Abstract
Background and objectives: In 2000, we reported the outcome of 101 children with a GFR <20 ml/min per 1.73 m2 at 0.3 yr of age (range 0.0 to 1.5 yr). Long-term data on such young children are scarce.
Design, setting, participants, & measurements: Mortality, treatment modalities, and growth were reanalyzed 9.9 yr later.
Results: Of the 101 patients, 28 died and three were lost to follow-up during 13.90 yr (range 0.03 to 22.90 yr). One-, 2-, 5-, 10-, 15-, 20-, and 22-yr survivals were 87, 81, 77, 75, 73, 72, and 64%, respectively. Fifty-one children had comorbidities. Sixty-six percent were tube fed for 1.7 yr (range 0.1 to 6.9 yr), 37% had a gastrostomy, and 13% had a Nissen fundoplication. Mean height SD score (SD) was −0.42 (2.33) at birth (n = 40), −2.07 (1.34) at 0.5 (n = 62), −1.93 (1.38) at 1 (n = 72), −1.14 (1.14) at 5 (n = 67), −1.04 (1.15) at 10 (n = 62), −1.84 (1.32) at 15 (n = 40), and −1.68 (1.52) at age ≥18 yr (n = 32). Comorbidities adversely influenced growth (P < 0.01) and final height (P = 0.02): Mean height SD score (SD) was −1.16 (1.38) in otherwise normal adults.
Conclusions: Growth and final height in infants with severe chronic kidney disease are influenced by comorbidity. Intensive feeding and early transplantation resulted in a mean adult height within the normal range in patients without comorbidities. Overall mortality is comparable to that of older children.
In the past 25 yr, renal replacement therapy (RRT) for infants who have other challenging treatment issues, such as prematurity and comorbid conditions, has become increasingly accepted. Medium-term data demonstrate satisfactory outcomes for growth, development, and transplantation (1–3), although comorbidity is recognized as a factor that affects success of treatment (1,4–6). Long-term outcome is unknown for infants who are treated from the inception of such programs. In 2000, we published the survival and growth of 101 children who were aged <2 yr and had severe chronic kidney disease (CKD) after up to 13 yr of follow-up (1). We present the outcome of these patients after up to 22.9 yr of follow-up. We hope that these unique data will help doctors inform families about the prognosis for similarly affected infants.
Materials and Methods
In 2000, we published the outcome of 101 children who were born between January 1, 1986, and December 31, 1997, were referred to our tertiary unit, received a renal diagnosis within the first 6 mo of life, and had a Cr-EDTA GFR <20 ml/min per 1.73 m2 or required RRT in the first 2 yr of life (1). The hospital database ensured that all who fulfilled these criteria were captured. The aim of this article was to reanalyze the outcome of this population 9.9 yr later.
Demographic characteristics, comorbidities, and renal diagnoses were collected in the original study (1). We divided the cohort into two groups according to the absence (group 1) or presence (group 2) of comorbidities. Comorbidity was defined as involvement of an organ other than the kidney (excluding mild developmental delay). Follow-up information was obtained from the notes, including age and cause of death; age at onset of dialysis; age at transplantation; donor type (living-related [LRT] or deceased-donor transplant [DDT]); transplant survival and function (eGFR by Schwartz formula with a K value of 33, calculated in our laboratory); age of onset and duration of tube feeding; gastrostomy and Nissen fundoplication; and yearly weight, height, body mass index [BMI], and intact parathyroid hormone (iPTH) levels.
Management Policy
Infants are seen in clinic on average twice weekly to enable a steady increase in feed and dialysis prescriptions with growth. Thereafter, frequency of appointments is reduced. The reviewing team includes a senior doctor, a renal dietician, and a nurse specialist. Care is paid to normalizing the iPTH, acid-base balance, albumin, and hemoglobin before and after transplantation (4,7). Tube feeding is introduced when expected growth is not achieved. A Nissen fundoplication is undertaken when vomiting persists. Dietary aims are to provide 100% of estimated average requirement for energy and 100% of the recommended nutrient intakes for protein (with a supplement for dialysis) on the basis of dietary reference values for a normal population. A whey-dominant formula is used before and a whole-milk protein feed after 2 yr of age, supplemented with carbohydrate and/or fat and protein (8). Peritoneal dialysis (PD) is the preferred modality for RRT; hemodialysis (HD) is used when PD fails (9). Recombinant human growth hormone (rhGH) is prescribed when the height SD score (HtSDS) is >−2 SD and height velocity SDS is <25th centile, after optimization of nutrition, metabolic control, and dialysis adequacy. rhGH is usually stopped at transplantation. Minimum weight for transplantation was 8 kg. Adult patients were traced (with ethical approval) using their NHS number to obtain their final heights.
Statistical Analysis
Results are described as mean (SD) or median (range). Heights are expressed as HtSDS and weights as BMI. Patient and graft survival were obtained using Kaplan-Meyer survival analysis. Comparison between survival functions was carried out with the log-rank test. Differences between variables were determined by the t test for parametric variables. The proportions were compared with χ2 test. When the data were matched by age, a paired t test was used. Significance was defined as P < 0.05. All analyses were performed using SPSS for Windows. Growth (HtSDS) has been analyzed according to nine different variables with univariate and multivariate regression models (gender, presence/absence of comorbidities, transplantation before or after dialysis, duration of dialysis or transplantation (<1 or ≥1 yr), number of transplantations, age at transplantation (<2 or ≥2 yr), LRT or DDT, and small for gestational age (SGA).
Results
Seventy-six children were male and 25 were female (gender ratio 3:1). Birth weights were available for 41 children. Median gestational age was 37 wk (range 30 to 44 wk). Twenty-six were preterm (<37 wk gestation), 18 were SGA, and 10 were preterm and SGA. Median age at presentation to our unit was 0.3 yr (range 0.0 to 1.5 yr). Twenty-nine received a diagnosis antenatally. Median follow-up was 13.90 yr (range 0.03 to 22.90 yr), with no difference between group 1 (13.80 yr [range 0.03 to 22.80 yr]) and group 2 (14.30 yr [range 0.22 to 22.90 yr]). Median age of the survivors at final follow-up was 17.8 yr (range 10.4 to 22.9 yr).
Fifty children did not (group 1) and 51 did (group 2) have comorbidities. Renal diagnoses were dysplasia with or without reflux or obstruction (n = 58), posterior urethral valve (n = 22), congenital nephrotic syndrome (CNS; n = 12), acute cortical necrosis (n = 5), renal venous thrombosis (n = 2), and nephronophthisis (n = 2). Comorbidities are represented in Table 1. Most (41 of 51) had more than one comorbidity.
Table 1.
Associated comorbidities
| Comorbidity | n |
|---|---|
| Heart disease | 10 |
| transposition of the great arteries | 1a |
| coarctation | 2 |
| aortic valve stenosis | 2 |
| pulmonary valve stenosis | 4 |
| ventricular septal defect | 1 |
| Pulmonary disease | 4 |
| congenital pulmonary dysplasia | 2 |
| cystic adenomatoid malformation of the lung | 1a |
| cystic fibrosis | 1 |
| Neurologic involvement | 24 |
| developmental delay with microcephaly | 9 (6a) |
| developmental delay associated with undiagnosed syndrome | 3 |
| developmental delay associated with syndrome | 2 |
| epilepsy | 5 |
| spastic diplegia/hemiplegia | 2 |
| intracranial hypertension | 1 |
| obstructive hydrocephalus | 1a |
| sacral agenesis and spinal cyst | 1 |
| Gastrointestinal disease | 7 |
| cleft palate | 2 |
| gut resection and ileostomy (ulcerative colitis/infarct) | 2 |
| gut malrotation | 1 |
| imperforate anus | 1 |
| Esophageal and duodenal atresia | 1 |
| Blindness/visual impairment | 6 |
| Deafness/hearing impairment | 2 |
| Diabetes | 1 |
| Hypogammaglobulinemia | 1a |
| Pauciarticular juvenile chronic arthritis | 1 |
| Vertebral abnormalities | 2 |
| Underlying syndromesb | 19 |
The majority (41 of 51) of the patients had more than one comorbidity.
Only one nonrenal comorbidity.
Galloway Mowatt (1), Down (1), Jeune (2), Alagille (1), CHARGE: Coloboma, Heart defects, Atresia (choanal), Retardation (of growth and/or development), Genital and/or urinary anomaly, and Ear anomaly (1), VATER: Vertebrae, Anus, Trachea, Esophagus, and Renal (2), BOR: Branchio-Oto-renal (2), Ivemark (1), Bardet-Biedl (3), prune belly (1), Asperger (1), and undiagnosed (3).
Patient Survival
Of the 101 patients, 28 died, 17 of whom had comorbidities (Table 2). In five, it was decided not to start RRT. Of these, four were otherwise normal infants whose parents decided that they did not want to inflict pain and suffering on their infant; all died in the first 6 mo of life. The fifth infant had microcephaly and developmental delay and died at 9.6 mo of age. Treatment was withdrawn for seven children, six on PD and one on HD. One had CNS with no comorbidities, and the others had cystic fibrosis (n = 1); pulmonary dysplasia (n = 1); Down syndrome (n = 1); microcephaly, blindness, and deafness (n = 1); and perinatal asphyxia (n = 2). Four died in the first 6 mo of life, one before 1 yr, one at 1.9 yr, and one at 5.2 yr.
Table 2.
Mortality (age, cause, and association with comorbidities)
| Parameter | Treatment not Started (n = 5) |
Treatment Withdrawal (n = 7) |
Actively Treated (n = 16) |
|||
|---|---|---|---|---|---|---|
| With Comorbidities | Without Comorbidities | With Comorbidities | Without Comorbidities | With Comorbidities | Without Comorbidities | |
| Number | 1 | 4 | 6 | 1 | 10 | 6 |
| Age at death | ||||||
| <6 mo | 0 | 4 | 4 | 1 | ||
| 6 mo to 1 yr | 1 | 0 | 1 | 1 | 1 | |
| 1 to 2 yr | 1 | 3 | 2 | |||
| 2 to 5 yr | 1 | 2 | ||||
| 5 to 10 yr | 1 | 1 | ||||
| 10 to 15 yr | 1 | |||||
| 15 to 20 yr | 1 | |||||
| >20 yr | 1 | |||||
| Modality of treatment at death | ||||||
| conservative | 2 | 2 | ||||
| PD | 5 | 1 | 4 | 1 | ||
| HD | 1 | 2 | ||||
| Transplant | 4 | 1 | ||||
| Cause of death | ||||||
| cot death | 2 | 3 | ||||
| sepsis | 3 | 1 | ||||
| mesenteric infarct/GI hemorrhage | 1 | 1 | ||||
| pulmonary edema | 1 | |||||
| hyperkalemia | 1 | |||||
| PTLD | 3 | |||||
GI, gastrointestinal; PTLD, posttransplantation lymphoproliferative disease.
Sixteen children died while actively treated. Ten had comorbidities: Seven were neurologic, including microcephaly and developmental delay (n = 3), Galloway Mowatt syndrome (n = 1), intracerebral events causing blindness and developmental delay (n = 2), GH deficiency (n = 1), and obstructive hydrocephalus (n = 1). The other three patients had Alagille syndrome, Jeune syndrome, and hypogammaglobulinemia. Causes of death are presented in Table 2.
Overall patient survivals at 1, 2, 5, 10, 15, 20, and 22 yr were 87, 81, 77, 75, 73, 72, and 64%, respectively. Thirteen of the 28 deaths were within the first year. Patient survival for group 1 was 87.7, 83.5, 81.3, 79.0, 79.0, 79.0, and 79.0% and for group 2 was 86.3, 78.4, 72.3, 70.2, 67.6, 64.5, and 48.0% (Figure 1). Although survival was lower in group 2 after the first year, this did not reach statistical significance (P = 0.13). Infants who were SGA (n = 18) had increased mortality (seven of 18 died; P < 0.01); this was not because of comorbidity (P = 0.69). The duration of dialysis did not affect mortality (P = 0.34), and there was no difference in duration of dialysis between groups (P = 0.06). Survival was better with preemptive transplantation (P = 0.015) and was unaffected by recipient age (before versus after age 2 yr; P = 0.95) or donor type (LRT versus DDT; P = 0.313).
Figure 1.
Patient survival.
Renal Outcome
Treatment modalities and outcome of all patients are shown in Figure 2. Of the 101, 48 started dialysis at age 1.3 yr (range 0.0 to 14.0 yr), 40 of whom received a transplant at age 3.2 yr (range 1.0 to 15.3 yr). Thirty-one received a preemptive transplant at age 5.5 yr (range 1.4 to 15.6 yr) and seven continued on conservative treatment. Three patients were lost to follow-up.
Figure 2.
Progress of treatment modality.
The 71 transplant patients received 91 transplants. Fifty-six first transplants were DDT and 15 were LRT. Sixteen required a second (14 DDT and two LRT) and four a third transplant (two DDT and two LRT).
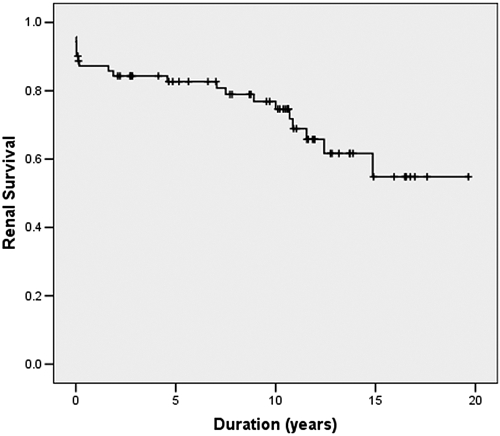
Of the 71 first transplants, 21 (30%) lost their graft after 1.9 yr (range 0.0 to 14.9 yr). First transplant survival was 75, 55, and 55% at 10, 15, and 20 yr, respectively (Figure 3). eGFR was 57 at 1 and 20 ml/min per 1.73 m2 at 15 yr after transplantation. Neither transplantation at age <2 yr (n = 13; P = 0.14) nor preemptive transplantation (n = 31; P = 0.44) affected transplant survival.
Figure 3.
First transplant survival.
Five-, 10-, and 15-yr transplant survivals for DDT were 76.2, 67.3, and 55.9% and for LRT were 100.0, 100.0, and 41.7% (P = 0.045, 0.025, and 0.140, respectively). Twenty-year survival was 55.9% for DDT. Because the practice of LRT started later, longer outcome data are not available.
Causes of transplant failure were vascular thrombosis (eight of 21) at a mean of 4 d (range 3 to 12 d) after transplantation and age of 3.5 yr (range 1.4 to 5.5 yr) and acute and/or chronic rejection (13 of 21) after 8.9 year (range 0.2 to 14.9 yr) at age 12.1 yr (range 1.2 to 19.3 yr). Seventeen patients received a second transplant at age 8.9 yr (range 2.5 to 20.5 yr); five were lost from acute and/or chronic rejection after 3.8 yr (range 0.9 to 9.2 yr) at age 11.5 yr (range 5.9 to 16.9 yr). Four patients received a third transplant at age 15.1 yr (range 7.0 to 18.3 yr), one of whom received temporary HD during pregnancy. At final follow-up, 60 patients had had a transplant, six had returned to dialysis, one was on PD and awaiting a first transplant, and three were still being treated conservatively.
Nutrition and Growth
Sixty-six children were tube fed for a median duration of 1.7 yr(range 0.1 to 6.9 yr). The median age at starting and stopping tube feeds is shown in Table 3 for each group. More children with comorbidities were tube fed, but this did not reach statistical significance (P = 0.24).
Table 3.
Nutritional support
| Support | All | With Comorbidities (n = 51) | Without Comorbidities (n = 50) |
|---|---|---|---|
| Tube fed (yr; median [minimum to maximum]) | 66 | 36 | 30 |
| duration | 1.7 (0.1 to 6.9) | 1.6 (0.1 to 6.9) | 1.7 (0.2 to 6.3) |
| age at start | 0.8 (0.0 to 4.9) | 1.0 (0.0 to 4.9) | 0.6 (0.0 to 2.2) |
| age at stop | 2.5 (0.1 to 8.7) | 2.6 (0.1 to 8.7) | 2.4 (0.4 to 6.6) |
| Gastrostomy | 37 | 24 | 13 |
| Nissen fundoplication | 13 | 9 | 4 |
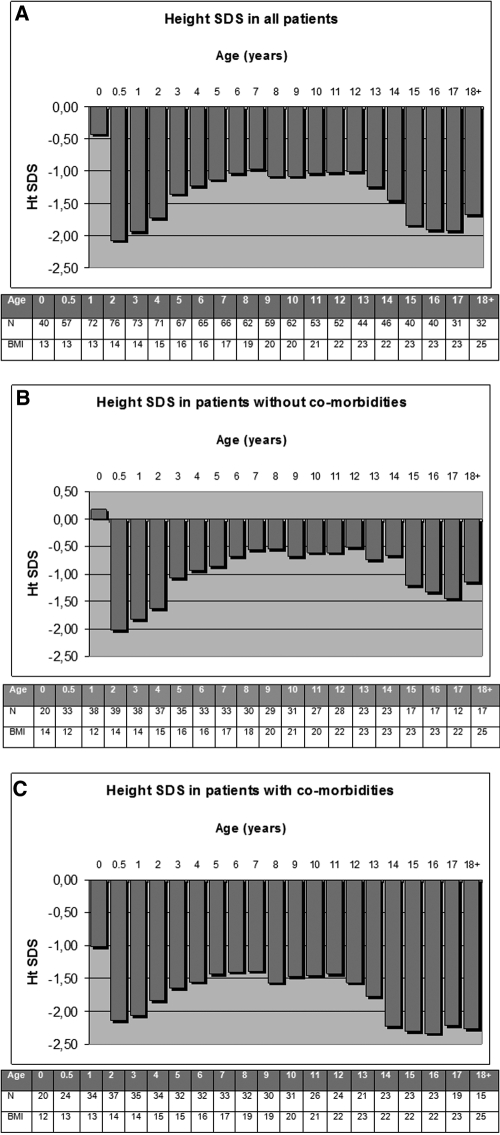
HtSDS (SD) with increasing age are shown in Figure 4A and were −0.42 (2.34) at birth (n = 40), −2.07 (1.34) at 0.5 (n = 57), −1.93 (1.38) at 1 (n = 72), −1.14 (1.14) at 5 (n = 67), −1.04 (1.15) at 10 (n = 62), −1.84 (1.32) at 15 (n = 40), and −1.68 (1.52) at ≥18 yr of age (n = 32). Fourteen received rhGH from age 6.3 yr (range 1.4 to 12.4 yr) for 3.2 yr (0.5 to 12.4 yr), four without and 10 with comorbidity. There was no difference in final HtSDS between those who did and did not receive rhGH (P = 0.581). The total duration of dialysis did not affect final HtSDS (P = 0.28).
Figure 4.
(A) HtSDS and BMI for all patients. (B) HtSDS and BMI for patients without comorbidities (group 1). (C) HtSDS and BMI for patients with comorbidities (group 2).
Delta and final HtSDSs were better in those without comorbidity (P < 0.01 and P = 0.02, respectively; Figure 4). BMI was <97th percentile at all ages, and there was no difference between groups (P = 0.87).
Table 4 shows heights for 32 of the 36 patients age >18 yr in whom this is available. Ten men and five women were below the normal range for adult height. Comorbidity adversely affected final height (P = 0.02).
Table 4.
Height (cm) of the patients older than 18 yr (n = 32/36)
| Gender | Comorbidities | n | Median (cm) | Minimum (cm) | Maximum (cm) | Third Percentile at 18 yr | Less than Third Percentile (n [%]) |
|---|---|---|---|---|---|---|---|
| Male | 24 | 167.2 | 143.2 | 188.0 | 163.6 | 10 (42) | |
| Without | 14 | 169.6 | 153.2 | 188.0 | 4 (29) | ||
| With | 10 | 162.4 | 143.2 | 183.0 | 6 (60) | ||
| Female | 8 | 149.0 | 145.0 | 168.0 | 152.2 | 5 (63) | |
| Without | 3 | 150.0 | 145.0 | 168.0 | 2 (66) | ||
| With | 5 | 148.0 | 147.0 | 161.2 | 3 (60) |
Of the 71 transplant patients, HtSDS (SD) at presentation was −1.97 (1.45), at transplantation was −1.46 (1.38), and at last assessment was −2.11 (1.53). Of 34 patients who still had their first transplant, HtSDS (SD) at entry was −1.87 (1.50), at transplantation was −1.39 (1.40), and at final follow-up was −2.04 (1.52).
Mean iPTH was normal in 64 (86%) patients, one to two times the upper limit of normal in 5 (7%), and more than two times the upper limit of normal in an addition 5 (7%) patients. The scatter of iPTH levels was too small to allow analysis of its effects on growth.
Discussion
This is the largest study from a single center to report outcome during up to 22.9 yr of follow-up of children who presented with stage 4 to 5 CKD before 2 yr of age. We have demonstrated the high incidence of comorbidity in this age group and its adverse effects on growth.
Although our study included infants who did not undergo dialysis, overall 1-yr mortality of 87% is similar to the 85% reported for infants who did undergo dialysis, although it might have been expected to be lower (10). This may be because we were able to capture all infants during the time studied by using our unit database, including even infants who did not go forward with RRT.
Death was due to withholding RRT, treatment withdrawal, or the complications of RRT. All infants for whom treatment was withheld died before their first birthday. The proportion of normal infants in this group is high and was due to the need for dialysis very early in life and family fears of future associated morbidity. Conversely, treatment withdrawal was almost exclusively in children with comorbidity, except for one child with CNS, and was due to a perceived unacceptably poor quality of life. Decisions about such ethical matters are made in discussion with the multidisciplinary team but principally respecting the views of the family. Again, most deaths were within the first year of life. Others have reported the importance of comorbidity on survival (5,6,11), and the difficult issue of offering RRT to such infants is a frequent cause of ethical debate (12–14); however, other than the very serious comorbidities that led to treatment withdrawal, milder ones, perhaps not surprising, did not affect mortality, so the death rate was comparable to that of otherwise normal children (15,16), and causes of death were similar to other reports, predominantly as a result of sepsis or biochemical- or fluid-related disturbances (1,17).
One interesting finding was the adverse influence of SGA on survival. It might be expected that this was due to comorbidity, but this was not the case. Another possible explanation is that the low nephron mass associated with low birth weight (18) resulted in reduced residual renal function, a recognized risk factor for mortality on dialysis (6); however, the causes of death were varied, and there is no obvious explanation for this finding.
As might be expected, comorbidity had a significant effect height potential. This is a factor that is rarely considered in the interpretation of registry data and growth studies. The difference between groups was not a reflection of a longer time on dialysis in the children with comorbidity, and although they had a lower incidence of preemptive transplantation, this did not affect graft outcome. The most critical time for loss of height potential is in the first 6 mo of life (1,19), a time that is particularly dependent on nutrition, which may be very hard to maintain because of prematurity, poor feeding, vomiting, and episodes of fasting as a result of surgery or sepsis. Infants with comorbidity are more likely to have these problems; however, tube feeding was associated with catch-up growth in children with and without comorbidity, although better growth was seen in the otherwise normal children. This was achieved without inducing obesity, because the BMIs of all of the children were normal. Previously, doubts have been cast about the ability of tube feeding to improve growth after the age of 2, but this study demonstrates that catch-up growth can be achieved throughout the prepubertal years, in association with tube feeding in many of the children (20).
Catch-up growth in infants has been reported in some but not all studies (8,21). Our use of tube feeding is higher than that used in other parts of the world: The International Pediatric Peritoneal Dialysis Network (http://www.pedpd.org/index.php?id=net_part) reports gastrostomy use of only 8%, compared with >40% in our patients, although Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines now recommend tube feeding when oral intake is insufficient to maintain growth, despite dietary manipulation and medication (22).
The decline in growth during the peripubertal years may have been due to pubertal delay as a result of steroid therapy, which is part of our posttransplantation immunosuppression. Unfortunately, we do not have adequate pubertal data to assess this; however, there was an improvement in HtSDS after the age of 18, particularly in the normal children, and some of these patients may not have yet achieved their final height, because we know that many continue to grow into their 20s (23). The heights of those who had reached 18 yr were within the normal range in the majority of group 1, although more were below normal in group 2. These final heights are comparable to those of other studies, which is very satisfactory given the early presentation of our patients. Previous reports give median final heights for women and men of 158.0 and 166.0 cm (24), 147.4 and 156.6 cm (25), and 156.0 and 165.0 cm, respectively (26), although, in the last study, the patients had received rhGH. We did not see a difference between those who were or were not treated with rhGH, but our use of rhGH was lower than in most centers. We were unable to examine the effect of iPTH control on growth, because the large majority of patients had normal iPTH levels; however, we can state that catch-up growth can occur with normal iPTH levels, as we previously reported (27,28).
The aim for our patients is early transplantation, which is believed by most authorities to provide the best option for survival, growth, and psychosocial development (29). This study, like others, shows that transplantation before the age of 2 yr is not associated with a poorer outcome than after this age (24,30–32). Indeed, our LRTs had 100% patient and graft survival after 10 yr. As previously reported, patients who received a preemptive transplant had better survival (33), although this did not affect transplant survival. Overall, graft survival was good, but it has to be noted that some children had already had three transplants by age 17.5 yr, and many had been around the circle of dialysis and transplant on more than one occasion.
What has to be remembered when analyzing a historical cohort of patients is that medical management is constantly evolving, and there is evidence for improving prognosis (34). It may be, therefore, that the prognosis now is better than when these infants presented two decades ago.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kari J, Gonzalez C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with chronic renal failure. Kidney Int 57: 1681–1687, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS: Long-term outcome of peritoneal dialysis in infants. J Pediatr 136: 24–29, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bunchman TE: Infant dialysis: The future is now. J Pediatr 136: 1–2, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Rees L: Management of the infant with end-stage renal failure. Nephrol Dial Transplant 17: 1564–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ellis EN, Pearson D, Champion B, Wood EG: Outcomes of infants on chronic peritoneal dialysis. Adv Perit Dial 9: 543–548, 1995 [PubMed] [Google Scholar]
- 6.Wood EG, Hand M, Briscoe DM, Donaldson LA, Yiu V, Harley FL, Warady BA, Ellis EN: North American Pediatric Renal Transplant Cooperative Study: Risk factors for mortality in infants and young children on dialysis. Am J Kidney Dis 37: 373–379, 2001 [PubMed] [Google Scholar]
- 7.Rees L: Long-term peritoneal dialysis in infants. Perit Dial Int 27[Suppl 2]: 180–184, 2007 [PubMed] [Google Scholar]
- 8.Rees L, Shaw V: Nutrition in children with CRF and on dialysis. Pediatr Nephrol 22: 1689–1702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff R, Wright E, Ledermann S, Hutchinson C, Rees L: Chronic hemodialysis in infants and children under 2 years of age. Pediatr Nephrol 18: 378–383, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Neu AM, Ho PL, McDonald RA, Warady BA: Chronic dialysis in children and adolescents: The 2001 annual report of the NAPRTCS. Pediatr Nephrol 17: 656–663, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann S: Long-term outcome of chronic dialysis in children. Pediatr Nephrol 21: 257–264, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Rees L: Management of the neonate with chronic renal failure. Semin Fetal Neonatal Med 13: 181–188, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Shooter M, Watson A: The ethics of withholding and withdrawing dialysis therapy in infants. Pediatr Nephrol 14: 347–351, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bunchman TE: The ethics of infant dialysis. Perit Dial Int 16[Suppl 1]: S505–S508, 1996 [PubMed] [Google Scholar]
- 15.McDonald SP, Craig IC: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS: Mortality and causes of death of end-stage renal disease in children: A Dutch cohort study. Kidney Int 61: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Coulthard MG, Crosier J: Outcome of reaching ESRF in children under 2 years of age. Arch Intern Med 87: 511–517, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moritz KM, Singh RR, Probyn ME, Denton KM: Developmental programming of a reduced nephron endowment: More than just a baby's birth weight. Am J Physiol Renal Physiol 296: F1–F9, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Karlberg J, Schaefer F, Hennicke M, Wingen AM, Rigden S, Mehls O: Early age-dependent growth impairment in chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10: 283–287, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ramage IJ, Geary DF, Harvey E, Secker DJ, Balfe JA, Balfe JW: Efficacy of gastrostomy feeding in infants and older children receiving chronic peritoneal dialysis. Perit Dial Int 19: 231–236, 1999 [PubMed] [Google Scholar]
- 21.Parekh RS, Flynn JT, Smoyer WE, Milne JL, Kershaw DB, Bunchman TE, Sedman AB: Improved growth in young children with severe chronic renal insufficiency who use specified nutritional therapy [published erratum appears in J Am Soc Nephrol 13: 1421–1422, 2002]. J Am Soc Nephrol 12: 2418–2426, 2001 [DOI] [PubMed] [Google Scholar]
- 22.KDOQI Work Group: KDOQI clinical practice guideline for nutrition in children with chronic kidney disease: 2008 update. Am J Kidney Dis 53: S11–S104, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Maxwell H, Haffner D, Rees L: Catch-up growth occurs following renal transplantation in children of pubertal age. J Pediatr 133: 435–440, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Rees L, Shroff R, Hutchinson C, Fernando O, Trompeter RS: Long-term outcome of paediatric renal transplantation: A follow-up of 300 children from 1973 to 2000. Nephron Clin Pract 105: c68–c76, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Broyer M, Le Bihan C, Charbit M, Guest G, Tete MJ, Gagnadoux MF, Niaudet P: Long-term social outcome of children after kidney transplantation. Transplantation 77: 1033–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Haffner D, Schaefer F, Nissel R, Wuhl E, Tonshoff B, Mehls O: Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343: 923–930, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Waller S, Ridout D, Cantor T, Rees L: Parathyroid hormone and growth in children with chronic renal failure. Kidney Int 67: 2338–2345, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Cansick J, Waller S, Ridout D, Rees L: Growth and PTH in prepubertal children on long-term dialysis. Pediatr Nephrol 22: 1349–1354, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rees L: Long-term outcome after renal transplantation in childhood. Pediatr Nephrol 24: 475–484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavers B, Najarian JS, Humar A: Kidney transplantation in infants and small children. Pediatr Transplant 11: 702–708, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Kari JA, Romagnoli J, Duffy PG, Fernando ON, Rees L, Trompeter RS: Renal transplantation in children under 5 years of age. Pediatr Nephrol 13: 730–737, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Khwaja K, Humar A, Najarian JS: Kidney transplants for children under 1 year of age: A single-center experience. Pediatr Transplant 7: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Jill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Carey WA, Talley LI, Sehring SA, Jaskula JM, Mathias RS: Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: A North American Pediatric Renal Trials and Collaborative Studies special analysis. Pediatrics 119: e468–e473, 2007 [DOI] [PubMed] [Google Scholar]