Abstract
Background and objectives: Previous studies of X-linked Alport syndrome demonstrated that “severe” COL4A5 mutations (large deletions and rearrangements, nonsense and frame-shift mutations, and glycine substitutions in the carboxy-terminal residues) were associated with early-onset renal failure, hearing loss, and lenticonus in affected male patients. This study examined whether severe mutations also resulted in the typical perimacular dot-and-fleck retinopathy.
Design, setting, participants, & measurements: Twenty unrelated families with X-linked Alport syndrome were studied for the causative mutation in the COL4A5 gene. Nineteen affected male and 22 affected female individuals aged at least 14 yr from these families were examined for clinical and, in particular, ophthalmologic features.
Results: Nineteen pathogenic mutations were identified in the COL4A5 gene in the 20 families using a thermal melt analyzer (HRM RotorGene 6000; Corbett) or direct sequencing of hair root or skin fibroblast cDNA. Fifteen mutations were classified severe and four as moderate. Severe mutations were associated with the central dot-and-fleck Alport retinopathy in male individuals (P = 0.0256) in addition to early-onset renal failure, hearing loss, and lenticonus (P = 0.0009, 0.009, and 0.009, respectively). Severe mutations did not correlate with clinical features in female individuals.
Conclusions: Severe mutations in male individuals with X-linked Alport syndrome are associated with the perimacular dot-and-fleck retinopathy. Furthermore, the retinopathy indicates that male individuals are at increased risk for renal failure before the age of 30 (P = 0.0007).
Alport syndrome is an inherited kidney disease characterized by hematuria, progressive renal failure, hearing loss, lenticonus, and a dot-and-fleck retinopathy (1). It affects between one in 5000 and one in 50,000 individuals (2) but is often unrecognized.
Approximately 85% of families with Alport syndrome have X-linked disease (OMIM 301050) (3), which is due to mutations in the COL4A5 gene (4). Male individuals are affected more severely than females, and hematuria is noted in early childhood, but many males develop renal failure before the age of 30 (“early-onset disease”). Most have a hearing loss, approximately 40% develop lenticonus, and 60% have retinopathy (5,6). The hearing loss is helped with hearing aids, the lenticonus eventually requires lens replacement, but the retinopathy does not affect vision. The retinopathy is nevertheless helpful diagnostically because it is specific for Alport syndrome and is usually obvious especially in retinal photographs. Affected female individuals with X-linked Alport syndrome typically have hematuria, and the likelihood of complications increases with increasing age, with approximately 30% eventually developing renal failure, hearing loss, or the ocular abnormalities (7,8). Clinical features in males are often consistent within families, reflecting the strong genetic influence (5). In females, the clinical features are more variable in families because of lyonization (7).
Not only is identification of the underlying COL4A5 mutations diagnostic for X-linked Alport syndrome, but also the location and nature of the mutations help to predict the likelihood of clinical complications. Thus, males with large deletions and nonsense and frame-shift mutations are more likely to develop renal failure before the age of 30 yr, as well as hearing loss and lenticonus (5). In addition, missense mutations close to the carboxy terminus result in a severe phenotype (6), but previous studies have not correlated severe COL4A5 mutations with an increased likelihood of retinopathy. The aim of this study was to identify mutations in our patients with X-linked Alport syndrome, confirm the previously proposed prognostic classification of COL4A5 mutations, extend the classification to include the dot-and-fleck retinopathy, and determine whether demonstration of the retinopathy itself had any clinical significance for predicting disease in other organs.
Materials and Methods
Patients
Twenty unrelated families with X-linked Alport syndrome were studied. Alport syndrome was diagnosed on renal biopsy in 18 (90%) families, and X-linked inheritance was confirmed with linkage studies in 15 (75%) (8). All participants were older than 14 yr, which is when ocular abnormalities are often first evident in male individuals.
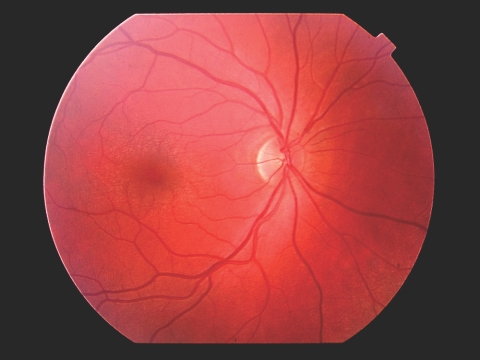
Clinical features, including the presence and age at onset of end-stage renal failure, and any clinically detectable hearing loss were noted. Participants were then examined by an ophthalmologist who was not aware of their disease status. They were examined for the “oil-droplet” sign of anterior lenticonus, their pupils were dilated with 1% tropicamide, and their optic fundi were examined for the perimacular dot-and-fleck retinopathy (Figure 1).
Figure 1.
Alport dot-and-fleck retinopathy with perimacular dots and flecks.
Mutation Detection Methods
Two mutation detection methods were used. These were a genomic DNA screening assay where variants were confirmed by direct sequencing and an assay where hair root or skin fibroblast cDNA was directly sequenced.
DNA Amplification and Screening.
DNA was prepared from peripheral venous blood using conventional methods. All 51 exons of COL4A5 plus flanking regions were amplified using primers based on published sequences (9) and screened for mutations using melting curve analysis (RotorGene 6000; Corbett). Amplicons with abnormal melt curves were then sequenced.
Sequencing of Hair Root or Skin Fibroblast COL4A5 cDNA.
mRNA was prepared from hair roots and immortalized fibroblast cell lines (10–12) using Trizol (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. mRNA was then transcribed using the Superscript III First-Strand Synthesis System (Invitrogen) using oligodT as recommended by the manufacturer. Hair root cDNA was amplified in nine overlapping fragments with primers designed using Primer3 design software (http://frodo.wi.mit.edu/primer3/). cDNA were then amplified using PCR and directly sequenced for mutations.
Sequencing
Amplicons were purified using the UltraClean GelSpin DNA purification kit (MoBio Laboratories, Carlsbad, CA) and sequenced using Big Dye Terminator (Applied Biosystems, Foster City, CA) and an ABI PRISM 3100 Genetic Analyser (Applied Biosystems).
Definition of Mutations
Variants were confirmed by sequencing in both directions. “Mutations” were variants that segregated with disease within families and were not present in 100 normal chromosomes. “Severe” mutations were large deletions and rearrangements, nonsense and frame-shift mutations, splicing mutations that affected the donor site, and missense mutations in exons 23 through 51. All other mutations were classified as “moderate.”
Correlation of Mutations with Clinical Features
Severe and moderate mutations were correlated with clinical features, namely the presence and age at onset of end-stage renal failure, and with the presence of hearing loss, lenticonus, and the perimacular retinopathy. Results were compared with Fisher exact test (two-tailed; http://www.graphpad.com/quickcalcs/contingency2.cfm). This study was approved by the Human Research Ethics Committee of Northern Health, and all participants provided signed informed consent.
Results
Mutations
Nineteen different mutations were identified in the 20 families (Table 1). G624D was present in two families who were not known to be related but were both of Italian origin. Fourteen mutations were novel. These included single-nucleotide substitutions, two in-frame deletions, a frame-shift insertion, and a large rearrangement. Eighteen mutations affected the coding region, and one affected an intronic sequence. Coding region mutations were nonsense (n = 6) or missense (n = 8), and six of the missense mutations affected a glycine. Fifteen (71%) of 19 mutations were classified severe and four as moderate. The mutations of moderate severity were c.142G>A, c.351_359delACCTCAAGC, c. 1165–1432del, and c.1498G>C.
Table 1.
COL4A5 mutations detected in X-linked Alport syndrome
| Exon | Nucleotide | Protein | Conserved Amino Acid | Type of Change | Collagenous Domain | Amino Acid Change | Severe |
|---|---|---|---|---|---|---|---|
| 2 | c.87C>Aa | p.C29X | Yes | Nonsense | Yes | Neutral to stop | Yes |
| 3 | c.142G>Aa | p.G48R | Yes | Missense | Yes | Neutral to neutral | No |
| 6 | c.351_359delACCTCAAGGa | NA | NA | In-frame deletion | Yes | NA | No |
| 19 | c.1117C>T | p.R373X | Yes | Nonsense | Yes | Basic to stop | Yes |
| 20 | c.1211_1212insAa | NA | NA | Frame-shift insertion | Yes | NA | Yes |
| 20/21 | c.1165–1432dela | p.G389_K474del | NA | In-frame deletion | Yes | NA | No |
| 22 | c.1498G>Ca | p.G500R | Yes | Missense | Yes | Neutral to basic | No |
| 24 | c.1690G>Ta | p.G564C | Yes | Missense | Yes | Neutral to neutral | Yes |
| 25 | c.1871G>A | p.G624D | Yes | Missense | Yes | Neutral to acidic | Yes |
| 27 | IVS26–18A>Ga | NA | NA | Intronic variant | Yes | NA | Yes |
| 30 | c.2473G>Ta | p.G825X | Yes | Nonsense | Yes | Neutral to neutral | Yes |
| 32 | c.2722G>Aa | p.G908R | Yes | Missense | Yes | Neutral to basic | Yes |
| 33 | c.2858G>T | p.G953V | Yes | Missense | Yes | Aliphatic (no change) | Yes |
| 43 | c.3925-?dela | p.G1309_?del | NA | Large rearrangement | Yes | NA | Yes |
| 44 | c.4006G>Ta | p.G1336X | Yes | Nonsense | Yes | Neutral to stop | Yes |
| 45 | c.4147C>Ta | p.Q1383X | Yes | Nonsense | Yes | Acid to stop | Yes |
| 48 | c.4549C>A | p.P1517T | Yes | Missense | No | Neutral to neutral | Yes |
| 49 | c.4702G>Ca | p. E1568Q | Yes | Missense | No | Neutral to neutral | Yes |
| 51 | c.5029C>T | p. R1677X | Yes | Nonsense | No | Neutral to neutral | Yes |
NA, not applicable.
Novel mutation.
Clinical Features
Forty-one affected individuals were studied from the 20 families. These included 19 males and 22 females. Five families had no affected adult male. Affected males had a median age of 38 yr (range 14 to 68). Nine (47%) of 19 had reached end-stage renal failure before the age of 30 yr, 15 (79%) of 19 had a clinically reported hearing loss, nine (53%) of 17 had lenticonus, and eight (50%) of 16 had a central retinopathy. The female individuals had a median age of 41 yr (range 14 to 80). One (5%) of 22 had developed end-stage kidney failure before 30 yr, three (14%) of 22 had a hearing loss, none had lenticonus, and three (14%) of 21 had a central retinopathy.
Correlation of Mutations with Clinical Features
Male individuals with severe mutations were more likely to develop early-onset renal failure, hearing loss, lenticonus, and retinopathy than those with moderate mutations (Table 2). The demonstration of the central retinopathy in males correlated with early-onset renal failure (P = 0.0070). Too few females developed renal failure or other clinical features to determine any effect of mutation severity; however all four female individuals who developed renal failure or the central retinopathy had severe mutations.
Table 2.
Frequency of clinical features associated with severe and moderate mutations
| Parameter | Severe Mutations | Moderate Mutations | P |
|---|---|---|---|
| Males | n = 12 | n = 7 | |
| renal failure before age 30a | 9/10 | 0/6 | 0.0009 |
| clinically reported hearing loss | 12/12 | 3/7 | 0.009 |
| lenticonus | 9/12 | 0/5 | 0.009 |
| central retinopathyb | 8/11 | 0/5 | 0.0256 |
| Females | n = 15 | n = 7 | |
| renal failure before age 30a | 1/15 | 0/7 | 1.000 |
| clinically reported hearing loss | 2/15 | 1/7 | 1.000 |
| lenticonus | 0/15 | 0/7 | 1.000 |
| central retinopathyb | 3/15 | 0/6 | 0.5263 |
Of those who had already reached the age of 30.
One adult had corneal scarring that precluded retinal examination, and three others did not have an ophthalmic examination.
Discussion
This study identified 19 different COL4A5 mutations in 20 families with X-linked Alport syndrome by using a melting curve mutation screening assay or by direct sequencing of the hair root transcript. Fourteen mutations were novel, and 15 were predicted to result in severe disease. Individuals with severe mutations were more likely to present for medical attention because they had renal failure or their clinical features were characteristic of Alport syndrome.
Two previous studies defined severe mutations in X-linked Alport syndrome and demonstrated that they correlated with early-onset renal failure, hearing loss, and lenticonus in male individuals; however, both studies failed to demonstrate a relationship with the perimacular dot-and-fleck retinopathy (5,6), possibly because their patients included children and because patients were examined in multiple centers by different ophthalmologists who were unfamiliar with variants of the Alport retinopathy (13). Although our study was small, it did not include children who were younger than 14 yr, which is when the retinopathy is often first obvious in male individuals, and all but three participants were examined by the same ophthalmologist. In many cases, retinal photographs were also examined. This study not only confirmed the previously described prognostic classification of COL4A5 mutations in male individuals with X-linked disease and that severe mutations correlated with early-onset renal failure, hearing loss, and lenticonus but also demonstrated that severe mutations correlated with the perimacular dot-and-fleck retinopathy. Furthermore, this study showed that the retinopathy in male individuals with X-linked Alport syndrome correlated with an increased likelihood of early-onset renal failure.
This study also demonstrated early-onset renal failure, lenticonus, and retinopathy were uncommon in affected females, but females with these features all had severe mutations. This has implications for the increased risk for early-onset renal failure in their male relatives but needs to be confirmed in a larger series. The development of renal failure and other clinical features in female individuals themselves is complicated by lyonization.
Not only does the demonstration of COL4A5 mutations in patients with suspected Alport syndrome confirm the diagnosis of X-linked disease, but also the location of the mutation and its nature (nonsense versus missense, frame-shift versus in-frame indel) helps to predict the clinical course in male individuals and, in particular, the likelihood of early-onset renal failure, lenticonus, and, as demonstrated here, perimacular retinopathy; however, mutation detection may not always be necessary, because the perimacular dot-and-fleck retinopathy both confirms the diagnosis of Alport syndrome and may predict increased risk for early-onset renal failure and hence the need for early and aggressive management.
Disclosures
None.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia; Kidney Health Australia; and the Northern Health Education, Equipment and Research Fund.
Preliminary results from this work were presented in abstract form at the annual meeting of the American Society of Nephrology; November 2 through 5, 2007.
We thank the many patients and their families who participated in these studies.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, Habib R: Alport's syndrome: A report of 58 cases and a review of the literature. Am J Med 70: 493–505, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Levy M, Feingold J: Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int 58: 925–943, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Feingold J, Bois E, Champret A, Broyer M, Gubler M-C, Grunfeld J-P: Genetic heterogeneity of Alport syndrome. Kidney Int 27: 672–677, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K: Identification of mutations in the COL4A5 gene in Alport syndrome. Science 24: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schroder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: Natural history in 195 families and genotype–phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Gross O, Netzer K-O, Lambrecht R, Seibold S, Weber M: Meta-analysis of genotype–phenotype correlation in X-linked Alport syndrome: Impact on clinical counselling. Nephrol Dial Transplant 17: 1218–1227, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schroder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC: X-linked Alport syndrome: Natural history and genotype–phenotype correlations in girls and women belonging to 195 families—A “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14: 2603–2610, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dagher H, Buzza M, Colville D, Jones C, Powell H, Fassett R, Wilson D, Agar J, Savige J: A comparison of the clinical, histopathological and ultrastructural phenotypes in carriers of X-linked and autosomal recessive Alport syndrome. Am J Kidney Dis 38: 1217–1228, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Martin P, Heiskari N, Zhou J, Leinonen A, Tumelius T, Hertz JM, Barker D, Gregory M, Atkin C, Styrkarsdottir U, Neumann H, Springate J, Shows T, Petterson E, Tryggvason K: High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol 9: 2291–2301, 1998 [DOI] [PubMed] [Google Scholar]
- 10.King K, Flinter FA, Green PM: Hair roots as the ideal source of mRNA for genetic testing. J Med Genet 38: E20, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tazon-Vega B, Ars E, Burset M, Santin S, Ruiz P, Fernandez-Llama P, Ballarin J, Torra R: Genetic testing for X-linked Alport syndrome by direct sequencing of COL4A5 cDNA from hair root samples. Am J Kidney Dis 50: 257.e1–257.e14, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Wang Y, Ding J, Yang J: Detection of mutations in the COL4A5 gene by analysing cDNA of skin fibroblasts. Kidney Int 67: 1268–1274, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Colville D, Wang YY, Tan R, Savige J: The retinal “lozenge” or “dull macular reflex” in Alport syndrome is associated with a severe retinopathy and early onset renal failure. Br J Ophthalmol 93: 383–386, 2009 [DOI] [PubMed] [Google Scholar]