Abstract
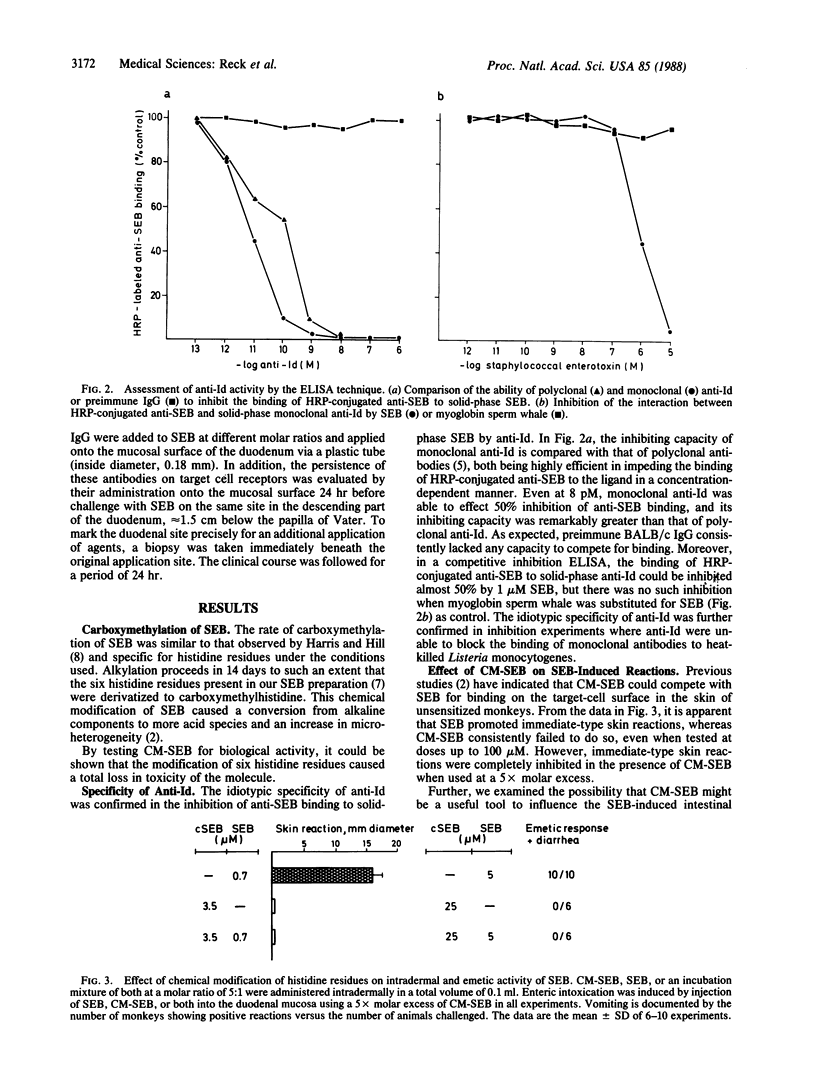
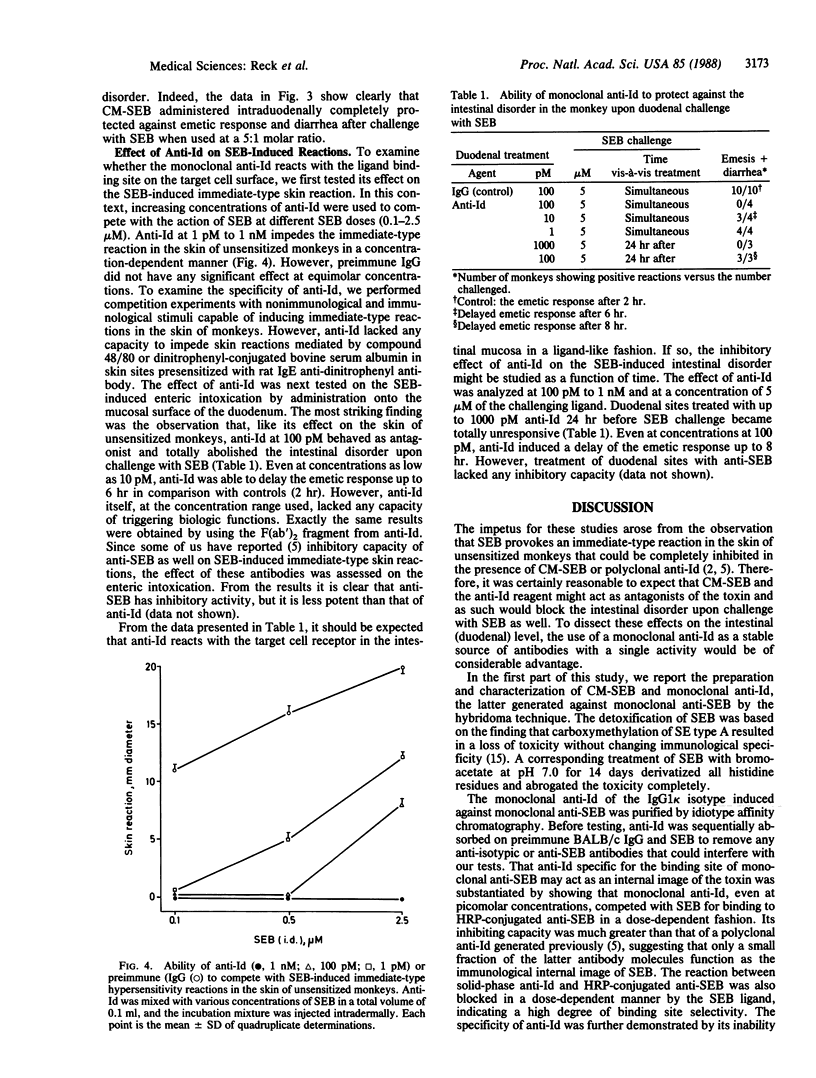
The staphylococcal enterotoxin serotype B (SEB)-induced enteric intoxication and the immediate-type reaction in the skin of unsensitized monkeys was used to define whether agents competing with SEB for target cell receptors may inhibit pathophysiological effects. For this purpose a duodenal provocation test was developed by use of a pediatric gastroscope, allowing the evaluation of the influence of antagonists on the intestinal disorder upon SEB challenge at the same duodenal site. First, carboxymethylation of histidine residues of SEB caused a complete loss of emetic and skin-sensitizing activity without changing the immunological specificity. However, carboxymethylated SEB is a strong inhibitor of enteric intoxications and immediate-type skin reactions upon SEB challenge. Second, after immunization of BALB/c mice with monoclonal anti-SEB antibodies, monoclonal antiidiotypic antibodies (anti-Id) were obtained by the "hybridoma technique" and purification by idiotype-affinity chromatography. Anti-Id specifically inhibited the binding of horseradish peroxidase-labeled anti-SEB to the ligand, and SEB blocked as well the interaction of these two antibody species, indicating a high degree of binding-site selectivity. Anti-Id completely protected against emetic response and diarrhea upon duodenal provocation with SEB and inhibited immediate-type skin reactions as well. Further, anti-Id acted as an antagonist without triggering biologic functions themselves. This shows that anti-Id constitute a useful tool to protect against a bacterial toxin-induced intestinal disorder.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger U., Scheuber P. H., Sailer-Kramer B., Bartsch K., Hartmann A., Beck G., Hammer D. K. Anti-idiotypic antibodies that inhibit immediate-type skin reactions in unsensitized monkeys on challenge with staphylococcal enterotoxin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7054–7058. doi: 10.1073/pnas.83.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2413–2419. [PubMed] [Google Scholar]
- Denzlinger C., Guhlmann A., Scheuber P. H., Wilker D., Hammer D. K., Keppler D. Metabolism and analysis of cysteinyl leukotrienes in the monkey. J Biol Chem. 1986 Nov 25;261(33):15601–15606. [PubMed] [Google Scholar]
- Ende I. A., Terplan G., Kickhöfen B., Hammer D. K. Chromatofocusing: a new method for purification of staphylococcal enterotoxins B and C1. Appl Environ Microbiol. 1983 Dec;46(6):1323–1330. doi: 10.1128/aem.46.6.1323-1330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehl R., Bartsch K., Hammer D., Himmelspach K. MOPC 104E IgM/anti-idiotype solid-phase inhibition assay as a model for screening myeloma proteins for ligand binding specificity. J Immunol Methods. 1981;42(2):243–249. doi: 10.1016/0022-1759(81)90154-x. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Kaveri S. V., Durieu O., Delavier C., Hoebeke J., Strosberg A. D. beta-Adrenergic agonist activity of a monoclonal anti-idiotypic antibody. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1781–1784. doi: 10.1073/pnas.82.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. M., Hillrl The carboxymethylation of human metmyoglobin. J Biol Chem. 1969 Apr 25;244(8):2195–2203. [PubMed] [Google Scholar]
- Homcy C. J., Rockson S. G., Haber E. An antiidiotypic antibody that recognizes the beta-adrenergic receptor. J Clin Invest. 1982 May;69(5):1147–1154. doi: 10.1172/JCI110550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Becker E. L. Anti-idiotype as antibody against the formyl peptide chemotaxis receptor of the neutrophil. J Immunol. 1982 Feb;128(2):963–968. [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Scheuber P. H., Denzlinger C., Wilker D., Beck G., Keppler D., Hammer D. K. Cysteinyl leukotrienes as mediators of staphylococcal enterotoxin B in the monkey. Eur J Clin Invest. 1987 Oct;17(5):455–459. doi: 10.1111/j.1365-2362.1987.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Scheuber P. H., Golecki J. R., Kickhöfen B., Scheel D., Beck G., Hammer D. K. Skin reactivity of unsensitized monkeys upon challenge with staphylococcal enterotoxin B: a new approach for investigating the site of toxin action. Infect Immun. 1985 Dec;50(3):869–876. doi: 10.1128/iai.50.3.869-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler E., Kolb H. Characterization of syngeneic anti-idiotypic antibody against the idiotype fo BALB/c myeloma protein J558. Eur J Immunol. 1977 Sep;7(9):649–654. doi: 10.1002/eji.1830070914. [DOI] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Stelma G. N., Jr, Bergdoll M. S. Inactivation of staphylococcal enterotoxin A by chemical modification. Biochem Biophys Res Commun. 1982 Mar 15;105(1):121–126. doi: 10.1016/s0006-291x(82)80019-3. [DOI] [PubMed] [Google Scholar]