Abstract
Background and objectives: No prospective study has reported the incidence of contrast-induced nephropathy (CIN) or the associated morbidity and mortality after contrast-enhanced computed tomography (CECT) in the outpatient setting.
Design, setting, participants, & measurements: We enrolled and followed a prospective, consecutive cohort (June 2007 through January 2009) of patients who received intravenous contrast for CECT in the emergency department of a large, academic, tertiary care center. Outcomes measured were as follows (1) CIN: An increase in serum creatinine ≥0.5 mg/dl or ≥25% 2 to 7 d after contrast administration; (2) severe renal failure: An increase in serum creatinine to ≥3.0 mg/dl or the need for dialysis at 45 d; and (3) renal failure as a contributing cause of death (consensus of three independent physicians) at 45 d.
Results: The incidence of CIN was 11% (70 of 633) among the 633 patients enrolled. Fifteen (2%) patients died within 45 d, including six deaths after study-defined CIN. Seven (1%) patients developed severe renal failure, six of whom had study-defined CIN. Of the six patients with CIN and severe renal failure, four died, and adjudicators determined that renal failure significantly contributed to all four deaths. Thus, CIN was associated with an increased risk for severe renal failure and death from renal failure.
Conclusions: CIN occurs in >10% of patients who undergo CECT in the outpatient setting and is associated with a significant risk for severe renal failure and death.
Contrast-induced nephropathy (CIN) is a known complication of intravenous, iodinated contrast; is a common cause of renal failure in the inpatient setting (1–5); and is associated with both short- and long-term adverse outcomes (6,7). Previous reports indicated that CIN occurs in 4 to 20% of patients after intra-arterial administration after coronary angiography (5–9). In the outpatient setting, the use of intravenous contrast to enhance (contrast-enhanced computed tomography [CECT]) imaging has increased sharply in recent years. Despite that >6% of all emergency department (ED) patients undergo CECT in the United States (10), no prospective data allow clinicians to estimate the rate of CIN or the associated morbidity and mortality after CECT in the outpatient setting in a heterogeneous population. Previous, retrospective work in outpatients who underwent CECT found the prevalence of CIN to be 5 to 13% (11–14) and indicates that patients without baseline renal insufficiency or chronic kidney disease may still be at risk for CIN in this population (11); however, these studies were limited by retrospective design and selection bias related to inclusion of inpatients with existing kidney disease (11–14). Thus, the absence of predicate literature required to estimate both the incidence and the clinical significance of CIN after CECT provided rationale for this work.
In this study, we sought to define prospectively the incidence of CIN in an unselected, consecutive, heterogeneous population of ED patients who received low-osmolar, nonionic contrast for a CECT study of any body region. We tested the hypothesis that the incidence of CIN in the ED population exceeds 4% and that CIN is associated with a high rate of severe renal failure and death (5–9,11).
Materials and Methods
Study Design and Setting
This was a prospective study of outpatients who received intravenous contrast for a CECT study from June 2007 through November 2008 from the ED. The study was performed at Carolinas Medical Center (Charlotte, NC), which is an urban, academic center with an annual ED volume >110,000 patients per year. As part of the Carolinas Healthcare System (CHS), the centralized medical record system provides access to records from 25 hospitals and >100 primary and specialty practice locations. The ED uses two Multi-Detector Siemens Somatom Sensation 64-slice scanners (Siemens Medical Solutions USA, Malvern, PA) 24 h/d, 7 d/wk, and images are interpreted, in real time, by on-site, board-certified radiologists. This institution uses Iopamidol-370 (Isovue-370; Bracco Diagnostics, Princeton, NJ) for all CECT scans. This study was approved by the institutional review board of CHS.
Selection of Participants
To test our hypothesis, we sought to enroll from an unbiased sample of ED patients who were receiving intravenous contrast for a CECT study. Accordingly, before the start of the study, we defined “consecutive” to require that a minimum of 85% of adults who were undergoing CECT in our ED during the period of enrollment would be approached for enrollment (15). Eligible patients were identified by research associates who reviewed the order-entry system (Centricity; GE Healthcare, Chalfont St. Giles, UK) in real time and approached patients while they were still in the ED, and written informed consent was obtained as soon as was practical after the CT order was placed.
We excluded patients with any of the following: (1) Age <18 yr; (2) hemodialysis or peritoneal dialysis within 45 d before enrollment or documented previous physician-directed plans to start dialysis within 45 d after enrollment; (3) kidney transplant before or planned at the time of enrollment; (4) intravenous contrast for any reason within 14 d before enrollment; (5) pregnancy or postpartum <48 h; (6) patients with immediately life-threatening injuries as classified by the institutional trauma classification guidelines; (7) the inability to provide written, informed consent; or (8) patient-stated unavailability for the follow-up blood draw. Patients who were enrolled but did not receive contrast (i.e., the CECT study was canceled or changed to a noncontrast study after the patient was enrolled) were also excluded.
Study Protocol
At enrollment, standard phlebotomy techniques were used to collect venous blood, and, within 30 min of collection, we measured a baseline serum creatinine (sCr) concentration (i-STAT; Abbott Point of Care, East Windsor, NJ). Clinical data were collected at the bedside, including literature-derived presumptive risk factors for CIN (14,16). We also recorded evidence of CIN prophylaxis: Prestudy saline hydration or institution-approved protocols for sodium bicarbonate infusion (17).
Patients who were not admitted to the hospital either physically returned to our hospital for study-required phlebotomy or had a study-sponsored home health nurse visit 2 to 7 d after enrollment for a follow-up blood draw for sCr measurement. A phlebotomist obtained the study blood sample from admitted patients who were inpatients at the time of follow-up (18).
Outcome Measures
The primary end point of this study was CIN defined as an increase in sCr of ≥0.5 mg/dl or ≥25% within 2 to 7 d of contrast administration (2,4,6,8,11,16). The secondary end point was the composite of either severe renal failure or death with renal failure as a contributing cause within 45 d. We followed patients for 45 d, using a combination of telephone and medical record review. The methods of follow-up have been previously published (19). Severe renal failure was defined as a rise in sCr to ≥3.0 mg/dl (3) or the need for dialysis. Death from renal failure was determined to be present or absent for all decedents within 45 d of contrast administration by the adjudicated consensus of two of three blinded physician reviewers, which included an ED physician and a nephrologist in all cases. The explicit definition used by reviewers was obvious evidence of renal failure defined by worsening azotemia with complications of renal failure including oliguria, pulmonary edema, hyperkalemia, pericardial effusion, or the need to initiate dialysis (hemodialysis or peritoneal dialysis) before death. In addition, adjudicators were asked to determine from a comprehensive medical record review whether any of these events significantly contributed to the death event.
Since the initiation of this study, published data suggest that an absolute rise in sCR of ≥0.3 mg/dl (regardless of a 25% increase in sCR) after contrast administration may be a more sensitive threshold for CIN and related complications (6). The use of this threshold is also supported for other sources of acute kidney injury (20). As a result, we included a subanalysis of the data using this definition.
Primary Data Analysis
Data were compiled into spreadsheet format (Microsoft Office Excel; Microsoft, Seattle, WA) for analysis. We performed all statistical analyses using STATSDirect 3.3 (Chesire, UK). We reported overall outcome incidence, population characteristics, and presumptive risk factors as proportions with associated 95% confidence intervals (95% CI) calculated using the Clopper-Pearson method. We also tested for interobserver reliability for outcomes determined by the physician adjudicators using the unweighted Cohen κ. The primary hypothesis was tested by assessing the lower limit of the 95% CI for the risk ratio of CIN, severe renal failure, and death. We also report the associated two-sided P value calculated using Fisher exact test. P < 0.05 was considered significant. Because adjudicators did not identify any occurrences for which renal failure contributed to deaths among CIN(−) patients (by either the primary or the alternative definition), 0.5 was substituted as the number of outcomes to calculate the risk ratio and associated 95% CI for this outcome.
Results
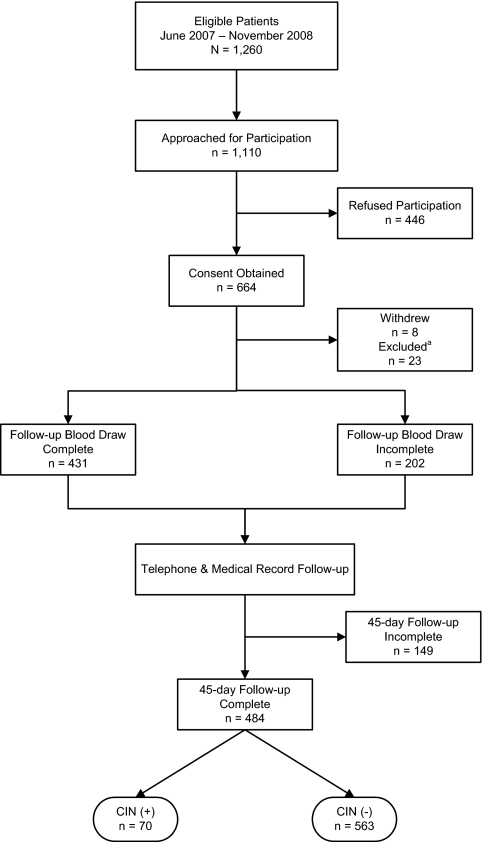
We approached 1110 (88%) of 1260 eligible ED patients and enrolled 664 patients. We excluded 23 patients whose CECT was cancelled or converted to a noncontrast study after enrollment, and eight patients requested to be withdrawn, leaving 633 patients included in this analysis. Figure 1 summarizes the enrollment of eligible patients for this study.
Figure 1.
Enrollment and follow-up of study participants. aPatients excluded after enrollment when CECT imaging was cancelled or converted to a noncontrast study.
We completed the follow-up blood draw for 431 (68%) of 633 patients (95% CI 64 to 72%) within the initial 2- to 7-d period, including 250 who were not inpatients at the time of follow-up. The clinical characteristics of study participants are summarized in Table 1. The follow-up blood sample was drawn between 48 and 71 h after contrast administration in 71% of the 431 patients with a follow-up sCR measurement; however, 128 patients had more than one sCR measurement within the 2- to 7-d postcontrast period, for which the peak sCR level was used to define the outcome of CIN. The peak sCR levels for the 431 patients with follow-up measurements occurred over the following distribution: 42% within 48 to 71 h after contrast administration, 20% between 72 and 95 h, 13% between 96 and 119 h, 10% between 120 and 144 h, and 15% between 145 and 168 h. Table 2 shows the breakdown of the anatomic regions studied by the CECT scans performed. The CECT study was performed for acute traumatic complaints in 10% of patients (n = 62) and for nontraumatic complaints in the remaining 90% of patients. The majority of enrolled patients (603 of 633; 95% CI 93 to 97%) stated that their primary access to follow-up care was within the CHS system, and 45-d follow-up was successfully completed for 484 patients (76%; 95% CI 73 to 80%) including 141 (70%) of 202 (95% CI 63 to 76%) patients who did not complete the follow-up blood draw. Thus, 572 (90%) of 633 patients (95% CI 88 to 93%) completed the initial blood draw and/or 45-d follow-up. There was no significant difference in the clinical characteristics of those who did and did not have follow-up phlebotomy. To provide a conservative estimate of the rate of CIN, we used n = 633 as the denominator for all calculations. Table 3 summarizes the results of the primary and secondary end points.
Table 1.
Comparison of clinical characteristics and presumptive risk factors for contrast-induced nephropathy (CIN) for CIN (+) and CIN(-) patients.
| Characteristic | CIN(+) (n = 70) | CIN(−) (n = 563) |
|---|---|---|
| Age (yr; mean ± SD) | 54 ± 14 | 46 ± 15 |
| Female gender (%; 95% CI) | 53 (41 to 65) | 58 (53 to 62) |
| Race (%; 95% CI) | ||
| white | 53 (41 to 65) | 52 (48 to 56) |
| black | 39 (27 to 51) | 40 (36 to 44) |
| other | 9 (3 to 18) | 8 (6 to 11) |
| Presumptive risk factors (%; 95% CI) | ||
| age >70 y | 13 (6 to 23) | 7 (5 to 9) |
| anemia | 10 (4 to 20) | 13 (10 to 16) |
| congestive heart failure | 16 (8 to 26) | 6 (4 to 9) |
| diabetes | 29 (18 to 41) | 16 (13 to 19) |
| vascular diseasea | 19 (10 to 30) | 9 (7 to 12) |
| baseline renal insufficiencyb | 7 (2 to 16) | 8 (6 to 11) |
Defined as a patient-reported history of cerebral, coronary, renal, or peripheral vascular disease.
Defined as a baseline GFR <60 ml/min per 1.73 m2 using the Modification of Diet in Renal Disease (MDRD) method (26).
Table 2.
Distribution of anatomic regions studied by CECT imaging
| Anatomic Region | Proportion (% [95% CI]) |
|---|---|
| Head and/or neck | 4 (2 to 5) |
| Chest | 27 (23 to 30) |
| Abdomen and/or pelvis | 54 (50 to 58) |
| Other (including extremities) | 16 (13 to 19) |
Table 3.
Summary of major outcomes
| CIN | Severe Renal Failurea | Death |
Total | |
|---|---|---|---|---|
| Any Cause | Renal Failureb | |||
| + | − | − | NA | 62 |
| + | − | + | − | 2 |
| + | − | + | + | 0 |
| + | + | − | NA | 2 |
| + | + | + | − | 0 |
| + | + | + | + | 4 |
| − | − | − | NA | 553 |
| − | − | + | − | 9 |
| − | − | + | + | 0 |
| − | + | − | NA | 1 |
| − | + | + | − | 0 |
| − | + | + | + | 0 |
| Total | 633 | |||
Defined as an increase in sCr from <3.0 mg/dl at enrollment to ≥3.0 mg/dl or the need for dialysis within 45 d.
Defined as renal failure that significantly contributed to death event, within 45 d of enrollment, and required the consensus of two of three blinded physician reviewers.
A total of 70 (11%) of 633 (95% CI 9 to 14%) developed CIN including, six (9%) of whom subsequently developed severe renal failure (95% CI 3 to 18%). Five of the six patients with CIN and severe renal failure required first-time hemodialysis or died, and the remaining patient had a sCr increase to more than 3 mg/dl but did not require hemodialysis within the follow-up period. In a single case, the patient did not have CIN 57 h after contrast administration and subsequently developed severe renal failure. The all-cause mortality rate among patients with CIN was 9% (six of 70; 95% CI 3 to 18%) compared with 2% (nine of 563; 95% CI 1 to 3%) among CIN(−) patients. In four (6%) of 70 (95% CI 2 to 14%) CIN(+) patients, adjudicators agreed (Cohen κ = 0.8; 95% CI 0.3 to 1.0) that renal failure significantly contributed to death. Renal failure was not identified as a significant contribution to any deaths that occurred in CIN(−) patients.
Thus, CIN was associated with a significantly increased risk of the secondary end point components of severe renal failure and renal failure as a contribution to death compared with patients who did not have CIN (relative risk [RR] 48.3 [95% CI 7.7 to 302.0; P < 0.01] and RR 64.4 [95% CI 6.1 to 675.3; P < 0.01], respectively). In addition, CIN was associated with an increased risk for death from any cause (RR 5.4; 95% CI 2.0 to 13.9; P = 0.02).
Although this report is not intended to present a comprehensive assessment of predictors of CIN, Table 1 presents a bivariate analysis of presumptive risk factors for CIN between patients with and without CIN. Three presumed risk factors (heart failure, diabetes, and vascular disease) were more common in the CIN(+) group, whereas baseline renal insufficiency and the presence of anemia were not. A total of 58 patients received prophylactic treatment for CIN: Two were treated with N-acetylcysteine (neither developed CIN), and the remaining received sodium bicarbonate (17). The rate of CIN was not significantly decreased among those who were treated with sodium bicarbonate compared with the 577 who were not (16% [95% CI 8 to 28%] and 11% [95% CI 8 to 13%], respectively).
Using the alternative definition of CIN, an absolute increase in sCR of ≥0.3 mg/dl (regardless of either criteria used by the traditional definition) over baseline resulted in 30 fewer cases of CIN(+) identified in this population: Incidence of 6% (40 of 633; 95% CI 5 to 8%). As with the traditional definition, six of seven patients who developed renal failure and all four patients with renal failure as a contribution to death were CIN(+). Compared with the traditional definition of CIN, the use of the alternative definition results in higher estimates of the associated risks for severe renal failure and death from renal failure (RR 88.9 [95% CI 14.3 to 55.2; P < 0.01] and RR 118.7 [95% CI 11.3 to 1239.4; P < 0.01], respectively). Incidentally, the risk for death from all causes remained similar to that of the standard definition (RR 7.4; 95% CI 2.7 to 19.3; P < 0.01).
Discussion
The most important finding of this study is that the incidence of CIN is probably higher than might be predicted for a low-risk, relatively young, ambulatory population. We base this assertion on the fact that the lower limit of the 95% CI for the incidence of CIN observed in this study (9%) was greater than twice the literature-reported 4% rate of CIN after coronary angiography in very low-risk populations and is within the range of moderate- to high-risk populations (5–9). In our population, 41% had none of the presumptive risk factors for CIN listed in Table 1, and 25% had only one risk factor. Among CIN(+) patients, the prevalence of risk factors was similarly low: 33% without any presumptive risk factor and 16% with only one risk factor. Nonetheless, patients with CIN had a significantly increased mortality risk. Recent reports suggested that the lifetime risk for fatal malignancy may be as high as 0.3% as a result of the exposure to the radiation required to obtain CT images of the chest or abdomen in patients who are younger than 40 yr (21). Thus, these findings suggest that the risk for death that is associated with renal failure after exposure to intravenous contrast that is required to obtain CECT images of any body part is at least as high as the risk for fatal malignancy as a result of the radiation exposure from the same study. Moreover, our data suggest that a low baseline GFR (<60 ml/min per 1.73 m2) may not independently predict the outcome of CIN after CECT ordered in the ED. Although some risk factors that are well established in the coronary angiography literature, including diabetes, vascular disease, and congestive heart failure did reach significance, the CIs remained wide, indicating that the low number of outcomes reduces the validity of using a multivariate model to test all predictors. This finding emphasizes the need for additional, multicenter data to define fully these risk factors for CECT in this setting.
Previous literature that examined CIN focused on populations that were undergoing coronary or limb angiography and focused primarily on patients with moderate to severe renal insufficiency. Several differences in administration technique and patient strata underscore the need for more data for more focused study of CECT in the ED. First, CECT requires an intravenous bolus of 80 to 150 ml of contrast material injected within 10 to 20 s, whereas organ or limb angiography studies use a series of smaller intra-arterial injections of contrast material, delivered over 10 to 20 min. This may result in important mechanistic differences in the development of CIN compared with intravenous administration (22). Many patients who undergo formal angiography are hospitalized, causing a different physiologic status than ambulatory patients. The urban ED patient may be more likely to have uncontrolled renal stresses such as undiagnosed or untreated hypertension or chronic hyperglycemia, together with a lower baseline hydration status. Moreover, hospitalized patients in a prolonged supine position may have a lowered rate of renin secretion, resulting in higher renal blood flow (23).
The discussion of the clinical importance of CIN invokes polarized opinions. Some experts argue that CIN is a laboratory-defined outcome with minimal biologic significance (9). Furthermore, the peak sCr level in many patients with CIN is in the normal range or returns to normal within 1 to 3 wk of exposure (2,4,8,16); however, as we demonstrated in this study, CIN, using the standard definition, is associated with significant morbidity and mortality: 11% (95% CI 5 to 21%) of patients with CIN went on to die or develop severe renal failure within 45 d. It is reasonable to hypothesize, therefore, that CIN may contribute to cumulative renal damage and increase the lifetime risk for ESRD (24).
These data also suggest that the standard definition of CIN in this population may not be the most appropriate. In fact, a definition that has been demonstrated to be more sensitive in other populations (6), an increase in serum sCR of ≥0.3 mg/dl, is actually less sensitive but may actually be more specific for major morbidity and mortality. Further study is required to determine whether this alternative definition is sensitive enough to identify those who are at risk of cumulative renal injury and ESRD in the longer term.
We believe that this study is the first prospective, noninterventional study to document the incidence of CIN after CECT that used contemporaneous contrast material (low osmolar and nonionic) in an outpatient setting and in a large, heterogeneous population. Although this study was performed at a single academic, urban ED and the results may vary in other settings, the population is relatively large compared with even the most recent study of outcomes from CIN (6,7). Only 60% of patients who were approached for enrollment agreed to participate. This may reflect the inclusion requirement for the patient to be willing and able to provide two blood samples, return to the ED or accommodate a home health visit, and agree to a 45-d telephone interview. The protocol excluded critically ill or severely injured patients, who account for approximately 20% of all CECT in our ED (25). Thirty-two percent of enrolled patients did not complete the follow-up blood draw; however, their clinical characteristics were not different from those of patients who did have repeat phlebotomy, and we were able to complete medical record and telephone follow-up for 70% of this subgroup, providing a reasonable knowledge of the occurrence of severe renal failure or death. It is also possible that the peak sCR level was missed by our follow-up methods; however, this effect is likely minimized in our sample with 15% of peak sCR levels measured as far out as 7 d, 128 patients with more than one measurement, and long-term follow-up designed to detect renal outcomes that require treatment. Each of these limitations represents sources of sampling bias that could affect the internal validity of our observed frequency of CIN. Taken together, if each limitation were treated as a mathematical vector (i.e., having direction and magnitude), then the net vector of all of the study limitations most likely caused an underestimation of the true proportion of CIN after CECT performed in the ED.
Conclusions
The incidence of CIN is high in an ED population undergoing CECT imaging and is associated with a significant risk for severe renal failure and death. Furthermore, the data suggest that the use of an alternative definition of CIN, an absolute increase in serum sCR of ≥0.3 mg/dl, may be equally sensitive and more specific for the outcomes of severe renal failure and death. Traditional risk factors may not adequately identify patients who are at risk for CIN in this population, which requires further study. Additional research is also needed to determine the potential for delayed complications in patients who have CIN and do not develop severe renal failure or death in the short term.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Briguori C, Tavano D, Colombo A: Contrast agent–associated nephrotoxicity. Prog Cardiovasc Dis 45: 493–503, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Schrader R: Contrast material-induced renal failure: An overview. J Interv Cardiol 18: 417–423, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Asif A, Preston RA, Roth D: Radiocontrast-induced nephropathy. Am J Ther 10: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Adam A, Becker CR, Davidson C, Lameir N, Stacul F, Tumlin J: Epidemiology and prognostic implications of contrast induced nephropathy. Am J Cardiol 98: 5K–13K, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloa CS, Sharma SK, Labinaz M, Gelormini JL, Barrett BJ: Contrast-induced nephropathy and long-term adverse events: Cause and effect? Clin J Am Soc Nephrol 4: 1162–1169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG: Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 64: 442–448, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Garovic VD: Contrast nephropathy after coronary angiography. Mayo Clin Proc 79: 211–219, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Rudnick M, Feldman H: Contrast-induced nephropathy: What are the true clinical consequences? Clin J Am Soc Nephrol 3: 263–272, 2008 [DOI] [PubMed] [Google Scholar]
- 10.McCaig LF, Burt CW: National Hospital Ambulatory Medical Care Survey: 2002 emergency department summary. Adv Data (340): 1–34, 2004 [PubMed] [Google Scholar]
- 11.Mitchell AM, Kline JA: Contrast nephropathy following computed tomography angiography of the chest for pulmonary embolism in the emergency department. J Thromb Haemost 5: 50–54, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hipp A, Desai S, Lopez C, Sinert R: The incidence of contrast-induced nephropathy in trauma patients. Eur J Emerg Med 15: 134–139, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Traub S, Tang A, Kancharla A, Cataldo L, Kelley-Stiles J, Kellum J: Risk factors for radiocontrast nephropathy after emergency department computerized tomography. Acad Emerg Med 15: S95–S96, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Barrett BJ, Katzberg RW, Thomsen HS, Chen N, Sahani D, Soulez G, Heiken JP, Lepanto L, Ni ZH, Nelson R: Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: A double-blind comparison of iodixanol and iopamidol. Invest Radiol 41: 815–821, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bossuyt PM, Reitsma JB, Bruns DE, Gastonis CA, Glaszione PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG: The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Ann Intern Med 138: W1–W12, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Harjai KJ, Raizada A, Shenoy C, Satter S, Orshaw P, Yeager K, Boura J, Aboufares A, Sporn D, Stapleton D: A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol 101: 812–819, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, VanMoore A, Simonton CA, Rittase RA, Norton HJ, Kennedy TP: Prevention of contrast-induced nephropathy with sodium bicarbonate: A randomized controlled trial. JAMA 291: 2328–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AM, Kline JA: A home health care agency is a novel resource for increasing the rate of successful follow-up in an emergency department study [Abstract]. Acad Emerg Med 15: S143, 2008 [Google Scholar]
- 19.Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB: Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med 12: 1127–1133, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levine A: Acute Kidney Injury Network (AKIN): Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einstein A, Henzlova M, Rajagopalan S: Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 298: 317–323, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rao QA, Newhouse JH: Risk of nephropathy after intravenous administration of contrast material: A critical literature analysis. Radiology 239: 392–397, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D: Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111: 1574–1582, 2005 [DOI] [PubMed] [Google Scholar]
- 24.McCullough PA, Soman SS. Contrast-induced nephropathy: Crit Care Clin 21: 261–280, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Mitchell AM, Kline JA: Systematic bias introduced by the informed consent process in a diagnostic research study. Acad Emerg Med 15: 225–230, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch J: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]