Abstract
Background and objectives: Hemoglobin (Hb) is the main carrier and buffer of nitric oxide. Evidence has been produced that Hb concentration is inversely related with endothelial function in human diseases. Testing whether this association exists also in diabetic patients stage 1 to 2 chronic kidney disease (CKD) is important because anemia in these patients starts at an earlier stage than in other renal diseases. The relationship was investigated between Hb and flow-mediated dilation (FMD) levels of the patients with diabetic nephropathy in a cross-sectional design.
Design, setting, participants, & measurements: Eighty-nine diabetics with mild to moderate proteinuria and normal to mildly reduced GFR who were normotensive, nondyslipidemic, and cardiovascular-events free were enrolled. None of the patients was taking metformin or renin-angiotensin system blockers.
Results: FMD was inversely related with Hb levels. Furthermore, there was an inverse link between proteinuria and FMD. However, further analysis of this association showed that the FMD-proteinuria link was confined to patients with proteinuria exceeding 150 mg/d, while no such association existed in patients with proteinuria <150 mg/d. Adjustment of the Hb-FMD relationship for pertinent Framingham risk factors, proteinuria, homeostasis model assessment (HOMA) index, and GFR levels had a modest influence on this association, which remained significant.
Conclusions: Endothelial function is inversely associated with Hb levels in diabetic patients with stage 1 to 2 CKD, and proteinuria is an effect modifier of this association. Overall, the observations of this study generate the hypothesis that proteinuria exposes a situation wherein Hb may limit the endothelium-mediated vasoregulation in diabetes.
Anemia is a common feature of advanced chronic kidney disease (CKD). Even though this complication is multifactorial in nature, deficiency of erythropoietin appears as the major factor responsible for low hemoglobin (Hb) levels in CKD patients. Treatment with erythropoiesis stimulating agents (ESA) has been a landmark achievement of modern nephrology, and this treatment is of unquestionable benefit in CKD patients with severe anemia. However, recent studies have shown that full anemia correction in stage 3 to 4 CKD patients may be harmful (1,2). Such an effect is currently attributed to the deleterious action of high doses of erythropoietin imposed by erythropoietin resistance in patients with systemic illness and inflammation (3). Even though a recent secondary analysis of one of these studies (4) offers solid support to this hypothesis, other mechanisms also may be at play (3). Because Hb is a well recognized carrier and buffer of nitric oxide (NO), which modulates the bioavailability of this compound at tissue level (5), disturbed regulation of endothelial function secondary to changes in Hb concentration appears to be a relevant pathophysiologic pathway whereby changes in red cell mass may exert adverse cardiovascular events (6). These biochemical effects may be of clinical relevance in type 2 diabetes. In a concerted effort by two leading European institutions in diabetes research, a strong inverse association between Hb and the vasodilatory response to acetylcholine (an endothelium-dependent phenomenon) was observed in these patients (7). This seminal study, which focused on patients without evidence of significant renal disease, included smokers and patients with New York Heart Association class I and II heart failure that were being treated with metformin or rosiglitazone or cardiovascular drugs, all factors that may disturb the interpretation of the Hb-endothelial function link. Testing whether this association exists also in diabetics with stage 1 to 2 CKD is important because in this condition anemia may antedate microalbuminuria and the GFR decline (8) and because it was claimed that treatment of anemia with ESA should be started early in diabetics with CKD (9,10). This claim contrasts with findings in a recent study (11) showing that normal Hb levels are associated with more pronounced endothelial function impairment in nondiabetic patients with moderate to severe CKD. However, the inverse link between Hb and endothelial function still needs to be specifically confirmed in type 2 diabetics with early nephropathy. With this background in mind, we investigated the relationship between Hb and the forearm blood flow mediated vasodilatory (FMD) response to ischemia in a sizable group of a well selected group of untreated, normotensive, nonobese, nonsmoker, nondyslipidemic, and cardiovascular events-free type 2 diabetics with stage 1 to 2 CKD.
Materials and Methods
Patients
The study group was selected from an overall population of 437 patients with type 2 diabetes referred to the nephrology and endocrinology outpatient clinics of Gülhane School of Medicine with stage 1 CKD (GFR ≥90 ml/min/1.73 m2 and kidney damage) or stage 2 CKD (GFR 0.89 to 60 ml/min/1.73 m2 and evidence of kidney damage). Among these patients, we selected 89 normotensive, nonobese, nonsmoker, nondyslipidemic, and cardiovascular events-free type 2 diabetics who had never received drugs interfering with the renin-angiotensin system nor metformin, i.e., agents that are commonly prescribed to diabetics that exert a direct effect on endothelial function. In detail, the exclusion criteria were as follows: systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg), active smoker, BMI >30 kg/m2, coronary heart disease (history of cardiovascular disease and/or past revascularization procedures and/or presence of ischemic ST-T alterations and voltage criteria for LVH on electrocardiogram), elevated liver enzymes (AST or ALT levels ≥40 U/L), dyslipidemia (total cholesterol >200 mg/dl), and the administration of one or more of the drugs indicated above. The presumed duration of diabetes mellitus in these cases was 52 ± 11 mo. All patients were on dietary treatment and/or on treatment with sulfonylureas, but none were being treated by insulin or other glucose-lowering medication. In detail, 82 of the patients were classified as having stage 1 CKD, and seven of the patients were classified as stage 2 CKD. Forty-two of these patients had minimal or no proteinuria, i.e., a 24-h protein excretion rate ≤150 mg (% 64 male gender, 50 ± 6 yr) and 47 patients had proteinuria (≥150 mg/d): 53% male gender, 50 ± 5 yr). Subjects underwent standard physical examination, chest x-ray, baseline electrocardiogram, two-dimensional echocardiography, and routine clinical laboratory tests, including liver and kidney function tests and 24-h urinary protein measurements.
All subjects gave informed consent for participating in the study. The ethics committee of Gülhane School of Medicine approved the study.
Arterial BP (the average value of three measurements) and venous blood sampling were performed in the morning hours, always after a 10- to 15-min resting period. In addition to standard routine biochemical tests, we measured HbA1C, C-reactive protein (CRP), and plasma insulin. To increase the precision of proteinuria estimates, 24-h urine collection was performed three times, and three 24-h proteinuria measurements on average were taken as representative of each participant's 24-h protein excretion rate.
Laboratory Procedures
Fasting plasma glucose (FPG), blood urea, serum creatinine (by the Jaffé method), total protein, serum albumin, total cholesterol, HDL cholesterol, and triglycerides were determined with the Olympus AU 600 autoanalyzer using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). Measurement of serum albumin is by using a dye-binding technique using bromocresol green (BCG). LDL cholesterol was calculated by Friedewald's formula (12). HbA1c was measured by inhibition of latex agglutination, using a DCA 2000 analyzer (Bayer, Elkhart, IN). Proteinuria was determined by a turbidimetric test with TCA (TCA). The serum basal insulin value was determined by the coated tube method (DPC-USA). In particular, an insulin resistance score (HOMA-IR) was computed with the formula: (HOMA-IR) = FPG (mg/dl) × immunoreactive insulin (IRI) (μIU/ml)/405 (13).
Patients were classified with respect to eGFR calculated according to the simplified version of the Modification of Diet in Renal Disease (MDRD) formula as defined by Levey et al. (14) (GFR = 186 × Pcr–1.154 × age–0.203 × 1.212 [if black] times 0.742 [if female]).
Serum CRP was measured by a high-sensitivity assay. The detection interval for CRP is 0.05 to 35 mg/L. Serum samples were diluted with a ratio of 1/101 with the diluent solution. Calibrators, kit controls, and serum samples were all added on each micro well with an incubation period of 30 min. After three washing intervals, 100 μl enzyme conjugate (peroxidase labeled anti-CRP) was added on each micro well for an additional 15-min incubation at room temperature in the dark. The reaction was stopped with a stop solution, and photometric measurement was performed at the 450 nm wavelength. The amount of serum samples was calculated as mg/L with a graphic that was made by noting the absorbance levels of the calibrators.
Vascular Assessment
FMD of the brachial artery was assessed noninvasively, using high-resolution ultrasound as described by Celermajer et al. (15). Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA) with a 12-Mhz probe. All vasoactive medications were withheld for 24 h before the procedure. The subjects remained at rest in the supine position for at least 15 min before the examination started. Subject's arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single 2-D frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. The maximum FMD dilation diameters were calculated as the average of the three consecutive maximum diameter measurements. The FMD were then calculated as the percent change in diameter compared with baseline resting diameters.
Statistical Analyses
All the statistical analyses were performed by using SPSS 11.0 (SPSS Inc., Chicago, IL) statistical package. Results are reported as the mean ± SD. Non-normally distributed data are summarized in terms of median and interquartile values. The Levene's test was used to evaluate the distribution characteristics of variables. Differences between the groups were tested for significance by Mann-Whitney U test and chi-square test, as appropriate. The relationship between variables was analyzed by Pearson's correlation. Relationships between study variables were tested by univariate and multivariate regression analysis. For multivariate analysis, we followed a hierarchical approach considering a series of major factors that may impact endothelial function in CKD patients (16), that is Framingham risk factors (age, gender, BP, cholesterol), the BMI, insulin sensitivity (HOMA), and proteinuria. To prevent multicolinearity problems, CRP was not included into the multiple regression analysis because it was highly intercorrelated with proteinuria. Differences and correlations were considered significant at P < 0.05.
Results
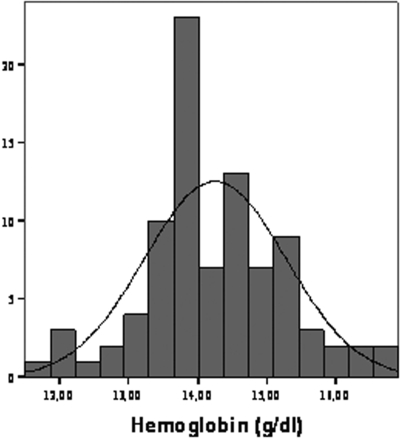
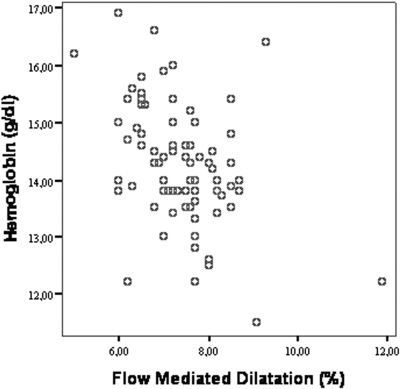
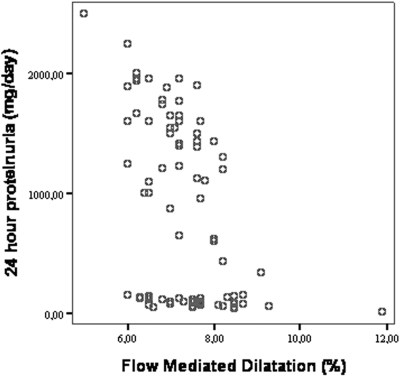
Overall, the distribution of Hb values in the study population was Gaussian and the prevalence of anemia (Hb <13 g/dl in male gender and <11.5 g/dl in female gender) was 9.6% and 0% (Figure 1). The demographic and clinical characteristics of the study population categorized in terms of Hb tertiles are reported in Table 1. Patients with Hb levels in the third tertile did not differ from those of the other tertiles as for age, gender, BMI, BP, serum glucose, insulin, HOMA index, HbA1c, estimated duration of diabetes, and GFR. The proportion of overweight patients (BMI ≥25<30 kg/m2) was similar in the three groups. However, patients in the third tertile exhibited higher hsCRP and a tendency for a lower GFR. In general, correlation analysis confirmed these associations and, in addition, showed significant associations between Hb and the GFR (direct association) and proteinuria (inverse association) (Table 1, last column). The hsCRP was related directly with proteinuria (r = 0.77, P < 0.001) and inversely with FMD (r = −0.48, P < 0.001). Of note, FMD was significantly less in patients in the third tertile than in those in the second and the first Hb tertile, an association that remained consistent and highly significant in linear regression analysis with Hb and FMD as continuous variables (Figure 2). Furthermore, the relationship between Hb and FMD held true also in an analysis considering only patients in the subgroup with GFR >90 ml/min (r = 0.40, P = 0.009). Notably, there was an overall inverse relationship between proteinuria and FMD (r = −0.47, P < 0.001). However, further analysis of this association showed that the FMD-proteinuria link was confined to patients with proteinuria exceeding 150 mg/24 h (r = −0.63, P < 0.001), while no such an association existed in patients with proteinuria below 150 mg/24 h (r = −0.186, P = 0.06) (Figure 3).
Figure 1.
The distribution hemoglobin (Hb) values in the study population.
Table 1.
Demographic, hemodynamic, and biochemical data in diabetic patients as categorized according to the hemoglobin tertiles and the correlations between the FMD and the other parameters
| All subjects (n = 89) | HbT1 (11.50 to 13.80 g/dl) (n = 34) | HbT2 (13.90 to 14.60 g/dl) (n = 28) | HbT3 (14.70 to 16.90 g/dl) (n = 27) | Pa | Correlations Pearson's r; Spearman's r(rS) | |
|---|---|---|---|---|---|---|
| Age (years) | 50 ± 5 | 50 ± 5 | 51 ± 6 | 49 ± 5 | 0.52 | r = −0.02, P = 0.85; rS = −0.01, P = 0.98 |
| Male gender, n (%) | 52 (58%) | 18 (53%) | 17 (61%) | 17 (63%) | 0.71 | r = −0.01, P = 0.94; rS = −0.02, P = 0.94 |
| BMI (kg/m2) | 26.9 ± 2.1 | 27.3 ± 2.2 | 26.6 ± 2.3 | 26.8 ± 1.8 | 0.41 | r = 0.05, P = 0.67; rS = 0.11, P = 0.31 |
| Systolic blood pressure (mmHg) | 131 ± 13 | 130 ± 8 | 130 ± 7 | 129 ± 8 | 0.78 | r = −0.18, P = 0.08; rS = −0.08, P = 0.45 |
| Diastolic blood pressure (mmHg) | 84 ± 4 | 84 ± 4 | 85 ± 3 | 83 ± 3 | 0.14 | r = 0.07, P = 0.50; rS = 0.01, P = 0.92 |
| Total cholesterol (mg/dl) | 173 ± 17 | 172 ± 18 | 175 ± 17 | 172 ± 16 | 0.76 | r = 0.14, P = 0.18; rS = 0.08, P = 0.46 |
| Glucose (mg/dl) | 137 ± 24 | 138 ± 24 | 140 ± 27 | 134 ± 23 | 0.71 | r = 0.05, P = 0.64; rS = 0.02, P = 0.88 |
| Insulin (UI/L) | 11.6 ± 4.9 | 12.4 ± 5.1 | 10.6 ± 3.9 | 11.8 ± 5.6 | 0.34 | r = −0.02, P = 0.89; rS = 0.02, P = 0.85 |
| HOMA index | 3.9 ± 1.6 | 4.2 ± 1.7 | 3.6 ± 1.3 | 3.8 ± 1.7 | 0.35 | r = 0.01, P = 0.92; rS = 0.05, P = 0.63 |
| HbA1c (%) | 8.5 ± 1.6 | 8.6 ± 1.9 | 8.4 ± 1.5 | 8.6 ± 1.4 | 0.93 | r = −0.04, P = 0.74; rS = −0.04, P = 0.75 |
| Uric acid (g/dl) | 4.8 ± 0.8 | 4.7 ± 0.9 | 4.8 ± 0.8 | 4.9 ± 0.9 | 0.77 | r = −0.16, P = 0.15; rS = −0.23, P = 0.03 |
| Duration of diabetes (months) | 52 ± 11 | 53 ± 11 | 51 ± 10 | 52 ± 10 | 0.86 | r = −0.13, P = 0.23; rS = −0.16, P = 0.13 |
| hsCRP (mg/L) | 9 (3 to 24) | 8 (4 to 20) | 8.5 (3 to 21) | 11 (4 to 24) | 0.04 | r = −0.48, P <0.001; rS = −0.53, P <0.001 |
| GFR (ml/min/1.73 m2) | 107 ± 9 | 105 ± 11 | 110 ± 7 | 105 ± 11 | 0.07 | r = 0.23, P = 0.03; rS = 0.18, P = 0.09 |
| 24 h proteinuria (mg/d) | 590 (10 to 2500) | 380 (10 to 1960) | 378 (30 to 1900) | 1000 (40 to 2500) | 0.60 | r = −0.47, P <0.001; rS = −0.52, P <0.001 |
| FMD (%) | 7.4 ± 0.9 | 7.7 ± 1.0 | 7.5 ± 0.7 | 6.8 ± 0.9 | <0.001 |
The comparison between the groups is performed by ANOVA. The data are presented as mean ± SD or percentage, where appropriate.
To convert total cholesterol in mg/dl to mmol/L, multiply by 0.02586; plasma glucose in mg/dl into mmol/L, multiply by 0.05551; eGFR in ml/min to ml/s, multiply by 0.01667; Hb and uric acid in g/dl into mmol/L, multiply by 10. HOMA, homeostasis model assessment; Hb, hemoglobin; FMD, flow-mediated dilation; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein.
Figure 2.
Scatter plot showing the correlation between flow-mediated dilation (FMD) and Hb in diabetic patients.
Figure 3.
Scatter plot showing the correlation between FMD and 24 h proteinuria in diabetic patients.
Multivariate Analyses
To further characterize the independent contribution of Hb to the FMD, we constructed a series of multiple regression models based on traditional and nontraditional risk factors impacting upon this response. In the unadjusted analysis (Table 2), the Hb and FMD levels were intercorrelated (β = −0.44, P < 0.001). Adjustment for pertinent Framingham risk factors did not influence the strength of the association. Further adjustment for proteinuria, HOMA index, and GFR levels had a modest influence on the strength of the association between Hb and FMD, which remained highly significant.
Table 2.
Multiple regression models of FMD in diabetic patients
| Unadjusted (β, P) | Model 1 (β, P) | Model 2 (β, P) | |
|---|---|---|---|
| Hemoglobin (g/dl) | −0.44 (<0.001) | −0.44 (<0.001) | −0.35 (<0.001) |
| Age (years) | −0.04 (0.69) | 0.01 (0.94) | |
| Gender (M/F) | −0.03 (0.81) | 0.02 (0.84) | |
| BMI (kg/m2) | 0.02 (0.89) | 0.02 (0.88) | |
| Systolic blood pressure (mmHg) | −0.20 (0.07) | −0.20 (0.02) | |
| Total cholesterol (mg/dl) | 0.14 (0.20) | 0.09 (0.43) | |
| HOMA index | 0.03 (0.78) | ||
| 24-h proteinuria (mg/d) | −0.102 (0.35) | ||
| Proteinuria × Hb | −0.43 (<0.001) |
To convert total cholesterol in mg/dl to mmol/L, multiply by 0.02586; Hb in g/dl into mmol/L, multiply by 10.
Of note, there was an interaction between Hb and proteinuria in the explanation of the variability of FMD (Table 2). No additional interactions were detected among the remaining risk factors. Due to the high intercorrelation between proteinuria and CRP, to prevent collinearity and overfitting problems, we did not enter CRP into the final model. However, in a model excluding proteinuria and including CRP, Hb maintained the independent link with FMD (β = −0.36, P = 0.01).
Discussion
This study shows that in uncomplicated, erythropoietin naive, type 2 diabetics with stage 1 to 2 CKD, hemoglobin levels are independently associated with endothelial function as assessed by FMD. Furthermore, proteinuria appears to be an effect modifier for the Hb-FMD relationship, suggesting that the interference of relatively higher Hb levels with endothelial function becomes more pronounced at higher levels of proteinuria.
The median Hb concentration in this study was 14 g/dl and the distribution of Hb values covered a wide range going from mild anemia to mild polycitemia (see Figure 1). Only a minority of male patients (about 10%) exhibited anemia as defined by current guidelines. The frequency of anemia in this series is of the same order of magnitude of that reported in a smaller series of type 2 diabetics without microalbuminuria and with normal GFR (8). Our study population was composed of untreated, uncomplicated, overweight (but not frankly obese) people with clear-cut insulin resistance, mild to moderate hyperglycemia, overt proteinuria, and normal or mildly reduced GFR, i.e., subjects with early type 2 diabetic nephropathy. Focusing on this population is important because it may provide information on whether the inverse link between Hb and endothelial function is influenced by renal dysfunction and may allow a better assessment on whether this link is independent of coexistent cardiovascular comorbidities and other risk factors. In a previous study, Natali (7) found a strong, inverse link between Hb and the forearm blood flow response to acetylcholine across all tested doses of this NO synthase stimulant. Patients in the Natali study were being treated with antihypertensive drugs and included cases with stage 1 to 2 heart failure and no detailed information on proteinuria, and the GFR was provided in this series, thus leaving open the question whether diabetic nephropathy modifies the Hb-FMD link. Interestingly, in the present series we found that proteinuria interacts with Hb in explaining the variability in the FMD response to ischemia. In fact, FMD was unrelated with Hb in patients without proteinuria (i.e., with urinary protein <150 mg/24 h), while this relationship appeared to be strong and inverse in those with frank proteinuria (urinary protein >150 mg/24 h). Both in the Natali study (7) and in a more recent study in essential hypertensives with an average GFR (average 84 ml/min) lower than type 2 diabetics in the present study (107 ml/min), Hb and the GFR were independently associated with FMD, with an additive effect but no interaction (17). Our findings for the first time show that frank proteinuria exposes a situation wherein Hb may limit the endothelium-mediated vasoregulation in type 2 diabetes. Proteinuria is an established determinant of disturbed endothelial function in patients with CKD (18,19). The endogenous inhibitor of NO synthase asymmetric dimethyl arginine (ADMA) is strictly associated with proteinuria in CKD (20) and is a strong marker of endothelial dysfunction in this population. Thus proteinuria underlies reduced NO bioavailability in CKD patients. Recent studies have shown that nitrosothiol (SNO) derivatives, including S-nitrosylated Hb, are among the most potent vasodilators known. These compounds form preferentially in the oxygenated Hb structure (SNO-Hb), whereas low pO2 favors release of NO groups, thus making Hb a carrier of systemic NO to peripheral tissues (6). Evidence has been produced that release of NO by erythrocytes may be impaired in subjects with diabetes because glycosylation of Hb hinders NO release and shifts the equilibrium among NO species toward irreversible compounds (heme-iron nitrosyl adduct with deoxy-Hb), which cannot release NO (21). It is therefore plausible that proteinuria, a factor which may per se reduce NO bioavailability, is a critical factor in the disturbed NO-dependent vasoregulation in type 2 diabetics. In this study, we did not measure endogenous inhibitors of NO system. However, given the strong link we previously described between ADMA and proteinuria in nondiabetic stage 1 CKD patients (20), this methylarginine may be a likely mediator of the Hb-proteinuria interaction as related to FMD. This possibility is in line with the hypothesis that red blood cells may participate in the regulation of the NO system by modulating ADMA levels (22).
This cross-sectional study has limitations. First, we cannot infer the direction of causality implied by the Hb-FMD relationship and therefore our data should be considered as hypothesis generating rather than as hypothesis testing. In this regard it is relevant to note that the endothelial NO-synthase null mouse, i.e., an animal model with severe systemic endothelial dysfunction, does not develop policytemia, an observation that indicates high hemoglobin as the trigger, rather than the result, of endothelial dysfunction (23). If so, findings in this study would suggest that it is highly unlikely that early treatment of anemia in type 2 diabetics may translate into favorable vascular effects. Second, our observations were made in a selected population of subjects with early type 2 diabetic nephropathy without overt cardiovascular disease, and therefore additional studies are needed to further explore the proteinuria-Hb interaction in diabetics with advanced nephropathy and with simultaneous cardiovascular complications.
In conclusion, in diabetic patients with stage 1 to 2 CKD, endothelial function is inversely associated with hemoglobin levels, and proteinuria is an effect modifier of this association. Overall, our observations generate the hypothesis that frank proteinuria exposes a situation wherein Hb may limit the endothelium-mediated vasoregulation in type 2 diabetes. Further studies are warranted to test the validity of this hypothesis not only in the diabetic nephropathy, but also in the other micro- and macro-vascular complications of diabetes mellitus.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Berns JS, Fishbane S: CHOIR, CREATE, and anemia treatment in patients with CKD. Semin Dial 20: 277–279, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Levin A: Understanding recent haemoglobin trials in CKD: Methods and lesson learned from CREATE and CHOIR. Nephrol Dial Transplant 22: 309–312, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Vaziri ND: Anemia and anemia correction: Surrogate markers or causes of morbidity in chronic kidney disease? Nat Clin Pract Nephrol 4: 436–445, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia L, Bonaventura C, Bonaventura J, Stamler JS: S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Singel DJ, Stamler JS: Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Natali A, Toschi E, Baldeweg S, Casolaro A, Baldi S, Sironi AM, Yudkin JS, Ferrannini E: Haematocrit, type 2 diabetes, and endothelium-dependent vasodilatation of resistance vessels. Eur Heart J 26: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Craig KJ, Williams JD, Riley SG, Smith H, Owens DR, Worthing D, Cavill I, Phillips AO: Anemia and diabetes in the absence of nephropathy. Diabetes Care 28: 1118–1123, 2005 [DOI] [PubMed] [Google Scholar]
- 9.van Dijk DJ, Boner G: The treatment of patients with advanced renal disease. In: Management of Diabetic Nephropathy, edited by Boner G, Cooper ME.Informa Health Care, 2003, pp 189–198 [Google Scholar]
- 10.Rossert J, Fouqueray B: Screening and management of patients with early chronic kidney disease. Acta Diabetol 41[Suppl 1]:S6–S12, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz MI, Sonmez A, Saglam M, Gulec M, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: Hemoglobin is inversely related with flow-mediated dilatation in chronic kidney disease. Kidney Int 75: 1316–1321, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, Deanfield JE: Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 22: 854–858, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C: Endothelial dysfunction in CKD: A new player in town? Nephrol Dial Transplant 23: 783–785, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Perticone F, Maio R, Mastroianni S, Greco L, Tripepi G, Mallamaci F, Zoccali C: Haemoglobin and endothelium-dependent vasodilation in uncomplicated, untreated subjects with essential hypertension (TH-PO251) [Abstract]. J Am Soc Nephrol 19 [Suppl]:165A, 2008 [Google Scholar]
- 18.Joles JA, Stroes ES, Rabelink TJ: Endothelial function in proteinuric renal disease. Kidney Int Suppl 71: S57–S61, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C: The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Caglar K, Yilmaz MI, Sonmez A, Cakir E, Kaya A, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Bilgi C, Oktenli C, Vural A, Zoccali C: ADMA, proteinuria, and insulin resistance in non-diabetic stage I chronic kidney disease. Kidney Int 70: 781–787, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Milsom AB, Jones CJ, Goodfellow J, Frenneaux MP, Peters JR, James PE: Abnormal metabolic fate of nitric oxide in Type I diabetes mellitus. Diabetologia 45: 1515–1522, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Billecke SS, Kitzmiller LA, Northrup JJ, Whitesall SE, Kimoto M, Hinz AV, D'Alecy LG: Contribution of whole blood to the control of plasma asymmetrical dimethylarginine. Am J Physiol Heart Circ Physiol 291: H1788–H1796, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC: Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]