Abstract
Background and objectives: An early histopathologic predictor of the renal prognosis, before the occurrence of advanced glomerular sclerosis/interstitial fibrosis and/or apparent renal dysfunction, remains to be established in IgA nephropathy (IgAN). This study aimed to determine whether the glomerular density (GD; nonsclerotic glomerular number per renal cortical area) of biopsy specimens obtained at an early stage of IgAN could predict the long-term renal outcome.
Design, setting, participants, & measurements: The predictive value of the factors at biopsy, including the GD, on the renal outcome was retrospectively analyzed for 98 patients who had IgAN with an estimated GFR of ≥60 ml/min per 1.73 m2 at biopsy (87 ml/min per 1.73 m2 on average).
Results: The individual value of GD in biopsy ranged from 1.2 to 8.1/mm2 (i.e., approximately a seven-fold variation), and the GD showed a close inverse correlation with mean glomerular volume. Among the various clinicopathologic factors involved, both a cellular/fibrocellular crescent and the GD were found to be significant predictors of progression in multivariate analyses. A low GD in the biopsy specimens was frequently associated with a steeper slope of the renal function and a synergistically enhanced risk for progression with the presence of cellular/fibrocellular crescent. The renal function, proteinuria, degrees of glomerulosclerosis, and interstitial fibrosis at biopsy were not independent predictors of the prognosis in these patients.
Conclusions: A strong predictive relationship of low GD with progression observed in this study suggests that GD may serve as an early histopathologic marker of long-term renal prognosis in IgAN.
The outcome of IgA nephropathy (IgAN) is highly variable among individuals (1–4). Some patients maintain only isolated urinary symptoms for many years, whereas other patients progress to ESRD. Previous studies have consistently identified various clinicopathologic parameters at the time of diagnosis, especially heavy proteinuria, reduced renal function, advanced glomerular sclerosis, or tubulointerstitial fibrosis, as independent risk factors for progression (4–7); however, these findings characterize already advanced renal injury rather than reflect the progression rate of renal diseases. The factors that may allow prediction of progression in early stage of IgAN remain to be fully elucidated.
We recently performed a study using pairs of serial biopsy specimens from 18 patients with progressive IgAN (8). In these patients, renal biopsies were performed both before and after the establishment of impaired renal function. We found a low glomerular density (GD; the number of nonsclerotic glomeruli per renal cortical area) in the first biopsy to be associated with both the already enlarged glomeruli and an increased susceptibility to subsequent renal scarring and a more rapid progression. A high GD in the first biopsy was associated with a relatively slow progression despite a large increase in the glomerular size in the second biopsy. These results were consistent with the currently proposed concept that kidneys with a reduced nephron number, presumably having less of a functional reserve, may cause glomerular enlargement, thereby becoming more susceptible to subsequent renal injury and functional decline (9–11). We therefore hypothesized that the GD in the early stage of “potentially progressive” renal diseases, such as IgAN, can predict subsequent renal adaptation and/or scarring and thus serve as an early marker of renal prognosis.
By analyzing a larger number of patients, this study aimed to clarify the relationship between GD and the long-term renal outcome of patients with IgAN and without any apparent renal dysfunction at the time of biopsy. In addition to the patients who showed a progressive loss of renal function, this study included patients whose renal function had been stable for a long time.
Materials and Methods
Selection of Patients
This study included patients who had IgAN, for whom renal biopsies were performed at the Jikei Hospital Tokyo during the years 1972 through 2001, and who were followed for >5 yr and/or those who had achieved a ≥50% reduction in estimated GFR (eGFR). As for the patients with a ≥50% reduction in eGFR, those with <5 yr of follow-up were also included. Any patients with moderately impaired renal function (eGFR <60 ml/min per 1.73 m2) at biopsy were excluded. Patients whose renal tissue specimens contained <10 glomeruli (including globally sclerotic glomeruli) were excluded.
From the outpatient population at our hospital, we recruited 125 patients who had been followed for >5 yr and/or who had achieved a ≥50% reduction in eGFR within 5 yr from the time of diagnosis. Fifteen patients who reached ESRD as a result of progression of IgAN were also recruited. Of these 140 patients, 30 showed moderately impaired renal function on diagnosis (eGFR <60 ml/min per 1.73 m2) and thus were excluded. Twelve of the remaining 110 renal biopsy samples contained <10 glomeruli and thus were excluded. Finally, 98 biopsies from 98 patients were included in this study. All 98 patients were Japanese. In this study population, some patients had a significantly large percentage of sclerotic glomeruli (seven of 98 patients had >40% global sclerosis). Such a minority of patients may represent compensated renal impairment; however, we did not exclude these patients because we did not know precisely what percentage of global sclerosis represents compensated renal impairment. In addition, such compensation would be differently determined for each individual with a different GD.
Definitions
The eGFR was calculated by applying a modified three-variable equation for estimating the GFR for Japanese (12) as follows: eGFR = 194 × age−0.287 × sCr−1.094 × 0.739 (if female), where sCr is serum creatinine. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg or use of antihypertensive medication. The progressors were defined as patients who had achieved a ≥50% reduction in the eGFR from the baseline or patients who had reached ESRD. We also used the percentage change in the eGFR from the baseline per year (⊿eGFR) to express the progression as the slope of renal function. The ⊿eGFR was calculated by applying the following formula: ⊿eGFR (%/yr) = [(eGFR at the last observation) − (eGFR at biopsy)] × 100/(eGFR at biopsy) × (time of observation in years).
Pathologic Analysis
All of the kidney tissue specimens were obtained by performing a percutaneous needle biopsy. The tissues were embedded in paraffin; cut into 3- to 4-μm sections; and stained with hematoxylin-eosin, periodic acid-Schiff, Masson's trichrome, and periodic acid-methenamine silver stain. The percentage of the glomeruli affected by global or segmental sclerosis, mesangial cell proliferation, and crescents were assessed. The presence of mesangial proliferation was defined when any of the mesangial areas contained more than three mesangial cells. Mesangial proliferation was presented as a percentage of the glomeruli involving any mesangial proliferation (exclusive of globally sclerotic glomeruli). A cellular crescent was defined as an extracapillary cell proliferation of more than two cell layers with ≥50% of the lesion occupied by cells. Fibrocellular crescent was defined as an extracapillary lesion composed of <50% cells and <90% matrix. Fibrous crescent was defined as an extracapillary lesion composed of ≥90% matrix. Interstitial fibrosis was semiquantitatively evaluated according to the percentage of cortical area involvement. The glomerular area (GA) was defined as the area described by the outer capillary loops of the tuft using a computed imaging analyzer (Scion Image). The mean GA was calculated by averaging the areas of all of the glomeruli. The mean glomerular volume (GV) was calculated from the measured GA as follows: GV = (GA)3/2Xβ/d, where β is a dimensionless shape coefficient (β = 1.38 for spheres) and d is a size distribution coefficient that is used to adjust for variations in glomerular size (13). We used d = 1.01 as in previous studies (14,15). The GD was determined by calculating the number of glomeruli that were not globally sclerotic per total renal cortical area, which was measured using a computed imaging analyzer (Scion Image). The measurement of GD is strongly influenced by the degrees of global sclerosis and interstitial fibrosis, especially in patients with impaired renal function; however, in terms of renal prognosis, similar results were obtained using GD with other definitions that include global sclerosis or that excluded an area of interstitial fibrosis (data not shown). This is probably because the majority of the patients in this study showed only mild to moderate degrees of global sclerosis and interstitial fibrosis. To simplify the data, we applied only one definition of the GD (the number of patent glomeruli per total renal cortical area).
Statistical Analysis
The continuous variables are expressed as means ± SD. The logistic regression was applied to assess the impact of the multiple categorical or continuous variables on the progression of renal impairment. We defined cutoffs of >50% for mesangial proliferation, >25% for global sclerosis, and >25% for interstitial fibrosis in a logistic regression analysis according to the method reported in previous studies (5,7,16). The univariate or the multivariate regression analysis was applied to determine the relationship between the continuous variables and the ⊿GFR. The clinically relevant parameters or the variables that were significantly associated on the basis of a univariate analysis were included in the multivariate analysis. Because the distribution of urinary protein excretion, global/segmental sclerosis, and cellular/fibrocellular crescent were skewed, these variables were log-transformed before performance of both univariate and multivariate regression analyses. Because the GD in individuals was normally distributed, we did not modify this value when performing the statistical analyses. P < 0.05 was considered to be statistically significant. All statistical analyses were performed using the SPSS software program.
Results
Baseline Characteristics and Clinical Outcome
Baseline characteristics and clinical outcome of patients are summarized in Table 1. Most patients showed mild to moderate degrees of proteinuria and were slowly progressive, which was demonstrated by the ⊿eGFR. The average length of the follow-up was 11 yr. At the end of the follow-up, 18 (18%) patients had achieved a ≥50% reduction in the eGFR and seven (7%) patients had progressed to ESRD.
Table 1.
Baseline characteristics and clinical outcome of patients
| Characteristic | Value |
|---|---|
| Patients | 98 |
| Age (yr) | |
| mean ± SD | 34 ± 13 |
| range | 12 to 64 |
| Male gender (%) | 45 |
| BMI (kg/m2) | |
| mean ± SD | 21.8 ± 2.9 |
| range | 15.5 to 31.6 |
| eGFR (ml/min per 1.73 m2) | |
| mean ± SD | 87 ± 18 |
| range | 61 to 132 |
| Hypertension (%) | 23 |
| Urinary protein excretion (g/d) | |
| mean ± SD | 1.10 ± 1.10 |
| range | 0.05 to 7.60 |
| Length of follow-up (yr) | |
| mean ± SD | 11.0 ± 5.2 |
| range | 2.5 to 34.0 |
| Corticosteroid treatment (%) | 28 |
| ACEI or ARB treatment (%) | 71 |
| Urinary protein excretion at the | |
| final (g/d) | |
| mean ± SD | 0.90 ± 1.40 |
| range | 0.02 to 9.80 |
| Changes of eGFR (%/yr) | |
| mean ± SD | −3.0 ± 3.3 |
| range | −19.8 to 2.5 |
| >50% reduction in eGFR (%) | 18 |
| ESRD (%) | 7 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index.
Histopathologic Findings
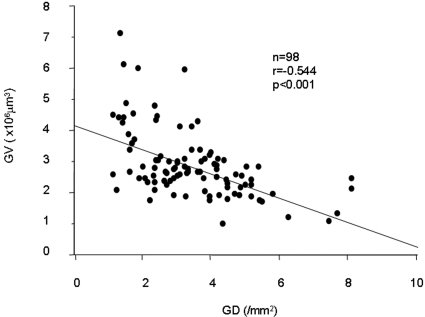
The histopathologic findings of the biopsies are summarized in Table 2. Most of the patients showed focal or diffuse mesangial alterations; some of these accompanied the various incidences of crescent formation. The majority of patients showed mild degrees of segmental/global sclerosis and interstitial fibrosis. Both the GV and the GD showed approximately seven-fold variations among individuals. The GV had a close inverse correlation with the GD (Figure 1).
Table 2.
Histologic characteristics of patients
| Characteristic | Mean ± SD (Range) |
|---|---|
| Total glomerular number | 19.4 ± 8.7 (10.0 to 51.0) |
| Total cortical area (mm2) | 5.4 ± 2.2 (1.7 to 12.7) |
| Global glomerular sclerosis (%) | 13.0 ± 14.8 (0.0 to 77.3) |
| Segmental glomerular sclerosis (%) | 6.2 ± 8.2 (0.0 to 45.5) |
| Mesangial proliferation (%) | 41 ± 23 (10 to 100) |
| Cellular/fibrocellular crescent (%) | 2.9 ± 5.4 (0.0 to 30.0) |
| Interstitial fibrosis (%) | 17.7 ± 12.3 (0.0 to 65.0) |
| Mean glomerular volume (×106μm3) | 2.9 ± 1.1 (1.0 to 7.1) |
| Glomerular density (/mm2) | 3.5 ± 1.5 (1.2 to 8.1) |
Figure 1.
Relationship between the GD and the mean GV in patients with IgAN and an eGFR of ≥60 ml/min per 1.73 mm2 at biopsy. The GD showed a close inverse correlation with the mean GV.
Univariate and Multivariate Analysis of Factors Associated with Progression
Both univariate and multivariate logistic analyses were performed to evaluate the impact of the potential predictors of progression (Table 3). Four (4%) of 98 patients at 5 yr and 14 (23%) of 60 patients at 10 yr showed progression with a ≥50% reduction in the eGFR. In these patients, proteinuria of ≥1 g/d, presence of cellular/fibrocellular crescent or segmental glomerular sclerosis, global glomerular sclerosis of >25%, and GD were statistically significant factors that were associated with the progression at 10 yr on the basis of a univariate analysis. In a multivariate analysis, the cellular/fibrocellular crescent and the GD were significant independent predictors of progression at 10 yr. The result of a logistic analysis with the percentage of crescents was less statistically significant in comparison with those with merely the presence or absence of crescents (data not shown).
Table 3.
Univariate and multivariate logistic analyses of the factors at biopsy that predicted progression during follow-up in patients with eGFR ≥60
| Variable | Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| 5 Yr (n = 98) |
10 Yr (n = 60) |
5 Yr (n = 98) |
10 Yr (n = 60) |
|||||
| P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | |
| eGFR | NS | – | NS | – | NS | – | NS | – |
| Urinary protein excretion (≥1 g/d) | NS | – | <0.01 | 7.1 (3.8 to 24.0) | NS | – | NS | – |
| Hypertension | NS | – | NS | – | NS | – | NS | – |
| Mesangial proliferation (>50%) | NS | – | NS | – | NS | – | NS | – |
| Cellular/fibrocellular crescent | NS | – | <0.01 | 8.0 (5.8 to 27.2) | NS | – | <0.05 | 10.4 (1.1 to 48.2) |
| Segmental glomerular sclerosis | NS | – | <0.05 | 5.2 (1.3 to 16.0) | NS | – | NS | – |
| Global glomerular sclerosis (>25%) | NS | – | <0.05 | 8.0 (3.8 to 29.2) | NS | – | NS | – |
| Interstitial fibrosis (>25%) | NS | – | NS | – | NS | – | NS | – |
| Glomerular density | NS | – | <0.01 | 4.7 (3.1 to 11.7) | NS | – | <0.05 | 23.1 (1.7 to 143.7) |
CI, confidence interval; OR, odds ratio.
Univariate and Multivariate Analysis of Factors Associated with the Slope of Renal Function
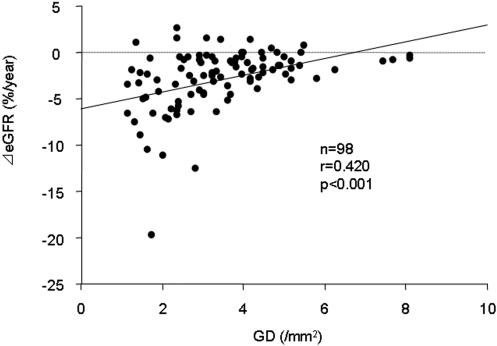
The continuous variables at biopsy were tested for the association with the ⊿GFR (Table 4). In the univariate analyses, urinary protein excretion, global glomerular sclerosis, and the GD were statistically significant. Variables that were associated with ⊿GFR or those that significantly correlated with the GD on the basis of a univariate analysis (Supplemental Table S1) were further evaluated by a multivariate analysis. As a result, only the GD was independently associated with the ⊿GFR. Figure 2 shows the relationship between the GD and the ⊿GFR. Patients with a low GD were frequently associated with a steeper slope of the renal function, whereas patients with a high GD showed a lower gradient of the renal function.
Table 4.
Factors at biopsy that influenced the slope of renal function (⊿eGFR) by univariate and multivariate regression analyses
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| r | P | t | P | |
| Age | −0.001 | NS | 1.070 | NS |
| eGFR | −0.014 | NS | −0.665 | NS |
| BMI | −0.128 | NS | 0.022 | NS |
| Mean arterial pressure | −0.105 | NS | −0.783 | NS |
| Urinary protein excretiona | −0.313 | <0.01 | −1.811 | NS |
| Mesangial proliferation | −0.134 | NS | 0.695 | NS |
| Cellular/fibrocellular crescenta | −0.270 | <0.01 | −1.627 | NS |
| Global glomerular sclerosisa | −0.107 | NS | 0.031 | NS |
| Segmental glomerular sclerosisa | −0.094 | NS | 0.275 | NS |
| Mean glomerular volume | −0.110 | NS | 0.525 | NS |
| Glomerular density | 0.420 | <0.001 | 3.398 | <0.01 |
BMI, body mass index.
Values were log-transformed before analysis.
Figure 2.
Relationship between the GD and the ⊿eGFR in patients with IgAN and an eGFR of ≥60 ml/min per 1.73 mm2 at biopsy. The patients with a low GD were frequently associated with a steeper slope of the renal function, whereas most of the patients with a high GD showed a lower gradient of renal function during the long-term follow-up.
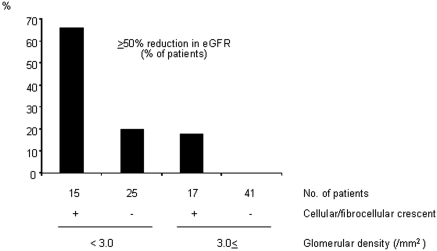
Synergistic Effects of Crescent Formation and the GD on the Progression
In our analyses, cellular/fibrocellular crescent and the GD were found to be plausible independent predictors of the progression. Figure 3 shows the effects of the cellular/fibrocellular crescent in patients with a lower (<3.0/mm2) and a higher GD (≥3.0/mm2) on the appearance of patients who progressed. The rate of patients with a ≥50% reduction in eGFR was synergistically enhanced by the presence of cellular/fibrocellular crescent, especially in patients with a lower GD.
Figure 3.
Synergistic effects of crescent formation and the GD on the progression of patients with IgAN and an eGFR of ≥60 ml/min per 1.73 mm2 at biopsy. The patients were divided into four groups according to the presence or absence of cellular/fibrocellular crescent and a low (<3/mm2) or a high (≥3/mm2) GD. The indices give the rate of the patients with ≥50% reduction in the eGFR in each group. The rate of progression was synergistically enhanced by the presence of cellular/fibrocellular crescent, especially in the patients with a lower GD.
Discussion
Impaired renal function, advanced glomerulosclerosis, and interstitial fibrosis at the time of diagnosis were proposed as adverse clinicopathologic findings that predict a poor renal outcome in IgAN (4–7,16); however, in our analysis of patients with an eGFR ≥60 ml/min per 1.73 m2 at biopsy, these factors were not consistently significant in terms of the progression. Furthermore, none of them was found to be a significant predictor of progression in the multivariate analysis. These results suggest that such chronic pathologic factors might no longer be independent markers of progression in patients with IgAN and without any apparent renal dysfunction at biopsy.
Proteinuria universally seems to be the most significant clinical factor for predicting a progression in IgAN, and the most generally accepted risk threshold is protein excretion of ≥1 g/d (4,7). In our preliminary study, proteinuria of ≥1 g/d was in fact associated with progression in the multivariate analyses of patients, including those with impaired renal function at biopsy (data not shown); however, in patients with an eGFR ≥60 ml/min per 1.73 m2 at biopsy, proteinuria was no longer associated with progression. This suggested that heavy proteinuria at presentation might partially reflect an already advanced renal injury and/or impaired renal function. Furthermore, arterial hypertension was found to be associated with progression of IgAN in several studies (4,5,7); however, in some studies from Japan, including this study, it was not found to be a predictor of progression (2,17,18). These differences may be due in part to the differences in the biopsy policies and to the stage of the diseases (19). Nevertheless, the most important finding of this study is that the prognostic value of GD, as well as the presence of the cellular/fibrocellular crescent, exceeded those of proteinuria and BP. This might be a persuasive appeal for performing renal biopsy early in the course of (putative) IgAN.
The individual value of GD ranged from 1.2 to 8.1 (approximately a seven-fold variation). Some explanations are possible for the causes of such a difference in GD even without any apparent renal dysfunction. First, it is possible that there was a large difference in the nephron number from the time of birth. Some studies have suggested that there is substantial variation in the nephron number in normal people without renal diseases (20,21). It is interesting that a low nephron number was shown to be associated with low birth weight, which was also shown to be associated with poor outcome of glomerular diseases, including IgAN (22–24). The second possibility is an effect of aging. It is known that glomerular number decreases as a person gets older (20). In fact, the GD showed a significant inverse correlation with age at biopsy, thus suggesting that aging itself may have some effect (Supplemental Table S1). The third possibility is the influence of glomerular scarring caused by IgAN. It is postulated that a large portion of the sclerosed glomeruli may disappear during a long-term period from the onset of IgAN. The fourth possibility is the effect of a sampling bias. It is expected that the GD will be significantly different as a result of the different parts of the renal cortex where the tissue sample was obtained. In a study of dogs, the inhomogeneous distribution of the glomeruli as sampled by a needle biopsy was reported to be up to 36% in individual patients (25). In addition, the estimation of GD may be influenced by the GV in individuals because larger glomeruli have a greater chance of being sampled.
Previous studies that used the animals of renal ablation model suggested that the glomerular hyperfiltration precedes the development of glomerular scarring (9,10,26,27), thus suggesting that the altered renal hemodynamics caused by the reduction of the nephron mass may be responsible for the progressive renal injuries. Our observation that the GD inversely correlated with the mean GV probably reflects the functional adaptation of the glomeruli in patients with a low GD or a small number of functioning nephrons. In addition, the GD inversely correlated with BP (Supplemental Table S1), indicating that the pathophysiologic role of a low GD is similar to that of a small number of nephrons in the development of hypertension (28,29). It is not clear whether the GD in biopsy simply represents the nephron number of the whole kidney, because data on the total cortical volume of the kidney in our patients were not available; however, our results indirectly support the possibility that GD could be interpreted as a surrogate marker of the total (patent) nephron number and could therefore be used as an important histologic predictor of the final outcome of progressive renal diseases, including IgAN. Finally, because this study did not include therapeutic intervention, it is difficult to interpret the relationship between the GD and the effects of the therapies in each individual. Notably, however, patients with a low GD progressed rapidly even though they tended to be better treated with corticosteroids or rennin-angiotensin system inhibitors (Supplemental Table S2). Future studies on the effects of therapies in individuals with various GDs may help to elucidate the mechanism of progression in relation to the nephron number in renal diseases, including IgAN.
Disclosures
None.
Acknowledgments
Parts of this study were presented at the annual meeting of the American Society of Nephrology; November 2, 2007; San Francisco, CA.
We thank Tomoko Hayakawa for valuable technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
References
- 1.Chauveau D, Droz D: Follow-up evaluation of the first patients with IgA nephropathy described at Necker Hospital. Contrib Nephrol 104: 1–5, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Koyama A, Igarashi M, Kobayashi M: Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis 29: 526–532, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Szeto CC, Lai FM, To K, Wong TY, Chow K, Choi PC, Lui S, Li PK: The natural history of immunoglobulin A nephropathy among patients with hematuria and minimal proteinuria. Am J Med 110: 434–437, 2001 [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Haas M: Histological subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Tomino Y, Sakai H: Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol 7: 93–97, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS: Histological grading of IgA nephropathy predicting renal outcome: Revisiting H.S. Lee's glomerular grading system. Nephrol Dial Transplant 20: 342–348, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Tsuboi N, Kawamura T, Ishii T, Utsunomiya Y, Hosoya T: Changes in the glomerular density and size in serial renal biopsies during the progression of IgA nephropathy. Nephrol Dial Transplant 24: 892–899, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM: Nephron adaptation to renal injury or ablation. Am J Physiol 249: F324–F337, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Shimamura T, Morrison AB: A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol 79: 95–106, 1975 [PMC free article] [PubMed] [Google Scholar]
- 11.Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR: Long-term follow-up after partial removal of a solitary kidney. N Engl J Med 325: 1058–1062, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Collaborators developing the Japanese equation for estimated GFR: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Weibel ER: Sterological Method: Practical Methods of Biological Morphometry, Vol. 1, London, Academic Press, 1979, pp 44–45, 131–134 [Google Scholar]
- 14.Fulladosa X, Moreso F, Narváez JA, Grinyó JM, Serón D: Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14: 2662–2668, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hughson MD, Samuel T, Hoy WE, Bertram JF: Glomerular volume and clinicopathologic features related to disease severity in renal biopsies of African Americans and whites in the southeastern United States. Arch Pathol Lab Med 131: 1665–1672, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Radford MG, Donadio JV, Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Okada H, Suzuki H, Konishi K, Sakaguchi H, Saruta T: Histological alterations in renal specimens as indicators of progression of IgA nephropathy. Clin Nephrol 37: 235–238, 1992 [PubMed] [Google Scholar]
- 18.Katafuchi R, Oh Y, Hori K, Komota T, Yanase T, Ikeda K, Omura T, Fujimi S: An important role of glomerular segmental lesions on progression of IgA nephropathy: A multivariate analysis. Clin Nephrol 41: 191–198, 1994 [PubMed] [Google Scholar]
- 19.Geddes CC, Rauta V, Gronhagen-Riska C, Bartosik LP, Jardine AG, Ibels LS, Pei Y, Cattran DC: A tricontinental view of IgA nephropathy. Nephrol Dial Transplant 18: 1541–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in multiracial study of kidneys at autopsy. Kidney Int Suppl (83): S32–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Zidar N, Cavic MA, Kenda RB, Koseelj M, Ferluga D: Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron 79: 28–32, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Duncan RC, Bass PS, Garrett PJ, Dathan JR: Weight at birth and other factors influencing progression of idiopathic membranous nephropathy. Nephrol Dial Transplant 9: 875, 1994 [PubMed] [Google Scholar]
- 24.Zidar N, Avgustin Cavic M, Kenda RB, Ferluga D: Unfavorable course of minimal change nephritic syndrome in children with intrauterine growth retardation. Kidney Int 54: 1320–1323, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Basgen JM, Steffes MW, Stillman AE, Mause SM: Estimating glomerular number in situ using magnetic resonance imaging and biopsy. Kidney Int 45: 1668–1672, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am J Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 27.Olson JL, Heptinstall RH: Nonimmunologic mechanisms of glomerular injury. Lab Invest 59: 564–578, 1988 [PubMed] [Google Scholar]
- 28.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Zanndi-Nejad K, Luyckx VA, Brenner BM: Adult hypertension and kidney disease: The role of fetal programming. Hypertension 47: 1–7, 2006 [DOI] [PubMed] [Google Scholar]