. Nephrol Dial Transplant 22:2686–2692, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ: Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol Dial Transplant 22:3028–3033, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Mallamaci F, Leonardis D, Tripepi R, Parlongo G, Catalano C, Tripepi G, Castronovo V, Ferini-Strambi L, Zoccali C: Sleep disordered breathing in renal transplant patients. Am J Transplant 1373–1381, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS GT, Kusek JW, Beck GJ, Group MS: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Jassal SV, Schaubel DE, Fenton SS: Baseline comorbidity in kidney transplant recipients: A comparison of comorbidity indices. Am J Kidney Dis 46:136–142, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A: A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects, Los Angeles, University of California at Los Angeles, Brain Information Service/Brain Research Institute, 1968 [Google Scholar]
- 18.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689, 1999 [PubMed] [Google Scholar]
- 19.Nakashima H, Katayama T, Takagi C, Amenomori K, Ishizaki M, Honda Y, Suzuki S: Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J 27:2317–2322, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hanly PJ, Pierratos A: Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 344:102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB: Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke 25:40–43, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Auckley DH, Schmidt-Nowara W, Brown LK: Reversal of sleep apnea hypopnea syndrome in end-stage renal disease after kidney transplantation. Am J Kidney Dis 34:739–744, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Langevin B, Fouque D, Leger P, Robert D: Sleep apnea syndrome and end-stage renal disease: Cure after renal transplantation. Chest 103:1330–1335, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Dolgos S, Hartmann A, Jenssen T, Isaksen GA, Pfeffer P, Bollerslev J: Determinants of short-term changes in body composition following renal transplantation. Scand J Urol Nephrol 43:76–83, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Wong HS, Boey LM, Morad Z: Body composition by bioelectrical impedance analysis in renal transplant recipients. Transplant Proc 36:2186–2187, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Coroas AS, Oliveira JG, Sampaio S, Borges C, Tavares I, Pestana M, Almeida MD: Body composition assessed by impedance changes very early with declining renal graft function. Nephron Physiol 104:115–120, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL, Ip MS, Lai KN: Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol 4:410–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S: The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep 24:96–105, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Hoffstein V, Mateika S: Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J 5:377–381, 1992 [PubMed] [Google Scholar]
- 31.Lam JC, Lam B, Lam CL, Fong D, Wang JK, Tse HF, Lam KS, Ip MS: Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 100:980–987, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM: Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med 162:893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Krieger J, Sforza E, Delanoe C, Petiau C: Decrease in haematocrit with continuous positive airway pressure treatment in obstructive sleep apnoea patients. Eur Respir J 5:228–233, 1992 [PubMed] [Google Scholar]
- 34.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD: High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 19:2271–2277, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Bazzano LA, Khan Z, Reynolds K, He J: Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 50:417–423, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Peppard PE, Young T, Palta M, Skatrud J: Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA: Obstructive sleep apnea as a cause of systemic hypertension: Evidence from a canine model. J Clin Invest 99:106–109, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 13:729–733, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, Budhiraja R, Punjabi NM, Buysse D, Newman AB: Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the sleep heart health study. Am J Kidney Dis 52: 305–313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]