Abstract
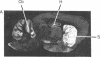
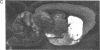
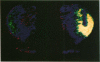
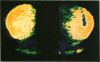
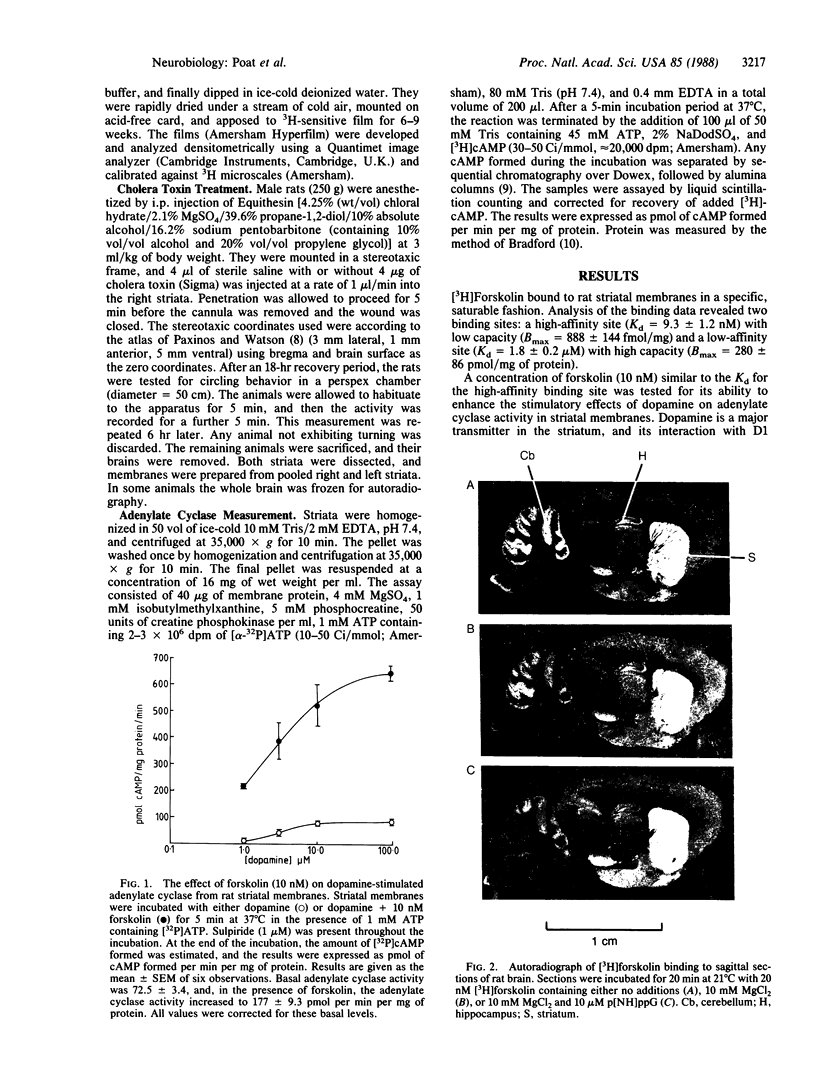
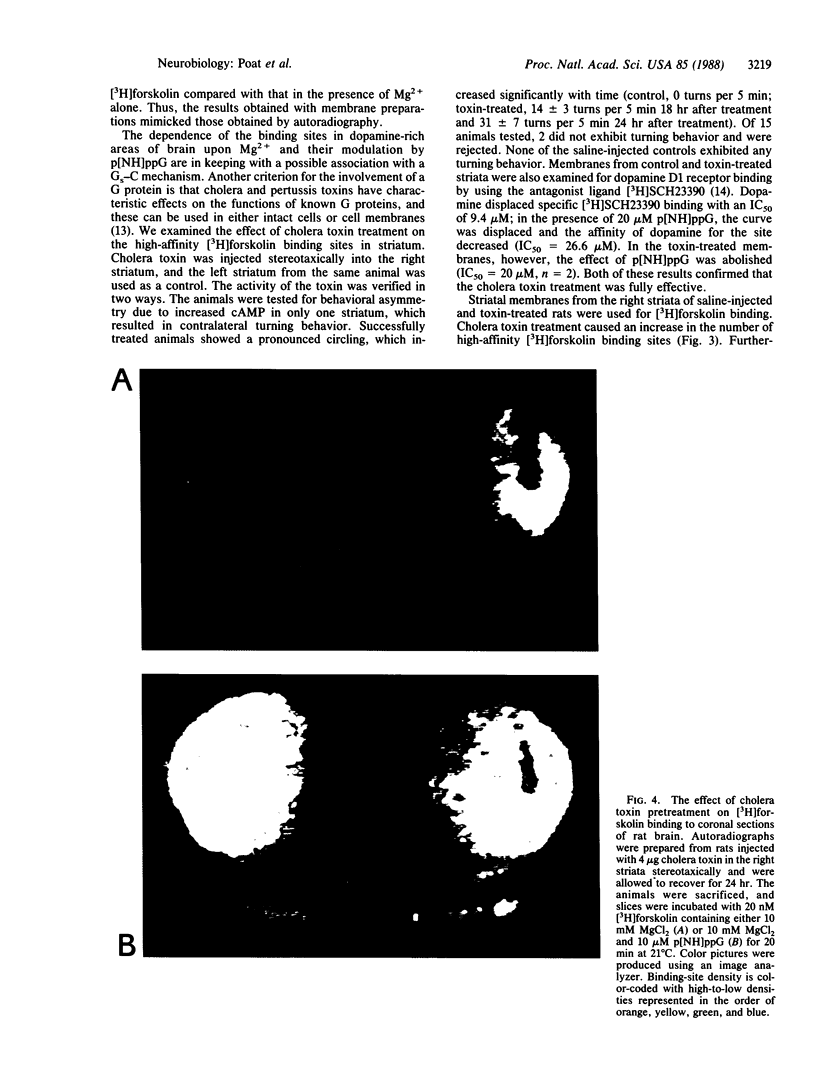
[3H]Forskolin bound to high- and low-affinity sites in the rat brain. The high-affinity site was discretely located, with highest densities in the striatum, nucleus accumbens, olfactory tubercule, substantia nigra, hippocampus, and the molecular layers of the cerebellum. This site did not correlate well with the distribution of adenylate cyclase. The high-affinity striatal binding site may be associated with a stimulatory guanine nucleotide-binding protein. Thus, the number of sites was increased by the addition of Mg2+ and guanylyl imidodiphosphate. Cholera toxin stereotaxically injected into one rat striatum increased the number of binding sites, and no further increase was noted following the subsequent addition of guanyl nucleotide. High-affinity forskolin binding sites in non-dopamine-rich brain areas (hippocampus and cerebellum) were modulated in a qualitatively different manner by guanyl nucleotides. In these areas the number of binding sites was significantly reduced by the addition of guanyl nucleotide. These results suggest that forskolin may have a potential role in identifying different functional/structural guanine nucleotide-binding proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barovsky K., Pedone C., Brooker G. Distinct mechanisms of forskolin-stimulated cyclic AMP accumulation and forskolin-potentiated hormone responses in C6-2B cells. Mol Pharmacol. 1984 Mar;25(2):256–260. [PubMed] [Google Scholar]
- Battaglia G., Norman A. B., Hess E. J., Creese I. Forskolin potentiates the stimulation of rat striatal adenylate cyclase mediated by D-1 dopamine receptors, guanine nucleotides, and sodium fluoride. J Neurochem. 1986 Apr;46(4):1180–1185. doi: 10.1111/j.1471-4159.1986.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Billard W., Ruperto V., Crosby G., Iorio L. C., Barnett A. Characterization of the binding of 3H-SCH 23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984 Oct 29;35(18):1885–1893. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Collins R. M., Spiegel A. Localization of mRNAs encoding the alpha-subunits of signal-transducing G-proteins within rat brain and among peripheral tissues. FEBS Lett. 1987 Sep 28;222(1):191–198. doi: 10.1016/0014-5793(87)80218-1. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Seamon K. B. Activation of cyclic AMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem. 1982 Feb;38(2):532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- Darfler F. J., Mahan L. C., Koachman A. M., Insel P. A. Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):11901–11907. [PubMed] [Google Scholar]
- Gehlert D. R., Dawson T. M., Yamamura H. I., Wamsley J. K. Quantitative autoradiography of [3H]forskolin binding sites in the rat brain. Brain Res. 1985 Dec 30;361(1-2):351–360. doi: 10.1016/0006-8993(85)91305-8. [DOI] [PubMed] [Google Scholar]
- Gehlert D. R. Regional modulation of [3H]forskolin binding in the rat brain by guanylyl-5'-imidodiphosphate and sodium fluoride: comparison with the distribution of guanine nucleotide binding sites. J Pharmacol Exp Ther. 1986 Dec;239(3):952–958. [PubMed] [Google Scholar]
- Hess E. J., Battaglia G., Norman A. B., Iorio L. C., Creese I. Guanine nucleotide regulation of agonist interactions at [3H]SCH23390-labeled D1 dopamine receptors in rat striatum. Eur J Pharmacol. 1986 Feb 11;121(1):31–38. doi: 10.1016/0014-2999(86)90389-4. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Schwertschlag U., Hackenthal E. Forskolin stimulates renin release from the isolated perfused rat kidney. Eur J Pharmacol. 1982 Oct 15;84(1-2):111–113. doi: 10.1016/0014-2999(82)90165-0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Vaillancourt R., Edwards M., Daly J. W. Binding of [3H]forskolin to rat brain membranes. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5081–5085. doi: 10.1073/pnas.81.16.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]