Abstract
Background and objectives: Although results of renal replacement therapy (RRT) in small children have improved during recent years, data about RRT in neonates are scarce.
Design, setting, participants, & measurements: In a retrospective study, we analyzed the outcome of infants who had chronic kidney disease and started RRT within their first year of life. Between 1997 and 2008, all 29 infants who were younger than 1 yr, had end-stage renal failure, and underwent RRT (dialysis or transplantation) at Hannover Medical School were analyzed for up to 12 yr.
Results: Twenty-seven of 29 infants with chronic kidney disease received peritoneal dialysis, starting at a mean age of 112 d; two children received preemptive renal transplantation (RTx). During follow-up, 21 of 29 children survived with RTx. The 5-yr patient and graft survival rate after RTx was 95.5%. Six of 29 children died, one with a functioning graft and five while on peritoneal dialysis. The main causes of death were severe cardiovascular and cerebral comorbidities. The mean GFR at last follow-up of patients who underwent RTx (mean time after RTx 5.1 yr) was 63.2 ml/min per 1.73 m2.
Conclusions: RRT in infants who are younger than 1 year offers excellent chances of survival and should be offered to all infants who do not have severe, life-limiting extrarenal comorbidity. Contrary to previous observations, the long-term outcome of infants may be comparable to that of older children who undergo RRT.
Renal replacement therapy (RRT) for children with ESRD has been used for several decades; however, data on infants who start RRT in the first year of life are sparse. Early reports on RRT in ESRD in infants were discouraging (1). During a European conference on RRT in infants held in Berlin in 1998, there was controversy as to whether all neonates and infants with ESRD should be treated with RRT. In the past, pediatric nephrologists offered RRT to children preferably older than 1 yr, and the parents' decision to refuse RRT was more often accepted by nephrologists when the patients were younger than 1 mo (2). The age group younger than 2 yr was described as a risk factor for transplantation, because mortality and graft failure were high (3,4). In recent years, RRT has also become a routine procedure in infants. Transplantation is regarded as the treatment of choice for all children with ESRD, regardless of age, offering the best potential for catch-up growth and development (5–9). Because data on the cause and outcome of infants with ESRD are scarce, we report on our experience with the treatment of 29 infants who required RRT in the first year of life.
Materials and Methods
Between January 1997 and December 2008, all infants who had stage 5 chronic kidney disease (CKD) and started RRT in the first year of life were recorded in a retrospective study at the Children's Hospital of Hannover Medical School. RRT included peritoneal dialysis (PD) as well as renal transplantation (RTx). The median period of follow-up was 7.2 yr (range 0.3 to 11.7). Seven of 29 patients had a very short follow-up <3 mo; six patients died during their first year of life, and one patient was born at the end of the observation time.
Patients
Between January 1997 and December 2008, a total of 33 patients who had stage 5 CKD were identified, and 29 infants were included in our analysis (22 boys and seven girls). Four patients were excluded: RRT was not offered to these four neonates and infants because of severe extrarenal comorbidity such as respiratory, gastrointestinal, cardiac, and cerebral failure, after counseling with parents and the local ethics committee.
Causes of CKD were renal hypo-/dysplasia (15 of 29), obstructive uropathy (7 of 29), polycystic kidney disease (2 of 29), congenital nephrotic syndrome (2 of 29), Denys-Drash syndrome (1 of 29), and unknown origin (2 of 29). Twenty-seven infants (22 boys and seven girls) received RRT before the age of 1 yr. Median gestational age of these infants was 36 wk (range 29.3 to 41.6); 17 of 29 neonates were preterm. Median birth weight was 2430 g (range 1126 to 4610 g).
Data Collection
Primary renal diseases, patient gender, GFR, start and duration of dialysis, age, weight, height at transplantation, age, and cause of death were documented. Follow-up data were collected every 6 mo. The Schwartz formula was used to calculate GFR (10). Creatinine was measured in μmol/L; therefore, a factor k of 38 was used for all infants
Statistical Analysis
Kaplan-Meier analysis was used to analyze patient and graft survival. Results are given as mean ± SD or median and range, depending on whether normal distribution was given (Kolmogorov-Smirnov test). P < 0.05 was considered significant.
Results
Treatment
Twenty-seven of 29 infants started PD at a mean age of 112 d; the youngest patient started PD on day 3 of life. Anuria was documented for seven patients; 20 patients had normal urine production before starting dialysis treatment. No infant had polyuria. The median period on PD before RTx was 320 d (range 17 to 924 d). All infants were treated with PD: 23 had continuous ambulatory PD initially, two were switched to continuous-cycling PD later, and four started with continuous-cycling PD. None switched to hemodialysis. Eleven of 27 infants experienced 23 episodes of peritonitis, resulting in a peritonitis incidence of 0.85 episodes/patient-year on dialysis. Three patients had two episodes of peritonitis, and three had four episodes.
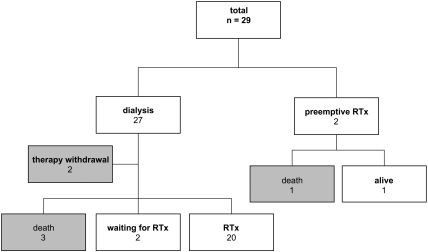
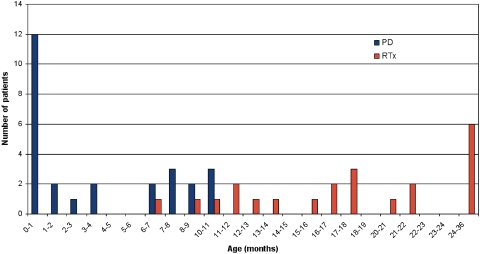
Twenty-two children successfully underwent transplantation, two of 22 underwent preemptive RTx, and two patients are still on PD awaiting RTx (Table 1, Figure 1). Figure 2 shows the age at start of PD and RTx. Five children died before RTx while on PD. The median age at transplantation was 1.4 yr (range 0.6 to 2.7 yr), median weight was 9.6 kg (range 6.2 to 20.5 kg), and median height was 78.3 cm (range 60.5 to 96.0 cm); the median age of donors was 32.5 yr (range 7.0 to 47.0 yr). Seven infants received a graft from a living-related donor. Five different immunosuppressive protocols were used: One patient was treated with cyclosporin A and prednisolone (1997); seven patients received basiliximab, cyclosporin A, and prednisolone (1997 through 2001); seven received cyclosporin A, mycophenolate mofetil, prednisolone, and basiliximab or placebo (2002 through 2005); four received basiliximab, cyclosporin A, mycophenolate mofetil, and prednisolone (2005 through 2006); and three received basiliximab, low-dosage cyclosporin A, everolimus, and prednisolone for 9 mo (2007 through 2008).
Table 1.
Primary renal diseases that caused stage 5 CKD in the first year of life
| Diagnosis | Patients (n [%]) | Death | Dialysis | RTx |
|---|---|---|---|---|
| Renal hypo-/dysplasia | 15 (52) | 2 | 13 | |
| Obstructive uropathy | 7 (24) | – | 1 | 6 |
| Polycystic renal disease | 2 (7) | 1 (with functioning graft) | 1 | 1 |
| Congenital nephrotic syndrome | 2 (7) | 1 | 1 | |
| Renal failure of unknown origin | 2 (7) | 1 | 1 | |
| Denys-Drash syndrome | 1 (3) | 1 | – |
Figure 1.
The outcome of 29 infants who had stage 5 CKD and started RRT in the first year of life.
Figure 2.
The age at start of PD and RTx.
Biopsy-proven acute rejection episodes were detected in four of 22 patients (all were treated with different immunosuppressive therapy). There was no graft loss. One girl developed a posttransplantation proliferative disease that was successfully treated with rituximab.
Outcome
During the study period, six of 29 patients died 93 to 365 d after start of RRT. Five of the six patients died during the study period while receiving PD, which had been started at the age of 3 to 208 d of life. PD was stopped in two of five infants because of extrarenal complications. In both cases, it was the consensus of parents, clinical teams, and the ethics committee to stop further therapy. One boy had multicystic kidney disease on one side and ureteral agenesis on the other side. He died of respiratory insufficiency at 93 d of age. He also suffered from the inability to tolerate enteral feeding and had received total parenteral nutrition all his life. The other boy, who had CKD of unknown origin and severe respiratory insufficiency, died on day 106.
Three infants died while on PD. One girl with Denys-Drash syndrome died of a stroke on day 167, one girl with mitchondriopathy and congenital nephrotic syndrome died on day 261 from cardiovascular dysfunction after aspiration, and one girl with renal dysplasia died after 279 d of a stroke (Table 2). One infant who had autosomal recessive polycystic kidney disease died after successful preemptive kidney transplantation with a functioning graft as a result of cerebro-arterial spasms of unknown origin.
Table 2.
Course of the disease in six deceased infants with RRT
| Patient | Diagnosis | Prematurity | Age at Start of PD (d) | Age at RTx (d) | Death (d) | Cause of Death |
|---|---|---|---|---|---|---|
| 1 | Autosomal recessive polycystic kidney disease, epilepsy, subarachnoid hemorrhage | No | – | 204 | 365 | Cardiovascular dysfunction and cerebral insult, no transplant failure |
| 2 | Renal dysplasia, respiratory distress syndrome III,a epilepsy | Yes | 3 | – | 279 | Cardiovascular dysfunction and cerebral insult |
| 3 | Congenital nephrotic syndrome, mitchondriopathy | Yes | 208 | – | 261 | Cardiovascular dysfunction after aspiration |
| 4 | Denys-Drash syndrome, epilepsy | Yes | 9 | – | 167 | Cerebral insult |
| 5 | Renal failure of unknown syndromic origin, cirrhosis of the liver, hypothyroidism, cutis laxa, muscle hypotonia | Yes | 6 | – | 106 | Treatment withdrawal, respiratory insufficiency |
| 6 | Renal dysplasia, respiratory distress syndrome II,a hypothyroidism | Yes | 24 | – | 93 | Treatment withdrawal, respiratory insufficiency |
Grade.
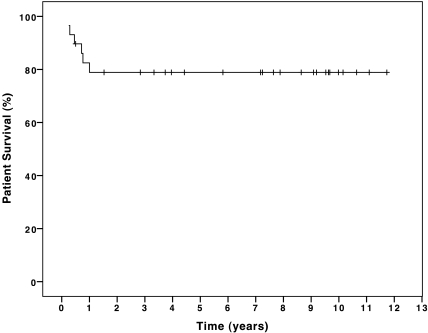
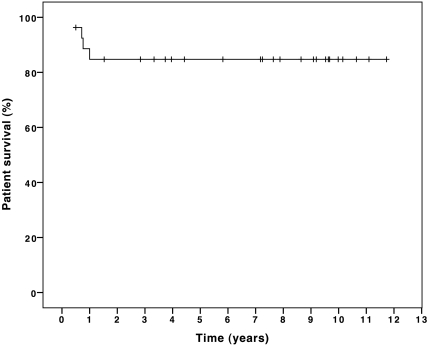
The estimated 1-, 2-, and 5-yr patient survival of all 29 infants with chronic renal failure (CRF) was 79% (Figure 3). Excluding the two patients for whom dialysis therapy was withdrawn, the patient survival rate of 23 of 27 infants was 85% at 1, 2, and 5 yr of age (Figure 4). Patient survival and graft survival of 22 infants who underwent RTx was 95.5% after 1, 2, and 5 yr. There was no difference in outcome of patients who started RRT in the first versus the last period of follow-up.
Figure 3.
Cumulative patient survival of 29 infants who had CRF and received RRT in the first year of life.
Figure 4.
Cumulative patient survival of 27 infants who had CRF and received RRT in the first year of life, excluding the two patients who had therapy withdrawn.
GFR
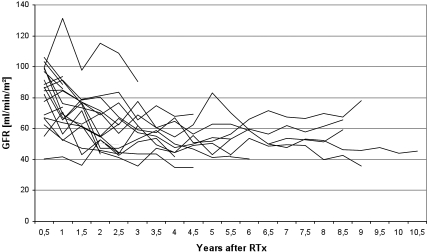
Figure 5 shows the course of GFR after successful kidney transplantation. Mean GFR at last follow-up of patients who underwent RTx (median time after RTx 5.6 yr; range 0.4 to 10.1 yr) was 63.2 ml/min per 1.73 m2 (range 3.4 to 123 ml/min per 1.73 m2).
Figure 5.
Individual course of GFR in infants who underwent RTx (n = 22).
Discussion
ESRD is a rare but important health problem in infants. The estimated incidence has remained stable in the past few decades (11,12). North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) reported an incidence of 0.045 cases of dialysis-treated neonatal ESRD per million population per year (13). No comparable data for newborns is available in Germany. The incidence for RRT in German children who were younger that 15 yr was 95 in 2006 (14). RRT in infants with ESRD has been established for several years, and the survival rate has increased steadily in the past 30 yr. Whereas in the early days of pediatric nephrology young age at start of dialysis had been shown to be a significant predictor of poor outcome (15–17), more recent data show no difference in mortality rates between patients who started dialysis during the neonatal period and those who began dialysis at the age of 2 to 24 mo (12).
Causes of CKD and gender distribution in our study were comparable to the results of other centers (11,12). Renal dysplasia and obstructive uropathy were the predominant diagnoses. Infants with CKD are also at risk for severe extrarenal congenital anomalies. It has been shown that lung hypoplasia had an adverse effect on survival (17).
Seventeen (59%) of 29 infants were preterm. This percentage is significantly higher than that of preterm infants in the total infant population of Germany (6%), confirming that congenital abnormalities of the kidneys or/and the urinary tract is a risk factor for prematurity.
PD is the most commonly used treatment in infants who require dialysis (18,19). Hemodialysis is rarely used in newborns because of the technical difficulties experienced with vascular access and a high extracorporeal blood volume, as well as the disadvantages of hospital dialysis. Among the problems of dialysis in infants are frequent infections, poor growth, and a higher mortality rate in contrast to older children (3,20). Shroff et al. (20) found a 2.7 times higher relative risk for death in infants who started dialysis treatment at ≤5 yr of age in contrast to those who started dialysis above 5 yr of age. Peritonitis episodes in our study were comparable to the results of Laakkonen et al. (21) with an incidence of 0.83 episodes per patient-year. A younger age at the start of RRT, especially for infants who are younger than 1 yr, has been a risk factor for mortality (15,16,20); however, early transplantation, in particular preemptive transplantation, has been shown to improve survival and reduce mortality in children who have CRF and are younger than 5 yr (7,22). Patients who underwent preemptive RTx showed better graft survival (23), probably because the donor was living related and because of shorter cold ischemia time (24).
Survival of infants who underwent RTx was comparable to that of older children. Elshiabi et al. (25) and McDonald et al. (26) reported patient survival at 1, 2, and 5 yr for grafts from cadaveric donors of 96.3, 95.2, and 91.4% and for grafts from living-related donors of 97, 96.5, and 94.5%, respectively. Furthermore, Najarian et al. (22,27) found no difference in patient or graft survival between children aged <1, 1 to 2, and 2 to 5 yr. More recent analyses showed equivalent results in age groups <1, 1 to 4, and 5 to 13 yr (28). Rees et al. (29) documented that the outcome of successful transplantation was unaffected by age at time of transplantation. The survival of living-related donor transplants was superior to deceased-donor transplants for the first 5 yr. The findings of our study confirm these results. Of 29 infants in our study, 22 underwent successful transplantation (two preemptive) with a 5-yr patient and graft survival of 95.5%. Graft function declined slowly after RTx, comparable to the results of Kari et al. (22).
In our study, most infants died in the first year of life as a result of severe extrarenal comorbidity. Several studies showed that extrarenal comorbidity had the most important influence on outcome (17,30,31). In the study of Shroff et al. (20), nonrenal comorbidity was a significant predictor of poor outcome with a 7.5 times increased risk for death. Kari et al. (30) and Neu et al. (32) found 1-, 2-, and 3-yr survival rates of 85, 74, and 68% for infants who were younger than 1 yr at initiation of dialysis compared with 79% after 1, 2, and 3 yr in our study. Excluding the two patients who had therapy withdrawn, 1-, 2-, and 3-yr patient survival was 85%. NAPRTCS reported patient survival for all dialysis patients (including acute renal failure and CRF) after 12 mo of 81.9% for the age group <1 yr, 92.2% for children aged 2 to 5 yr, and 97.6% for children aged >12 yr (33).
Compared with other published data, our study includes all infants who received PD since birth, regardless of duration of PD. In the majority of other studies, only infants who underwent PD for a continuous period of ≥3 mo were included, and children who received PD in the first days of life were excluded; therefore, different survival rates were documented for neonates who were treated with RRT.
We showed improved survival of neonates and infants with ESRD with early initiation of RRT (34) in contrast to other reports (1,3,15,16). There was no difference in outcome of patients who started RRT in early versus late time of follow-up. Center politics concerning guidelines and clinical approach were not changed during study time. Most patients died from comorbidities rather than RRT. All deaths occurred in the first year of RRT, which means that the prognosis is excellent beyond 1 yr of age. Survival for infants who underwent RTx was comparable to that of older children. Our findings may help to support the decision of parents and nephrologists to offer RRT to even the youngest patients. PD treatment is an acceptable option when preparing for successful transplantation. Intensive pretransplantation care is necessary, including dietetic care (including tube feeding); early therapy with growth hormone (35); and medical, surgical, and psychological care. Although survival was excellent, there may be other outcome measures, such as quality of life, growth, or development, that have not been well defined but are important to consider when discussing the long-term picture with families.
Conclusions
RRT in neonates and infants who do not have severe comorbidity and start therapy at <1 yr of age offers good chances of survival and should therefore be offered routinely. In children with severe comorbidities, individual decisions have to be made when deciding on whether to start RRT. The decision should be based on a consensus among parents, neonatologists, attending pediatric nephrologists, and the ethics committee.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Moel DI, Butt KM: Renal transplantation in children less than 2 years of age. J Pediatr 99: 535–539, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Geary DF: Attitudes of pediatric nephrologists to management of end-stage renal disease in infants. J Pediatr 133: 154–156, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Warady BA, Hebert D, Sullivan EK, Alexander SR, Tejani A: Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents: The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 11: 49–64, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Ehrich JH, Rizzoni G, Brunner FP, Fassbinder W, Geerlings W, Mallick NP, Raine AE, Selwood NH, Tufveson G: Renal replacement therapy for end-stage renal failure before 2 years of age. Nephrol Dial Transplant 7: 1171–1177, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Offner G, Hoyer PF, Ehrich JH, Pichlmayr R, Brodehl J: Paediatric aspects of renal transplantation: Experience of a single centre. Eur J Pediatr 151[Suppl 1]: S16–S22, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Aschendorff C, Offner G, Winkler L, Schirg E, Hoyer PF, Brodehl J: Adult height achieved in children after kidney transplantation. Am J Dis Child 144: 1138–1141, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Najarian JS, Almond PS, Mauer M, Chavers B, Nevins T, Kashtan C, Matas AJ: Renal transplantation in the first year of life: the treatment of choice for infants with end-stage renal disease. J Am Soc Nephrol 2[Suppl]: S228–S233, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Vester U, Offner G, Hoyer PF, Oldhafer K, Fangmann J, Pichlmayr R, Brodehl J: End-stage renal failure in children younger than 6 years: Renal transplantation is the therapy of choice. Eur J Pediatr 157: 239–242, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Nissel R, Brazda I, Feneberg R, Wigger M, Greiner C, Querfeld U, Haffner D: Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 66: 792–800, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 11.Coulthard MG, Crosier J: Outcome of reaching end stage renal failure in children under 2 years of age. Arch Intern Med 87: 511–517, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey WA, Talley LI, Sehring SA, Jaskula JM, Mathias RS: Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: A North American Pediatric Renal Trials and Collaborative Studies special analysis. Pediatrics 119: e468–e473, 2007 [DOI] [PubMed] [Google Scholar]
- 13.North American Pediatric Renal Trials and Collaborative Studies: NAPRTS annual report 2006. Available at: https://web.emmes.com/study/ped/annlrept/annlrept2006.pdf Accessed June 15, 2009
- 14.Frei U, Schober-Halstenberg HJ: Nierenersatztherapie in Deutschland, Bericht 2006/2007, QuaSi-Niere. Available at: http://www.bundesverband-niere.de/files/QuaSi-Niere-Bericht_2006-2007.pdf Accessed June 15, 2009
- 15.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS: Mortality and causes of death of end-stage renal disease in children: A Dutch cohort study. Kidney Int 61: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
- 16.McDonald SP, Craig JC: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Wood EG, Hand M, Briscoe DM, Donaldson LA, Yiu V, Harley FL, Harley FL, Warady BA, Ellis EN; North American Pediatric Renal Transplant Cooperative Study: Risk factors for mortality in infants and young children on dialysis. Am J Kidney Dis 37: 573–579, 2001 [PubMed] [Google Scholar]
- 18.Leonard MB, Donaldson LA, Ho M, Geary DF: A prospective cohort study of incident maintenance dialysis in children: An NAPRTC study. Kidney Int 63: 744–755, 2003 [DOI] [PubMed] [Google Scholar]
- 19.van der Heijden BJ, van Dijk PC, Verrier-Jones K, Jager KJ, Briggs JD: Renal replacement therapy in children: Data from 12 registries in Europe. Pediatr Nephrol 19: 213–221, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann S: Long-term outcome of chronic dialysis in children. Pediatr Nephrol 21: 257–264, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Laakkonen H, Holtta T, Lonnqvist T, Holmberg C, Ronnholm K: Peritoneal dialysis in children under two years of age. Nephrol Dial Transplant 23: 1747–1753, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kari JA, Romagnoli J, Duffy P, Fernando ON, Rees L, Trompeter RS: Renal transplantation in children under 5 years of age. Pediatr Nephrol 13: 730–736, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Ishitani M, Isaacs R, Norwood V, Nock S, Lobo P: Predictors of graft survival in pediatric living-related kidney transplant recipients. Transplantation 70: 288–292, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Cransberg K, Smits JM, Offner G, Nauta J, Persijn GG: Kidney transplantation without prior dialysis in children: The Eurotransplant experience. Am J Transplant 6: 1858–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Elshihabi I, Chavers B, Donaldson L, Emmett L, Tejani A: Continuing improvement in cadaver donor graft survival in North American children: The 1998 annual report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Transplant 4: 235–246, 2000 [DOI] [PubMed] [Google Scholar]
- 26.McDonald R, Donaldson L, Emmett L, Tejani A: A decade of living donor transplantation in North American children: The 1998 annual report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Transplant 4: 221–234, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Najarian JS, Almond PS, Gillingham KJ, Mauer SM, Chavers BM, Nevins TE, Kashtan CE, Matas AJ: Renal transplantation in the first five years of life. Kidney Int Suppl 43: S40–S44, 1993 [PubMed] [Google Scholar]
- 28.Chavers B, Najarian JS, Humar A: Kidney transplantation in infants and small children. Pediatr Transplant 11: 702–708, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rees L, Shroff R, Hutchinson C, Fernando ON, Trompeter RS: Long-term outcome of paediatric renal transplantation: Follow-up of 300 children from 1973 to 2000. Nephron Clin Pract 105: c68–c76, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with severe chronic renal failure. Kidney Int 57: 1681–1687, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS: Long-term outcome of peritoneal dialysis in infants. J Pediatr 136: 24–29, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Neu AM, Ho PL, McDonald RA, Warady BA: Chronic dialysis in children and adolescents: The 2001 NAPRTCS Annual Report. Pediatr Nephrol 17: 656–663, 2002 [DOI] [PubMed] [Google Scholar]
- 33.North American Pediatric Renal Trials and Collaborative Studies: NAPRTS 2008 annual report. Available at: https://web.emmes.com/study/ped/annlrept/Annual%20Report%20-2008.pdf Accessed June 15, 2009
- 34.Wedekin M, Ehrich JH, Offner G, Pape L: Aetiology and outcome of acute and chronic renal failure in infants. Nephrol Dial Transplant 23: 1575–1580, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Franke D, Zivicnjak M, Ehrich JH: Growth hormone treatment of renal growth failure during infancy and early childhood. Pediatr Nephrol 24: 1093–1096, 2009 [DOI] [PubMed] [Google Scholar]