Abstract
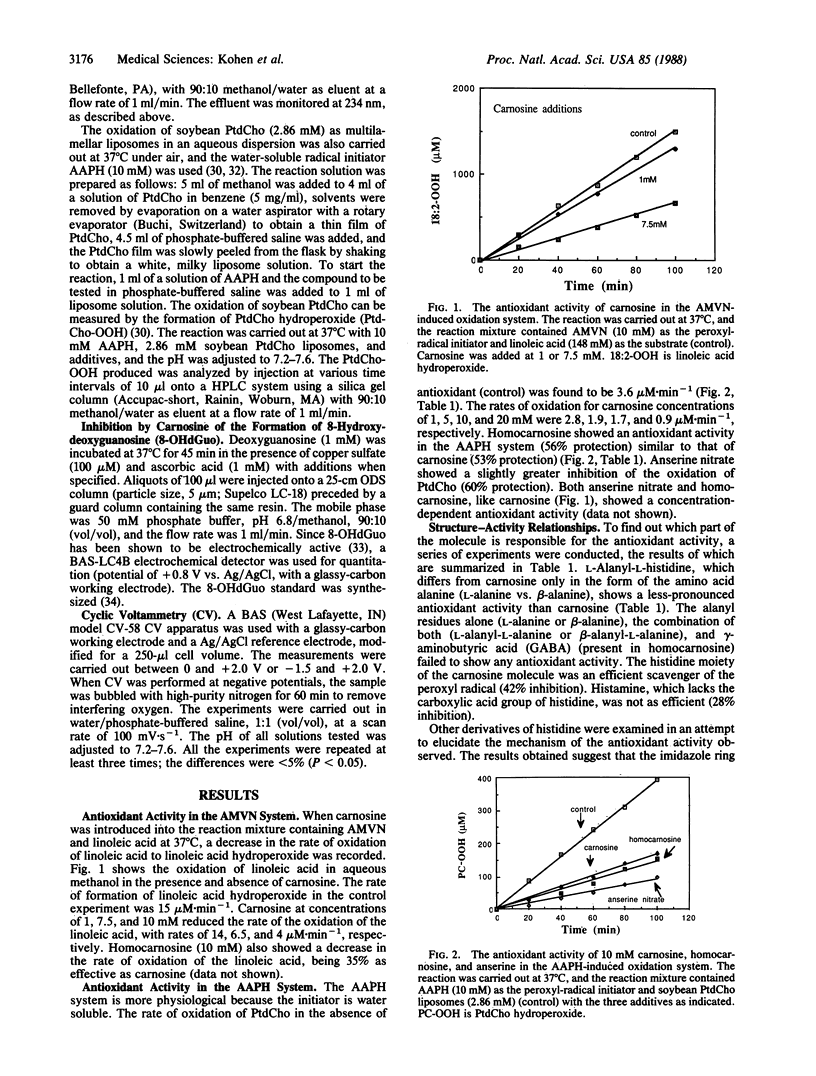
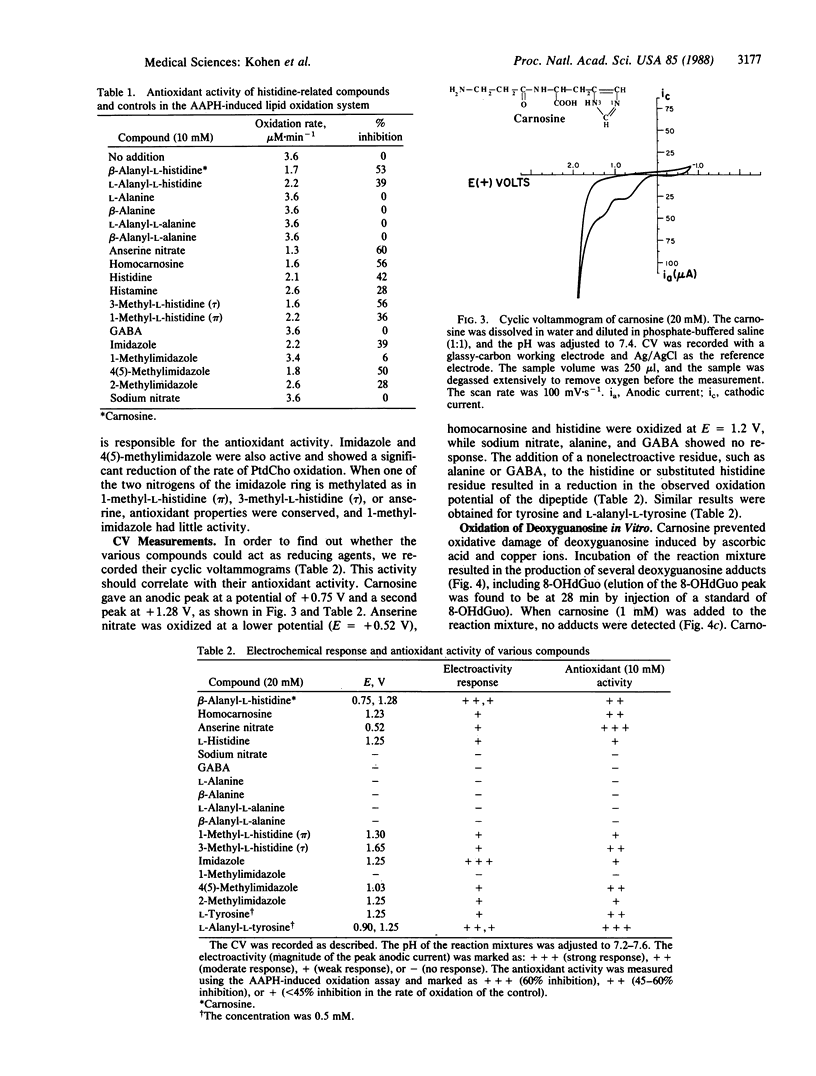
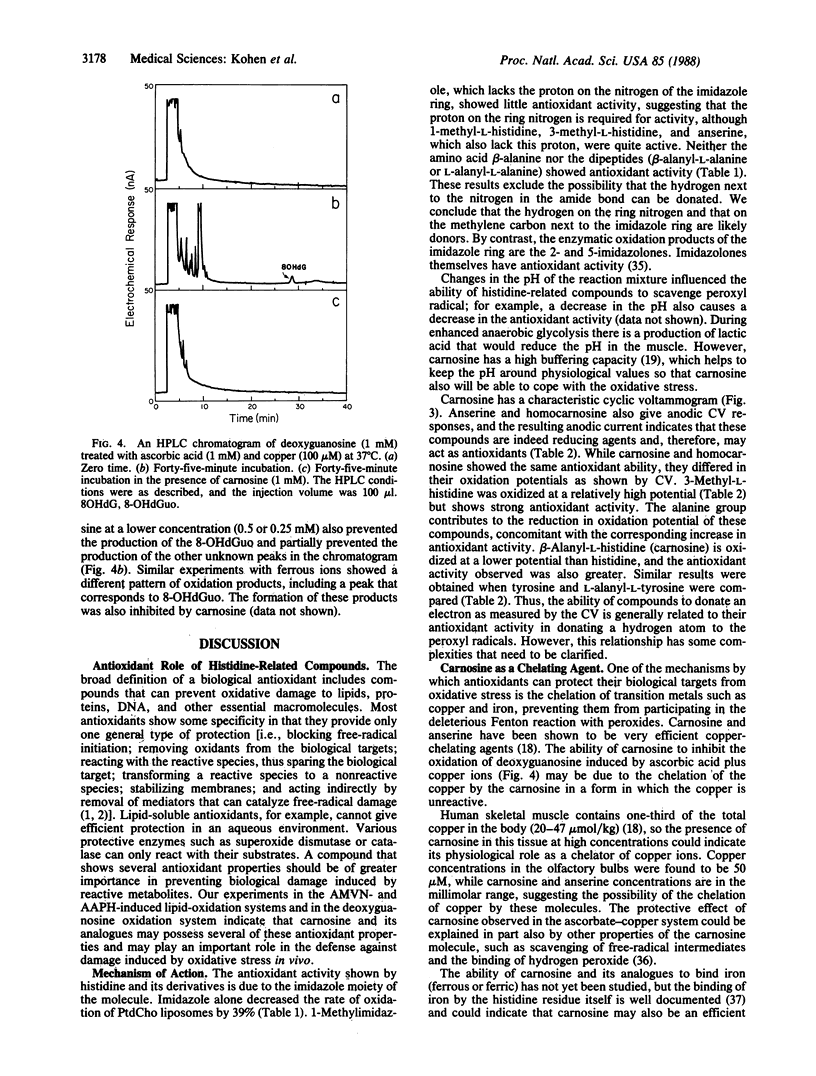
Carnosine, homocarnosine, and anserine are present in high concentrations in the muscle and brain of many animals and humans. However, their exact function is not clear. The antioxidant activity of these compounds has been examined by testing their peroxyl radical-trapping ability at physiological concentrations. Carnosine, homocarnosine, anserine, and other histidine derivatives all showed antioxidant activity. All of these compounds showing peroxyl radical-trapping activity were also electrochemically active as reducing agents in cyclic voltammetric measurements. Furthermore, carnosine inhibited the oxidative hydroxylation of deoxyguanosine induced by ascorbic acid and copper ions. Other roles of carnosine, such as chelation of metal ions, quenching of singlet oxygen, and binding of hydroperoxides, are also discussed. The data suggest a role for these histidine-related compounds as endogenous antioxidants in brain and muscle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM D., PISANO J. J., UDENFRIEND S. The distribution of homocarnosine in mammals. Arch Biochem Biophys. 1962 Nov;99:210–213. doi: 10.1016/0003-9861(62)90002-4. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bondarenko T. I., Makletsova M. G., Sukhomovskii B. I. Soderzhanie svobodnykh aminokislot i dipeptida gomokarnozina v likvore detei s gidrotsefaliei. Zh Nevropatol Psikhiatr Im S S Korsakova. 1983;83(10):1484–1488. [PubMed] [Google Scholar]
- Bondarenko T. I., Mendzheritskaia L. G., Khodakova A. A. Rol' nekotorykh mediatorov v vozrastnoi chuvstvitel'nosti zhivotnykh k giperbaricheskoi oksigenatsii. Fiziol Zh SSSR Im I M Sechenova. 1980 Aug;66(8):1251–1255. [PubMed] [Google Scholar]
- Brown C. E. Interactions among carnosine, anserine, ophidine and copper in biochemical adaptation. J Theor Biol. 1981 Jan 21;88(2):245–256. doi: 10.1016/0022-5193(81)90073-4. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M. Radiation-induced decomposition of the purine bases within DNA and related model compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):127–143. doi: 10.1080/09553008514550201. [DOI] [PubMed] [Google Scholar]
- Crush K. G. Carnosine and related substances in animal tissues. Comp Biochem Physiol. 1970 May 1;34(1):3–30. doi: 10.1016/0010-406x(70)90049-6. [DOI] [PubMed] [Google Scholar]
- DAVEY C. L. The effects of carnosine and anserine on glycolytic reactions in skeletal muscle. Arch Biochem Biophys. 1960 Aug;89:296–302. doi: 10.1016/0003-9861(60)90058-8. [DOI] [PubMed] [Google Scholar]
- Ferriero D., Marogolis F. L. Denervation in the primary olfactory pathway of mice. II. Effects on carnosine and other amine compounds. Brain Res. 1975 Aug 22;94(1):75–86. doi: 10.1016/0006-8993(75)90878-1. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gercken G., Bischoff H., Trotz M. Myokardprotektion durch eine Carnosin-gepufferte kardioplegische Lösung. Arzneimittelforschung. 1980;30(12):2140–2143. [PubMed] [Google Scholar]
- Gutteridge J. M. Age pigments and free radicals: fluorescent lipid complexes formed by iron- and copper-containing proteins. Biochim Biophys Acta. 1985 Apr 25;834(2):144–148. doi: 10.1016/0005-2760(85)90149-3. [DOI] [PubMed] [Google Scholar]
- Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975 Sep 30;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kimura K., Hama T., Tamaki N. Activation of rabbit muscle fructose 1,6-bisphosphatase by histidine and carnosine. J Biochem. 1980 Jan;87(1):179–185. doi: 10.1093/oxfordjournals.jbchem.a132723. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: correlation with vitamin E content. Lipids. 1980 May;15(5):315–322. doi: 10.1007/BF02533546. [DOI] [PubMed] [Google Scholar]
- Krichevskaia A. A., Bondarenko T. I., Makletsova M. G., Lobova E. F. Soderzhanie gomokarnozina v otdelakh mozga i krovi krys v sostoianii gipoksii i posle deistviia giperbaricheskoi oksigenatsii. Vopr Med Khim. 1985 Jan-Feb;31(1):74–76. [PubMed] [Google Scholar]
- Krichevskaia A. A., Bondarenko T. I., Makletsova M. G., Mikhaleva I. I. Protektornoe deistvie neiropeptida gomokarnozina pri giperbarooksigenatsii. Vopr Med Khim. 1985 Jul-Aug;31(4):75–79. [PubMed] [Google Scholar]
- Margolis F. L., Grillo M., Hempstead J., Morgan J. I. Monoclonal antibodies to mammalian carnosine synthetase. J Neurochem. 1987 Feb;48(2):593–600. doi: 10.1111/j.1471-4159.1987.tb04134.x. [DOI] [PubMed] [Google Scholar]
- Neidle A., Kandera J. Carnosine--an olfactory bulb peptide. Brain Res. 1974 Nov 15;80(2):359–364. doi: 10.1016/0006-8993(74)90701-x. [DOI] [PubMed] [Google Scholar]
- PISANO J. J., WILSON J. D., COHEN L., ABRAHAM D., UDENFRIEND S. Isolation of gamma-aminobutyrylhistidine (homocarnosine) from brain. J Biol Chem. 1961 Feb;236:499–502. [PubMed] [Google Scholar]
- Parker C. J., Jr, Ring E. A comparative study of the effect of carnosine on myofibrillar-ATPase activity of vertebrate and invertebrate muscles. Comp Biochem Physiol. 1970 Dec 1;37(3):413–419. doi: 10.1016/0010-406x(70)90569-4. [DOI] [PubMed] [Google Scholar]
- Parkhouse W. S., McKenzie D. C., Hochachka P. W., Ovalle W. K. Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol (1985) 1985 Jan;58(1):14–17. doi: 10.1152/jappl.1985.58.1.14. [DOI] [PubMed] [Google Scholar]
- Schubert J., Watson J. A., Baecker J. M. Formation of a histidine-peroxide adduct by H2O2 or ionizing radiation on histidine: chemical and microbiological properties. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jan 17;14(6):577–583. doi: 10.1080/09553006914551771. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Reeves J. C., Dage R. C., Schnettler R. A. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem Pharmacol. 1987 May 1;36(9):1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Brain peptides as neurotransmitters. Science. 1980 Aug 29;209(4460):976–983. doi: 10.1126/science.6157191. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Niki E., Kamiya Y., Shimasaki H. Oxidation of lipids. 7. Oxidation of phosphatidylcholines in homogeneous solution and in water dispersion. Biochim Biophys Acta. 1984 Sep 12;795(2):332–340. [PubMed] [Google Scholar]



