Abstract
Background and objectives: Hematopoietic growth factor–inducible neurokinin 1 (HGFIN), also known as Gpnmb and osteoactivin, is a transmembrane glycoprotein that is expressed in numerous cells, including osteoclasts, macrophages, and dendritic cells. It serves as an osteoblast differentiation factor, participates in bone mineralization, and functions as a negative regulator of inflammation in macrophages. Although measurable at low levels in monocytes, monocyte-to-macrophage transformation causes substantial increase in HGFIN expression. HGFIN is involved in systemic inflammation, bone demineralization, and soft tissue vascular calcification.
Design, setting, participants, & measurements: We explored HGFIN expression in monocytes and monocyte-derived macrophages in 21 stable hemodialysis patients and 22 control subjects.
Results: Dialysis patients exhibited marked upregulation of colony-stimulating factor and IL-6 and significant downregulation of IL-10 in intact monocytes and transformed macrophages. HGFIN expression in intact monocytes was negligible in control subjects but conspicuously elevated (8.6-fold) in dialysis patients. As expected, in vitro monocyte-to-macrophage transformation resulted in marked upregulation of HGFIN in cells obtained from both groups but much more so in dialysis patients (17.5-fold higher). Upregulation of HGFIN and inflammatory cytokines in the uremic monocyte-derived macrophages occurred when grown in the presence of either normal or uremic serum, suggesting the enduring effect of the in vivo uremic milieu on monocyte/macrophage phenotype and function.
Conclusions: Uremic macrophages exhibit increased HGFIN gene and protein expression and heightened expression of proinflammatory and a suppressed expression of anti-inflammatory cytokines. Further studies are needed to determine the role of heightened monocyte/macrophage HGFIN expression in the pathogenesis of ESRD-induced inflammation and vascular and soft tissue calcification.
Hematopoietic growth factor–inducible neurokinin 1 (HGFIN; also known as glycoprotein nonmetastatic melanoma protein b [gpnmb], dendritic cell–associated heparan sulfate proteoglycan integrin ligand [DC-HIL], and osteoactivin) is a type 1 transmembrane glycoprotein (1). Its extracellular domains include a heparin-binding domain, an integrin-binding arginine-glycine-aspartic acid motif, and a polycystic kidney disease sequence (1–4). It is expressed in many cells and tissues, including embryonic nervous system, developing nephrons, basal layer of skin, germinal cells of hair follicles, osteoblasts, osteoclasts, myocytes, retinal pigment epithelium, renal tubules, macrophages, and dendritic cells (1,5–9). In bone, it plays an essential role in terminal osteoblast differentiation and bone mineralization (6,10,11).
HGFIN expression is enriched in monocytes and is strongly upregulated during macrophage differentiation. Upon macrophage activation, increased HGFIN is associated with a reduction of the proinflammatory cytokine IL-6, suggesting that it may play a role in mitigating inflammatory responses (12). In fact, mice with an inactivation mutation in HGFIN exhibit elevated numbers of peritoneal macrophages and higher levels of proinflammatory cytokines in response to LPS (12). HGFIN has also been shown to inhibit T cell activation by antigen-presenting cells by binding syndecan 4 (a heparan sulfate proteoglycan) (13,14). Thus, it seems that HGFIN functions as a negative regulator of inflammation.
ESRD is associated with systemic inflammation, arteriosclerosis, bone demineralization, and soft tissue vascular calcification/ossification (15–19). Vascular calcification in ESRD is not a passive process but an active one that resembles bone mineralization (20). Because HGFIN is involved in inflammation and mineralization, processes that are upregulated in ESRD, we explored its expression in circulating monocytes and monocyte-derived macrophages in a group of hemodialysis-dependent patients and age-matched control subjects.
Materials and Methods
The study protocol was approved by the human subjects institutional review board of the University of California Irvine and completed with the assistance of the University of California General Clinical Research Center. Informed consent was obtained from all patients who participated in the study.
Participants
Twenty-one stable patients who had ESRD and were on maintenance hemodialysis for a minimum of 3 mo were recruited for the study. Hemodialysis therapy was performed thrice weekly using cellulose acetate dialyzers. Individuals with evidence of acute or chronic infection or acute intercurrent illnesses were excluded. Medical history, systolic and diastolic BP, body weight, interdialytic weight change, routine monthly laboratory data and dialysis prescription including dialyzer type, and medications were recorded. Predialysis blood samples were obtained. A group of 22 normal age-matched individuals were used as control subjects.
Blood Sampling
Predialysis blood was obtained from hemodialysis patients after cannulation of the vascular access but before the administration of heparin and the initiation of dialysis. Samples from control subjects were collected from a peripheral vein.
Cell Culture
Mononuclear cells were isolated by density gradient centrifugation over lymphocyte separation medium (Cellgro). The peripheral blood mononuclear cells were plated onto six-well plates and incubated with Iscove's modified Dulbecco's medium supplemented with 2% heat-inactivated FBS for 16 h. After 16 h, suspension cells (predominantly lymphocytes) were removed by aspiration, and the medium was replaced with Iscove's modified Dulbecco's medium (ATCC), supplemented with 10% heat-inactivated normal serum or 10% heat-inactivated uremic serum obtained from hemodialysis patients, and 20 ng/ml macrophage colony-stimulating growth factor (M-CSF; PeproTech Inc.), 20 U/ml penicillin, and 20 μg/ml streptomycin (Invitrogen Life Technologies). Cells were cultured at 37°C in a humidified tissue culture incubator with 5% CO2. For culture periods longer than 3 d, 50% of the culture medium was removed and replaced with fresh medium.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from cells with Mini-RNA isolation kit (ZEMO Research). One microgram of total RNA was converted to DNA using SuperScript III (Invitrogen). After the reverse transcription reaction, all samples were diluted 1:50 in double-distilled water, and 2 μl was subjected to real-time PCR analysis with SYBR Green PCR Master Mix (Applied Biosystems). Primers (Realtimeprimers) were used at a concentration of 1 μM in a total reaction volume of 10 μl. The cycling conditions were as follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles each consisting of 95°C for 15 s and 60°C for 1 min. Incorporation of SYBR green dye into the PCR products was monitored with Mx 300 real-time PCR system (Stratagene). The integrity and specificity of the amplified PCR products were confirmed by dissociation curve analysis Mx 300 software (Stratagene). To normalize the degradation of total RNA used in cDNA synthesis, the threshold cycle values were determined for target genes and corresponding housekeeping genes (human β-actin) in each sample.
Relative abundance of HGFIN, IL-6, IL-10, CSF, and TNF mRNA levels in the ESRD group are presented as a ratio of the corresponding values found in the control cells at both day 1 and day 7 using the Pfaffl method (21). All measurements were conducted in duplicate, and three independent experiments were done.
Immunocytochemistry
Mononuclear cell preparations were inoculated at 1 × 105 cells/ml in eight-well Lab-Tek chamber slides (Nunc) at 0.4 ml/well. After 16 h, suspension cells (predominantly lymphocytes) were removed by aspiration and the adherent cells (monocytes) were cultured. For studies of monocyte-derived macrophages, the medium was replaced with complete growth medium with M-CSF (see the Cell Culture section). Half of the medium was replaced after 3 d. After 7 d, cells were washed with PBS and fixed with 4% formaldehyde in PBS for 20 min at 20°C. The cells were incubated for 16 h with or without primary antibody (mouse anti-GPNMB antibody; R&D Systems Inc., Minneapolis, MN). Antibodies were diluted 1:50 with PBS that contained 1% BSA to block nonspecific reactivity. The cells were then washed three times (5 min each) in PBS, and the slides were incubated for 2 h at room temperature with Alexa Fluor 594 goat anti-mouse IgG (Invitrogen-Molecular Probes, Carlsbad, CA) diluted in 1% normal goat serum and 1% BSA in PBS. After three washes in PBS, the slides were mounted with DAPI (Vectashield, Vector Laboratories, Burlingame, CA) and viewed by fluorescence microscopy.
Western Blot
Cultured cells were lysed for 5 min on ice in lysis buffer (10 mM Tris [pH 7.6], 150 mM NaCl, and 1% Triton X-100 and supplemented with protease inhibitors; Sigma). Protein concentrations were determined by BSA assay kit (Pierce), and 50 μg of total protein from each pooled sample was loaded into 10% Tris-SDS gel. Proteins were blotted to a nitrocellulose membrane, followed by incubation with anti-Gpnmb antibody (1:200 dilution; R&D systems) and/or anti-actin (1:10,000 dilution; Sigma). The appropriate horseradish peroxidase–conjugated secondary antibodies (Sigma) were used at a dilution of 1:5000, and membranes were visualized with Chemiluminescent HRP Substrate (Denville Scientific Inc.).
Statistical Analysis
Data are expressed as mean ± SD unless otherwise specified and analyzed with t test and regression analysis using SPSS; P < 0.05 was considered significant.
Results
General Data
Data are summarized in Table 1. Gender and mean ages were similar in both control and ESRD groups. The underlying causes of ESRD were diabetic nephropathy in five, hypertension in six, chronic glomerulonephritis in four, urologic causes in two, and unknown in four patients. The types of vascular access included arteriovenous fistulas in 16, arteriovenous grafts in three, and tunneled central catheter in two patients. As expected, serum creatinine and blood urea nitrogen concentrations were significantly higher in patients with ESRD compared with the control group. Likewise, serum concentrations of phosphorous and parathyroid hormone were significantly elevated in the patients with ESRD. Blood hemoglobin concentration was significantly lower in patients with ESRD, whereas serum calcium, total white cell count, and percentage of neutrophils, lymphocytes, and monocytes were not significantly different from those observed in the control group. The mean Kt/V value in the patients with ESRD was 1.43, reflecting the adequacy of the dialysis regimen in the study participants.
Table 1.
Clinical and biochemical parameters in normal control subjects and patients with ESRD
| Parameter | Control (n = 22) | ESRD (n = 21) | P |
|---|---|---|---|
| Age (yr) | 45 ± 13 | 49 ± 16 | NS |
| BUN (mg/dl) | 13.3 ± 1.0 | 67.9 ± 18.8 | <0.001 |
| Creatinine (mg/dl) | 0.85 ± 0.18 | 9.70 ± 3.70 | <0.001 |
| Hemoglobin (g/dl) | 14.3 ± 1.3 | 11.1 ± 1.4 | <0.001 |
| Kt/V | 1.42 ± 0.27 | ||
| Calcium (mg/dl) | 9.14 ± 0.24 | 8.80 ± 1.30 | NS |
| Phosphorous (mg/dl) | 3.20 ± 0.16 | 5.70 ± 2.00 | <0.001 |
| PTH ( pg/ml ) | 40.00 ± 3.84 | 504.30 ± 605.80 | <0.001 |
| WBC count (thousand/μl) | 6.2 ± 1.4 | 7.5 ± 2.9 | NS |
| Neutrophils (%) | 63.4 ± 6.9 | 62.5 ± 10.4 | NS |
| Lymphocytes (%) | 27.2 ± 5.9 | 23.9 ± 8.8 | NS |
| Monocytes (%) | 7.2 ± 1.9 | 7.2 ± 2.0 | NS |
Data are means ± SD. BUN, blood urea nitrogen; PTH, parathyroid hormone; WBC, white blood cell.
Cell Culture
Monocytes and macrophages were identified by the presence of specific morphologic characteristics. Monocytes appeared as homogeneous populations of small, circular, compact cells, whereas macrophages appeared as larger, fusiform-shaped cells that developed elongated processes, enlarged cytoplasm with a vacuolated appearance, and large, irregular, flattened, laterally positioned nucleus.
Overnight adhering monocytes from normal control subjects appear no different from control monocytes under light microscopy. As expected, some of the monocytes that were grown for 7 d with normal serum differentiated into macrophages, whereas some remained in dividing colonies. The addition of uremic serum to the culture medium resulted in macrophage differentiation of almost all of the cultured monocytes by day 7. Images are shown in Figure 1.
Figure 1.
Microscopic examination of normal monocytes after overnight culture (A) and after 7 d of culture using normal serum (B) and uremic serum (C).
Cytokine and Growth Factor Gene Expression
Patients with ESRD exhibited upregulation of IL-6 and TNF (proinflammatory cytokines) and downregulation of IL-10 (anti-inflammatory cytokine) mRNA levels in transformed monocytes that were grown in either uremic or normal serum. Normal control individuals exhibited comparable upregulation of IL-6 and TNF only in monocytes that were cultured with uremic serum. In contrast to the observation in patients with ESRD, normal control individuals exhibited upregulation of IL-10 mRNA levels in transformed monocytes that were grown in either uremic or normal serum. Data are shown in Figure 2. Uremic serum enhanced the expression of CSF (a marker/mediator of macrophage transformation) in normal monocytes after culture for 7 d, whereas uremic monocytes exhibited enhanced CSF expression in both normal and uremic serum. Data are shown in Figure 2. Taken together, these data demonstrate that monocyte-transformed macrophages derived from patients with ESRD express a proinflammatory cytokine phenotype and that monocyte-transformed macrophages derived from normal individuals can be transformed to express a proinflammatory cytokine phenotype by exposing them to uremic serum.
Figure 2.
mRNA expression of proinflammatory cytokines (IL-6, TNF), anti-inflammatory cytokines (IL-10), and CSF (a marker/mediator of macrophage transformation) in monocytes from normal control subjects (CTL; A) and patients with ESRD (B) after overnight culture (day 1) and after macrophage transformation (day 7) grown in either control (CS) or uremic serum (US). mRNA levels are presented as a ratio of corresponding values found in the control cells at both day 1 and day 7. *P < 0.05.
HGFIN Gene and Protein Expression
HGFIN mRNA expression in adhering monocytes was negligible in control subjects but elevated 8.6-fold in patients with ESRD. In vitro monocyte-to-macrophage transformation resulted in upregulation of HGFIN in both groups but much more in the patients with ESRD (17.5-fold). Data are shown in Figure 3. Culture with normal serum failed to normalize the HGFIN mRNA expression in uremic monocyte-derived macrophages, and culture with uremic serum did not increase HGFIN mRNA expression in the monocyte-derived macrophages taken from healthy control subjects (Figure 4).
Figure 3.
Comparison of HGFIN mRNA expression in monocytes obtained from control subjects (CTL) and patients with ESRD after overnight culture (day 1; A) and macrophage-transformation (day 7; B). Comparison of HGFIN mRNA levels in ESRD (C) and CTL cells (D) at day 1 and at day 7 after monocyte-macrophage transformation. *P < 0.05.
Figure 4.
mRNA expression of HGFIN in monocyte-derived macrophages from normal control subjects (CTL) and patients with ESRD grown in either control (CS) or uremic serum (US). mRNA levels are presented as a ratio of the corresponding values found in the control cells at both day 1 and day 7. *P < 0.05.
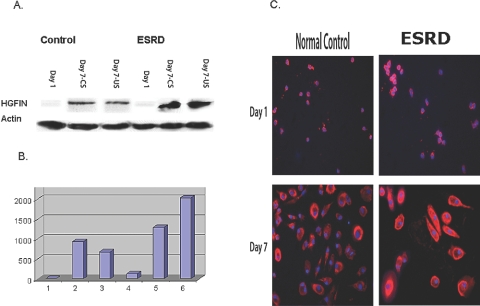
HGFIN protein expression in adhering monocytes was negligible in control subjects but elevated in patients with ESRD. As expected, in vitro monocyte-to-macrophage transformation resulted in upregulation of HGFIN protein expression in both groups but much more in the patients with ESRD when compared with normal monocytes after overnight culture. Normal serum failed to normalize the HGFIN protein abundance in uremic monocyte-derived macrophages, and uremic serum did not change the expression of HGFIN protein levels in the normal cells. These data are shown in Figure 5.
Figure 5.
HGFIN protein expression in control and ESRD cells. After overnight incubation (day 1) and macrophage transformation (day 7), cells were grown in normal serum (CS) and uremic serum (US). (A) Representative Western Blot. (B) Group data. (C) Immunocytochemical images of HGFIN protein expression in control and ESRD cells after overnight incubation (day 1) and macrophage transformation (day 7).
Immunocytochemical images confirm the Western blot data (Figure 5). Uremic monocytes (cultured in standard growth medium) exhibited increased HGFIN protein expression at 1 d and after in vitro macrophage transformation at 7 d when compared with normal control cells.
Correlations and Multivariate Analyses
A weak, statistically insignificant correlation was found with Kt/V and HGFIN gene expression (r = 0.37, P > 0.05). No significant correlations were found between markers of inflammation and severity of azotemia.
Discussion
This study shows for the first time that HGFIN gene and protein expressions are significantly increased in cultured uremic monocytes and macrophages. This is associated with heightened expression of proinflammatory and suppressed expression of anti-inflammatory cytokines. The upregulation of HGFIN and inflammatory cytokines in the uremic monocyte-derived macrophages occurs when grown in the presence of either normal or uremic serum, suggesting an enduring proinflammatory effect of the in vivo uremic milieu on circulating uremic monocytes. The precise implication of the presence of a macrophage marker on the surface of circulating uremic monocytes is not known. ESRD is characterized by a storm of inflammatory insults that are linked to many uremia-induced complications, including anemia, malnutrition, and vascular calcification (22). Vascular calcification and inflammation seem to be inextricably linked because vascular calcification results in inflammation and inflammation promotes vascular calcification (23–25). HGFIN, a growth factor initially cloned and characterized as osteoactivin in osteoblasts (1), has been identified as a regulator of macrophage activity and inflammation (12). As monocytes transform to macrophages, they exhibit upregulation of HGFIN, which is coupled with the reduction of IL-6 production and inflammation (12). In fact, mice with an inactivation mutation in HGFIN exhibit elevated numbers of peritoneal macrophages and higher levels of proinflammatory cytokines in response to LPS (12).
Because HGFIN is involved in inflammation, a process that is upregulated in ESRD, we explored its expression in circulating monocytes and monocyte-derived macrophages. We hypothesized that the patients with ESRD, who, in general, express a proinflammatory phenotype, would have reduced levels of HGFIN, a negative regulator of inflammation. Surprising, we observed upregulation of HGFIN associated with elevated levels of proinflammatory markers in hemodialysis patients. The mechanisms underlying these counterintuitive observations are unclear. It is possible that increased expression of HGFIN may represent a compensatory response to the prevailing inflammatory state in ESRD.
The functions of elevated HGFIN in monocytes and macrophages of patients with ESRD are unclear; however, because of its role in osteoblast and osteoclast maturation and mineralization, because osteoclasts and osteoblasts are derived from macrophage precursors, and because of the contribution of these cells to vascular calcification, it is tempting to theorize that there is an association with changes in HGFIN expression and vascular calcification in ESRD. Vascular calcification in patients with ESRD is associated with inflammation and marked perturbations of calcium, phosphorus, parathyroid hormone, and vitamin D metabolism (26,27). Vascular calcification is an active process in which osteoblast-like cells control the course through multiple paracrine signals, and detailed studies have also confirmed the role of bone morphogenic protein 2 (BMP-2), a potent morphogenic factor, in its pathogenesis (28,29). HGFIN has been shown to play an important role in osteoblast differentiation and bone mineralization (6,10,11), and its effects on osteoblasts seem to be regulated by BMP-2. Treatment of osteoblasts with BMP-2 results in increased HGFIN mRNA expression, and HGFIN acts as a downstream mediator of BMP-2 (10). If similar signaling pathways are activated in macrophages within the artery wall, then uremia-induced upregulation of HGFIN may serve as a mediator of vascular calcification in patients with ESRD.
To explore the effects of the uremic milieu on HGFIN expression, we cultured mature uremic monocytes with normal and uremic serum. The observed increase in HGFIN and proinflammatory cytokine expression in uremic monocyte-derived macrophages was preserved when these cells were grown in normal serum. These findings suggest that mature circulating monocytes from hemodialysis patients undergo “uremic programming” that is not rapidly reversible. The enduring effect of uremia observed in the monocyte-derived macrophages that were obtained from patients with ESRD may be consistent with their programmability and extended lifespan.
Conclusions
We report that uremic macrophages exhibit increased HGFIN gene and protein expression associated with heightened expression of proinflammatory and a suppressed expression of anti-inflammatory cytokines. The upregulation of HGFIN and inflammatory cytokines in the uremic monocyte-derived macrophages occurs when grown in the presence of either normal or uremic serum, suggesting an enduring effect of the in vivo uremic milieu.
Further studies are needed to determine the role of heightened monocyte/macrophage HGFIN expression in the pathogenesis of ESRD-associated inflammation and vascular calcification. We have initiated a study designed to compare HGFIN expression in calcified arteries obtained from patients who have ESRD and are undergoing kidney transplants with samples obtained from the normal donors. The planned studies might shed light on the potential role of HGFIN in chronic kidney disease–induced vascular calcification.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Safadi F, Xu J, Smock S, Rico M, Owen T, Popoff S: Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem 84: 12–26, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Haralanova-Ilieva B, Ramadori G, Armbrust T: Expression of osteoactivin in rat and human liver and isolated rat liver cells. J Hepatol 42: 565–572, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Shikano S, Bonkobara M, Zukas PK, Ariizumi K: Molecular cloning of dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparin sulfate. J Biol Chem 271: 8125–8134, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bandari PS, Qian J, Yehia G, Joshi D, Maloof PB, Potian J, Oh H, Gascon P, Harrison J, Rameshwar P: Hematopoietic growth factor inducible neurokinin-1 (HGFIN) gene: A transmembrane protein that is similar to neurokinin-1 interacts with substance. Regul Pept 111: 169–178, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bachner D, Schroder D, Gross G: mRNA expression of the murine glycoprotein (transmembrane) nmb (Gpnmb) gene is linked to the developing retinal pigment epithelium and iris. Brain Res Gene Expr Patterns 1: 159–165, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Selim A, Abdelmagid S, Kanaan R, Smock S, Owen T, Popoff S, Safadi F: Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro. Crit Rev Eukaryot Gene Expr 13: 265–275, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Owen T, Smock S, Prakash S, Pinder L, Brees D, Krull D, Castleberry T, Clancy Y, Marks S, Jr, Safadi F, Popoff S: Identification and characterization of the genes encoding human and mouse osteoactivin. Crit Rev Eukaryot Gene Expr 13: 205–220, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Weterman M, Ajubi N, van Dinter I, Degen W, van Muijen G, Ruitter D, Bloemers H: nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer 60: 73–81, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Nomiyama H, Egami K, Wada N, Tou K, Horiuchi M, Matsusaki H, Miura R, Yoshie O, Kukita T: Identification of genes differentially expressed in osteoclast-like cells. J Interferon Cytokine Res 25: 227–231, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Abdelmagid S, Barbe M, Arango-Hisijara I, Owen T, Popoff S, Safadi F: Osteoactivin acts as downstream mediator of BMP-2 effects on osteoblast function. J Cell Physiol 210: 26–37, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT: Transcriptional profiling of bone regeneration: Insight into the molecular complexity of wound repair. J Biol Chem 277: 30177–30182, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume D: GPNMB is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol 178: 6557–6566, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chung J, Sato K, Dougherty I, Cruz P, Jr, Ariizumi K: DC-HIL is a negative regulator of T lymphocyte activation. Blood 109: 4320–4327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung JS, Dougherty I, Cruz PD, Jr, Ariizumi K: Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J Immunol 179: 5778–5784, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Efstratiadis G, Tziomalos K, Mikhailidis DP, Athyros VG, Hatzitolios A: Atherogenesis in renal patients: A model of vascular disease? Curr Vasc Pharmacol 6: 93–107, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Kalantar-Zadeh K: Novel targets and new potential: Developments in the treatment of inflammation in chronic kidney disease. Expert Opin Investig Drugs 17: 451–467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Mehrotra R, Kalantar-Zadeh K: Battleground: Chronic kidney disorders mineral and bone disease—Calcium obsession, vitamin D, and binder confusion. Clin J Am Soc Nephrol 3: 168–173, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 20.Vaziri ND: Oxidative stress in chronic renal failure: The nature, mechanism and consequences. Semin Nephrol 24: 469–473, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl MA: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jofre R, Rodriguez-Benitez P, Lopez-Gomez JM, Perez-Garcia R: Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 17[Suppl 3]: S274–S280, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Peripheral vascular calcification in long-haemodialysis patients: Associated factors and survival consequences. Nephrol Dial Transplant 24: 948–955, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Porazko T, Kuzniar J, Kusztal M, Kuzniar TJ, Weyde W, Kuriata-Kordek M, Klinger M: IL-18 is involved in vascular injury in end-stage renal disease patients. Nephrol Dial Transplant 24: 589–596, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Al-Aly Z: Arterial calcification: A tumor necrosis factor-alpha mediated vascular Wnt-opathy. Transl Res 151: 233–239, 2008 [DOI] [PubMed] [Google Scholar]
- 26.London GM, Marchais SJ, Guerin AP, Metivier F: Arteriosclerosis, vascular calcifications, and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens 14: 525–531, 2005 [DOI] [PubMed] [Google Scholar]
- 27.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, deVernejoul MC: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL: Bone morphogenic protein expression in human atherosclerotic lesions. J Clin Invest 91: 1800–1809, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towler DA: Calciotropic hormones and arterial physiology: “D”-lightful insights. J Am Soc Nephrol 18: 369–373, 2007 [DOI] [PubMed] [Google Scholar]