Abstract
Background and objectives: Fibroblast growth factor-23 (FGF23) levels are elevated in ESRD and have been associated with adverse outcomes. The effects of various treatments for secondary hyperparathyroidism on FGF23 levels in ESRD have not been examined in a clinical trial.
Design, setting, participants, & measurements: We assessed intact FGF23 levels in 91 subjects over the course of the ACHIEVE trial, which was designed to compare escalating doses of Cinacalcet plus fixed low-dose calcitriol analogs (Cinaclcet-D) with titration of calcitriol analogs alone (Flex-D) to suppress parathyroid hormone. Between-group and within-group changes in log-transformed FGF23 levels were analyzed. Factors associated with change in FGF23 were assessed using a multiple regression model.
Results: Intact FGF23 levels were markedly elevated in subjects at baseline. A statistically significant difference in percent change in log FGF23 levels was observed between treatment groups (P < 0.002). The Cinacalcet-D group had a significant decrease in percent change in log FGF23 levels (corrected P = 0.021), whereas FGF23 levels trended toward an increase in the Flex-D group. Change in FGF23 level was significantly associated with changes in levels of phosphate (P < 0.0001) and calcium (P = 0.0002) but not parathyroid hormone.
Conclusions: Treatment with Cinacalcet plus low-dose calcitriol analogs results in lower FGF23 levels compared with a treatment regimen using calcitriol analogs alone in ESRD. The mechanisms underlying the differential effects of these treatment regimens on FGF23 levels and the clinical impact of these changes on FGF23 remain to be defined.
Fibroblast growth factor-23 (FGF23) is a circulating factor that plays an important role in the regulation of phosphate and vitamin D homeostasis (1–3). FGF23 is predominately secreted by osteocytes (4) and targets a number of tissues through FGF receptor–Klotho complexes in cell membranes. The kidney is the major target for FGF23, where this phosphaturic and vitamin D–regulating hormone increases renal phosphate (P) excretion through inhibition of sodium-dependent phosphate reabsorption and decreases 1,25(OH)2D production by inhibiting 1α-hydroxylase and by stimulating 24-hydroxylase activity in the proximal tubule (2,5–8). FGF23 also targets other tissues expressing FGFR:Klotho, including the parathyroid and pituitary glands and the choroid plexus. In hemodialysis (HD) patients, circulating FGF23 levels are typically several orders of magnitude greater than in individuals with intact renal function (9–11). Although the clinical significance of FGF23 in the absence of functioning kidneys is not fully understood, several studies have implicated elevated FGF23 levels with refractory hyperparathyroidism (10), progression of renal failure (12), left ventricular hypertrophy (13), vascular calcification (14), and increased mortality (15).
Given the emerging importance of FGF23 in disordered mineral homeostasis, it is important to know how treatments for secondary hyperparathyroidism (SHPT) affect FGF23 levels. In some, but not all, clinical studies, FGF23 levels have been strongly and independently correlated with levels of P (16,17), calcium (Ca) × P product (17), and parathyroid hormone (PTH) (16,17), suggesting the possibility that SHPT treatments that alter circulating levels of Ca, P, and PTH may affect FGF23 levels. Additionally, use of calcitriol analogs is associated with increased FGF23 levels in humans with ESRD (10,18), consistent with observations that calcitriol increases FGF23 gene transcription (8). Cinacalcet, an allosteric modulator of the calcium sensing receptor (19), was introduced several years ago as a calcitriol analog-sparing therapy for SHPT (20–24). However, the effect of Cinacalcet on FGF23 levels in a clinical trial of HD patients has not been studied.
To assess the effects of treatment with Cinacalcet and calcitriol analogs on circulating FGF23 levels in ESRD patients, we measured FGF23 levels in participants of the ACHIEVE study, a randomized controlled clinical trial of HD patients with SHPT designed to test whether treatment with Cinacalcet plus low-dose calcitriol analogs (Cinacalcet-D) would compare with treatment with calcitriol analogs alone in (Flex-D) in attainment of National Kidney Foundation K/DOQI mineral metabolic parameters (25). In this secondary analysis, we used prospectively collected samples from a subset of participants to study change in FGF23 levels in response to the two treatment strategies.
Materials and Methods
Subjects
Subjects in this study were a subset of the participants of ACHIEVE, a phase 4, open-label, placebo-controlled, multicenter randomized controlled trial. Subject enrollment for ACHIEVE has been described elsewhere (25); in brief, subjects were eligible if they had been receiving some form of calcitriol analog on HD for at least 3 mo. Intact PTH had to be between 150 and 800 pg/ml within the previous 6 mo, with “postwashout” PTH >300 pg/ml. The subjects were randomized to receive either escalating doses of Cinacalcet and fixed low doses of paricalcitol or doxercalciferol (Cinacalcet-D) or escalating doses of paracalcitriol or doxercalciferol (Flex-D).
Subjects in this study consisted of individuals enrolled in ACHIEVE in whom plasma for FGF23 levels was successfully collected prospectively at multiple time points. The entire ACHIEVE study sample was unable to be used because (1) some study sites did not receive timely institutional review board approval to collect samples for FGF23, (2) some sites closed enrollment before institutional review board approval for FGF23 sampling, (3) some subjects withdrew from the original ACHIEVE trial (amounting to ∼25% of the participants), and (4) some subjects did not have samples properly collected by their study sites. Subjects in this study therefore represented approximately one half of the original ACHIEVE sample.
The study was conducted in accordance with the principles of the Declaration of Helsinki. Approval for participation was obtained from participating sites and the University of Kansas Medical Center.
Experimental Design
As reported in detail elsewhere (25), subjects underwent a 6-wk screening phase. This period included a 3-wk washout phase in which participants were withdrawn from their calcitriol analogs (i.e. either paricalcitol or doxercalciferol, depending on the prescribing patterns of the study site). After randomization, there was a 27-wk intervention period, consisting of a 16-wk medication titration phase followed by an 11-wk assessment phase. In one treatment group, subjects reinitiated their previous calcitriol analogs only, which were reintroduced at a maximum of 2 μg paricalcitol or 1 μg doxercalciferol thrice weekly and that were titrated upward; no maximum dose was set. The other group was initiated on Cinacalcet 30 mg daily and was reinitiated on a fixed maximum dose of 2 μg paricalcitol or 1 μg doxercalciferol thrice weekly; Cinacalcet could be titrated up monthly. By design, the intent was to keep the calcitriol analog dose relatively low (e.g. ≤2 μg of intravenous paricalcitol or the equivalent three times per week) and constant in this arm.
This investigator-initiated study built on the ACHIEVE protocol by adding a prospective measure of FGF23. FGF23 measurements were obtained from plasma samples successfully collected at baseline and week 27 from the ACHIEVE study sample; some participants also had samples collected at screening (i.e. before drug washout) and week 16 (the start of the assessment phase). The investigators were blinded to the results of treatment assignment until after FGF23 samples were assayed.
FGF23 Measurements
Plasma samples were collected prospectively for measuring FGF23 levels at screening, postwashout baseline, and 16 (i.e. the beginning of the study assessment phase) and 27 wk (i.e. at end of study), shipped cold to the central ACHIEVE study laboratory (Covance, Inc.), aliquoted, and shipped cold to the University of Kansas Medical Center. FGF23 levels were measured in triplicate using the full-length intact human FGF23 ELISA (Kainos, Tokyo, Japan), as used by many other investigators (10,11,17,26). Ca, P, and PTH were measured as described elsewhere (25). As described in the original ACHIEVE trial, during the course of study, the Nichols Allegro Biointact assay (Nichols Institute, San Clemente, CA) was withdrawn from the market, necessitating a change to the Bayer Advia Intact assay (Bayer Healthcare, Morristown, NJ). As a result, a conversion factor, determined by studies by Amgen, was applied to transform the results and enable consistent comparisons between the two assays.
Statistical Analysis
A descriptive analysis of the treatment group baseline demographic and mineral metabolic parameters was conducted. Differences in racial and gender distributions between treatment groups were evaluated with χ2 tests. Subject's age, duration of dialysis, and baseline averages of PTH, Ca, P. and Ca × P product were examined between treatment groups with an ANOVA. Raw baseline FGF23 levels were analyzed with a Wilcoxon rank-sum test because of the significant expected departure from normality. A log transformation was used to normalize variability of the FGF23 data distribution; log transformation occurred before entry into the analysis. An attempt was made preserve clinical information by presenting FGF23 levels in both raw and transformed unit scales where appropriate. The longitudinal behavior of the log of FGF23, PTH, Ca, and P between screening and baseline was analyzed with a paired t test. Group differences in the magnitude of the percent relative change across the screening–baseline interval were also examined using a two-sample t test.
After descriptive comparisons, a between-group analysis of the percent relative change in Ca, P, PTH, and log-transformed FGF23 was performed to illustrate the effect of treatment with ANOVA. Percent relative change measures were specifically selected for analysis to mitigate the biasing effect of the subject's baseline mineral metabolic levels (i.e. the phenomenon in which individuals with higher baselines levels are able to exhibit a greater degree of physiologic change in comparison to individuals closer to a physiologic floor). The percent relative change was computed for each mineral metabolic parameter with the following expression: (week 27 measurement – baseline measurement)/baseline measurement) × 100. The log FGF23 percent relative change was calculated similarly: (log week 27 measurement – log baseline measurement)/log baseline measurement) × 100. Direction of the relative change in mineral metabolic parameters within each treatment group was evaluated with a one-sample t test. All P values in the within-group analysis were adjusted for multiple comparisons using the Holms method.
For modeling the percent relative change of the log FGF23, the mineral metabolic analytes, the mean calcitriol analog dose, and the demographic variables were entered into a preliminary stepwise multiple regression analysis. The variable criterion for entry into the model was set to enter P = 0.1 and retained at P = 0.05 controlling for treatment in every model step. Factors identified as significant in predicting the change of log FGF23 were entered into a final linear regression model controlling for treatment. All analyses were conducted with SAS 9.1 (SAS Institute, Cary, NC). A type I error rate of 5% was used to determine statistical significance.
Results
Subject characteristics are shown in Table 1. Relative to the general ACHIEVE study sample, subjects of our study were very similar in demographic and mineral metabolic parameters (25), and there were no significant differences between treatment groups at baseline. The wide intersubject variability in FGF23 levels was consistent with previously published findings.
Table 1.
Baseline subject characteristics
| Characteristic | Total Sample (n = 91) | Cinacalcet-D (n = 48) | Flex-D (n = 43) | P Valuea |
|---|---|---|---|---|
| Age (yr) | 58.7 ± 14.5 | 57.9 ± 15.7 | 59.5 ± 13.1 | 0.61 |
| Male [n (%)] | 53 (58) | 29 (60) | 24 (56) | 0.66 |
| Race [n (%)] | 0.69 | |||
| African American | 35 (39) | 19 (40) | 16 (37) | |
| White | 28 (31) | 13 (27) | 15 (35) | |
| Hispanic | 25 (28) | 15 (31) | 10 (23) | |
| Other | 3 (35) | 1 (2) | 2 (5) | |
| Dialysis duration (mo) | 47.4 ± 44.4 | 46.5 ± 34.9 | 48.4 ± 53.3 | 0.84 |
| Log FGF23 (pg/ml) | 7.6 ± 1.7 | 7.9 ± 1.8 | 7.4 ± 1.6 | 0.19 |
| FGF23 (pg/ml) | 2631 (494–8263) | 3850 (787–8912) | 1306 (427–6546) | 0.15 |
| PTH (pg/ml) | 611 ± 218 | 595 ± 219 | 628 ± 219 | 0.48 |
| P (mg/dl) | 5.3 ± 1.7 | 5.4 ± 1.7 | 5.2 ± 1.8 | 0.59 |
| Ca (mg/dl) | 9.6 ± 0.5 | 9.6 ± 0.6 | 9.7 ± 0.4 | 0.54 |
| Ca × P (mg2/dl2) | 50.3 ± 16.0 | 50.9 ± 15.0 | 49.6 ± 17.1 | 0.70 |
The χ2 test was used for comparisons of proportions among gender and race, whereas ANOVA was used for comparisons of age, dialysis duration, log FGF23, PTH, P, Ca, and Ca × P; these values are shown as mean ± SD.
The Wilcoxon rank-sum test was used to compare medians of untransformed FGF23; these values are shown as median and interquartile range.
Comparison of Cinacalcet-D versus Flex-D treatment groups.
As designed, mean weekly dose of calcitriol analogs during the assessment phase differed significantly between treatment groups, with Cinacalcet-D subjects receiving a mean of a 5.8 μg/wk of paricalcitol equivalents and Flex-D subjects receiving 14.8 μg/wk (P < 0.0001); these levels were comparable to the doses reported to have been received by the entire study sample in ACHIEVE (6.8 and 13.9 μg/wk, respectively).
The differential effect of treatment on percent change in log FGF23, PTH, Ca, and P over the study duration is shown in Table 2. Examination of between-group changes in FGF23 levels shows that the percent change of log FGF23 differed significantly between treatment groups (P = 0.002). Within the Cinacalcet-D subjects, the percent change of log FGF23 showed a significant decrease (P = 0.021 corrected for multiple comparisons; data not shown); in contrast, there was no significant difference in FGF23 in the Flex-D group, although there was a trend toward an increase. Table 2 also shows that there were no significant differences in percent change of PTH between groups (P = 0.20), although PTH levels decreased >29% in Cinacalcet-D group and <11% in the Flex-D group.
Table 2.
Percent changes in mineral metabolic parameters over the course of the study
| Metabolic Parameter | Cinacalcet-D (% Change) | Flex-D (% Change) | P Value of Change Between Groups |
|---|---|---|---|
| Log FGF23 | −9.7 ± 18.2 | 4.1 ± 16.5 | 0.002 |
| PTH | −29.4 ± 72.9 | −10.8 ± 47.2 | 0.20 |
| Ca | −6.8 ± 9.0 | 2.0 ± 6.1 | <0.0001 |
| P | 4.4 ± 32.9 | 4.0 ± 31.8 | 0.96 |
Values shown as mean ± SD.
Within the Cinacalcet-D subjects, percent decreases in log FGF23 and Ca were significant (P = 0.021 and 0.008, respectively); there were no significant within-group changes in the Flex-D subjects.
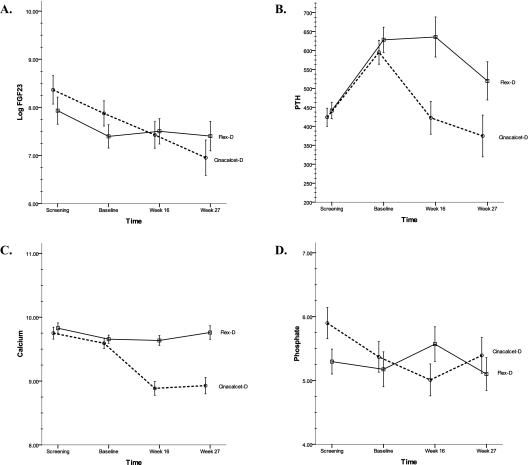
Graphical representation of the mean levels of log FGF23, PTH, Ca, and P are shown in Figure 1. Figure 1A shows how log FGF23 levels changed over time; significant between-group differences seem to be attributable to a significant decline in FGF23 levels in the Cinacalcet-D subjects in the face of relatively stable levels of FGF23 in the Flex-D subjects. Figures 1B–1D show the behavior of PTH, Ca, and P, respectively.
Figure 1.
Changes of circulating levels log FGF23 (A), PTH (B), calcium (C), and phosphate (D) between treatment groups over the course of the study. By 27 wk, there was a significant change between treatment groups in log FGF23 (P = 0.002) and calcium (P < 0.001) but no change in PTH or phosphate. Data shown as mean ± SEM; data points are staggered for clarity so that error bars do not overlap.
In a smaller set of subjects (n = 57), both screening (prewashout) and baseline (postwashout, prerandomization) samples were available. The behavior of log FGF23 levels was examined in detail during this washout period. Log FGF23 levels dropped significantly (P < 0.001) in the prerandomized subjects as a whole, whereas PTH increased significantly (P < 0.0001), and both Ca (P = 0.0005) and P (P = 0.02) decreased significantly. The subjects were stratified by the treatment group to which they were eventually randomized. Change in log FGF23 between screening and baseline was not significantly different between groups (P = 0.73), a finding reflected graphically by the similar slopes in the two groups during the washout period (Figure 1A). Changes in Ca, P, and PTH during washout also did not differ significantly between groups.
Next, we performed a stepwise linear regression model to identify factors that were associated with the change of log FGF23 (Table 3). None of the subject demographic variables met modeling retention criteria for continued evaluation, nor did the mean calcitriol analog dose during the assessment phase. Of the variables tested (e.g. percent change in PTH, Ca, P, mean calcitriol analog dose, and the subject demographic characteristics), only the percent changes of P (P < 0.0001) and Ca (P = 0.0002) were identified as significant factors associated with percent change of log FGF23. The percent changes in P and Ca accounted for ∼47% of the variance of the percent change in log FGF23, controlling for treatment condition.
Table 3.
Multiple linear regression model showing association of percent change in log FGF23 with mineral metabolic parameters and treatment assignment
| Variable | ß Estimate | SE | P Value | 95% CI |
|---|---|---|---|---|
| Percent change in P | 0.323 | 0.057 | <0.0001 | 0.21 to 0.44 |
| Percent change in Ca | 0.905 | 0.220 | 0.0002 | 0.46 to 1.35 |
| Treatment | 7.420 | 3.890 | 0.062 | −0.37 to 15.20 |
Because baseline log FGF23 levels have been associated with degree of responsiveness to SHPT (27), we separately explored whether there was a univariate association between baseline log FGF23 levels and final PTH both in each treatment group and in the pooled subjects; no significant relationship was found.
Discussion
Ongoing studies are attempting to determine the optimal combination of calcimimetics and calcitriol analogs for the treatment of SHPT. The ACHIEVE study recently compared the effects of Cinacalcet plus fixed low-dose calcitriol analogs with flexible doses of calcitriol analogs on PTH, Ca, and P levels (25). In this study, we used samples from ACHIEVE to perform a per-protocol analysis of the effects of treatment on FGF23, a factor associated with poor outcomes in ESRD (15). We found that the treatment regimen using Cinacalcet plus fixed low-dose calcitriol analogs results in a relative decrease in FGF23 levels compared with an approach using escalating doses of calcitriol analogs alone. This study is the first report of the disparate effects of different treatment strategies on FGF23 levels in HD patients.
The existence of complex interactions between treatment agents and mineral metabolic parameters make it difficult to ascribe the observed differences in FGF23 to a specific therapeutic component of, or biochemical response to, the tested treatments. Nonetheless, given the current knowledge of the regulation of FGF23 expression, there are several potential explanations for our findings. Because calcitriol is known to stimulate FGF23 production in osteoblasts (8) and calcitriol administration increases FGF23 levels in hemodialysis patients (18), the lower overall exposure to calcitriol analog dose in the Cinacalcet-D group (amounting to about one half compared with the Flex-D group) might account for the observed behavior of FGF23. In addition, the reduction in FGF23 after discontinuation of calcitriol analog therapy during the washout supports an important role of calcitriol in regulating FGF23. It is possible that FGF23 levels, which showed only modest upward trend in the Flex-D arm, would have increased had higher doses of calcitriol analogs been used in this arm to suppress PTH more robustly. However, the association between calcitriol analog dose with log FGF23 levels in univariate analysis was not sufficient to justify its retention in multivariate regression modeling; these latter findings suggest that additional factors may also contribute to the differences we observed in FGF23 levels.
Changes in PTH, Ca, or P, or even the administration of Cinacalcet itself, might also account for the differential effects of these treatment on FGF23 levels, although PTH, Ca, and P have not been shown to directly regulate FGF23 transcription in osteoblasts (8). Phosphate restriction has been shown to lower FGF23 levels in several clinical settings (28,29), but we found no difference in the percent change of P between the two treatment groups, and the relationship between change in P and change in FGF23 was comparable in each group, making it unlikely that P is principally responsible for our observed changes in FGF23. Alteration in PTH is such another potential factor. Since studies have shown a direct correlation between elevated levels of PTH and FGF23 (30), reductions in PTH might lead to lower endogenous synthesis of 1,25 vitamin D. Significant reductions in FGF23 are also reported to occur following parathyroidectomy (17). The more robust suppression of PTH in the Cinacalcet-D subjects could be posited to decrease endogenous synthesis of 1,25 vitamin D, thereby lowering FGF23 levels. Without measurement of 1,25 vitamin D levels, this possibility cannot be discounted. However, because there was no statistically significant difference in the behavior of PTH between groups (in the face of significant between-group changes of FGF23), we cannot cite evidence to support this potential mechanism. Changes in Ca could also be invoked to explain our findings, but there is no published evidence, to our knowledge, that changes in Ca or the administration of Cinacalcet affect FGF23 levels, and decrements in Ca do not directly downregulate FGF23 expression in osteoblasts (8). We did observe a suggestive, albeit nonsignificant, association of Cinacalcet treatment itself with lower FGF23 levels in our regression model. It seems plausible that a fall in Ca was in reality a “signal” that Cinacalcet was having a biologic effect on SHPT. We believe that, ultimately, different study designs will be needed to ascertain which therapeutic component and/or changes in mineral metabolic parameters are responsible for the changes in FGF23 levels.
The clinical significance of our findings is also uncertain, in part because of remaining questions regarding the biologic significance of elevated FGF23 levels in ESRD and the fact that the absolute changes in FGF23 levels between groups were small, particularly compared with changes after parathyroidectomy (17). However, there is a significant association between elevated FGF23 levels and increased mortality in ESRD, where relatively small increments in the absolute FGF23 levels are associated with reduced survival in patients previously unexposed to calcitriol analogs (15). Given the apparent survival benefits associated with use of calcitriol analogs (31,32) and lower FGF23 levels (15), a “mortality trade-off” may exist in dialysis patients, in which multiple competing factors influencing survival may need to be considered in selecting different therapeutic regimens. In fact, a potential paradox may exist between apparent stimulation of FGF23 levels (accompanied by elevations of Ca and P) by calcitriol analogs at high doses and the beneficial effects of calcitriol analog treatment on survival (31–34). The recent findings that the survival benefit of calcitriol analogs is partially attenuated at high doses of these agents (33), coupled with our finding that lower FGF23 levels are associated with a calcitriol analog-sparing regimen, suggest that studies examining potential survival benefits of various SHPT treatment regimens (consisting of combinations of both calcitriol analogs and calcimimetics) should be undertaken. In particular, the potentially interactive effects calcitriol analogues and calcimimetics on mortality must be explored in detail.
There are several important limitations of our study. First, our subjects represent a subset of about one half of the larger ACHIEVE trial participants. Inadequate sample volume and missed collection time points were the main reason that not all subjects were included in our study. Nevertheless, we found that the baseline characteristics, mean administered dose of calcitriol analogs, and response to the therapeutic interventions in this subgroup were very similar to the overall study sample. It is unlikely that the smaller sample size would involve a systematic bias favoring one outcome over another. Additionally, our analytic design required that FGF23 levels be measured at the beginning and the end of the study; as such, we studied only drug “tolerators” who completed the study and we lack information on subjects who dropped out. Because of this, our per-protocol analysis cannot be generalized to drug-naïve patients initiating therapy or to all patients with SHPT in whom treatment is being considered. Also, as with ACHIEVE, we do not have information on duration and previous treatment of SHPT in the trial participants, but the inclusion criteria, washout period, and randomization process were designed to reduce residual confounding and foster similarities between participants of the two treatment groups. Although the ACHIEVE trial was not originally designed and powered to study FGF23 levels as a primary outcome, our study limitations are probably counterbalanced by the prospective sample acquisition (collected in real time and shipped to our laboratory for the purpose of FGF23 measurement), which capitalized on a randomized trial, as well as by the breadth of the recruited population and our use of a high-quality, full-length intact assay for FGF23.
In summary, a treatment strategy of Cinacalcet plus low-dose calcitriol analogs results in greater diminution of FGF23 levels compared with treatment with higher doses of calcitriol analogs alone. Whether this is because of Cinacalcet per se, lower exposure to calcitriol analogs, superior overall control of SHPT afforded by decrements in PTH, or other mechanisms cannot be definitively determined in this study. Further studies are warranted to determine how different components of complex treatment regimens impact FGF23 levels and whether treatment-induced alterations in FGF23 levels have clinical significance.
Disclosures
J.B.W. receives honoraria from Amgen. L.D.Q. receives honoraria from and serves on the advisory board of Amgen.
Acknowledgments
The authors thank Amgen for funding this study. We thank William Goodman, MD, for his comments and suggestions and Connie Wang, MD, for her technical assistance. Additional funding for this study came from R01 AR 45955 awarded to L.D.Q.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Second Chances in Mineral Metabolism,” on pages 1–3.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Yamashita T, Yoshioka M, Itoh N: Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277: 494–498, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T, Konishi M, Miyake A, Inui K, Itoh N: Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 277: 28265–28270, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD: Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278: 37419–37426, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N: Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem 278: 2206–2211, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Perwad F, Zhang MY, Tenenhouse HS, Portale AA: Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293: F1577–F1583, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Imanishi Y, Kobayashi K, Kawata T, Inaba M, Nishizawa Y: Regulatory mechanisms of circulating fibroblast growth factor 23 in parathyroid diseases. Ther Apher Dial 11 Suppl 1: S32–37, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M: Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int 67: 1120–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T: Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 9: 336–339, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P: Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hsu HJ, Wu MS: Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337: 116–122, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant 24: 948–955, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y: FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65: 1943–1946, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Tominaga Y, Ueki T, Goto N, Matsuoka S, Katayama A, Haba T, Uchida K, Nakanishi S, Kazama JJ, Gejyo F, Yamashita T, Fukagawa M: Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis 44: 481–487, 2004 [PubMed] [Google Scholar]
- 18.Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M: Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101: c94–c99, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, Barri YM, Cohen RM, Coburn JW: The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 13: 1017–1024, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lindberg JS, Moe SM, Goodman WG, Coburn JW, Sprague SM, Liu W, Blaisdell PW, Brenner RM, Turner SA, Martin KJ: The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney Int 63: 248–254, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, McCary LC, Liu W, Turner SA, Bushinsky DA: The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol 14: 575–583, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Messa P, Macario F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazao JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Wilkie M, Zani VJ, Molemans B, Carter D, Locatelli F: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3: 36–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishbane S, Shapiro W, Moe S, Goodman B, Ling X, Turner S.C C: Cinacalcet HCl with low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients versus vitamin D alone - the ACHIEVE study. Clin J Am Soc Nephrol 3: 1718–1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe S, Yoshizawa M, Nakanishi N, Yazawa T, Yokota K, Honda M, Sloman G: Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J 131: 1137–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M: Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int 67: 1171–1178, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70: 1858–1865, 2006 [DOI] [PubMed] [Google Scholar]