Abstract
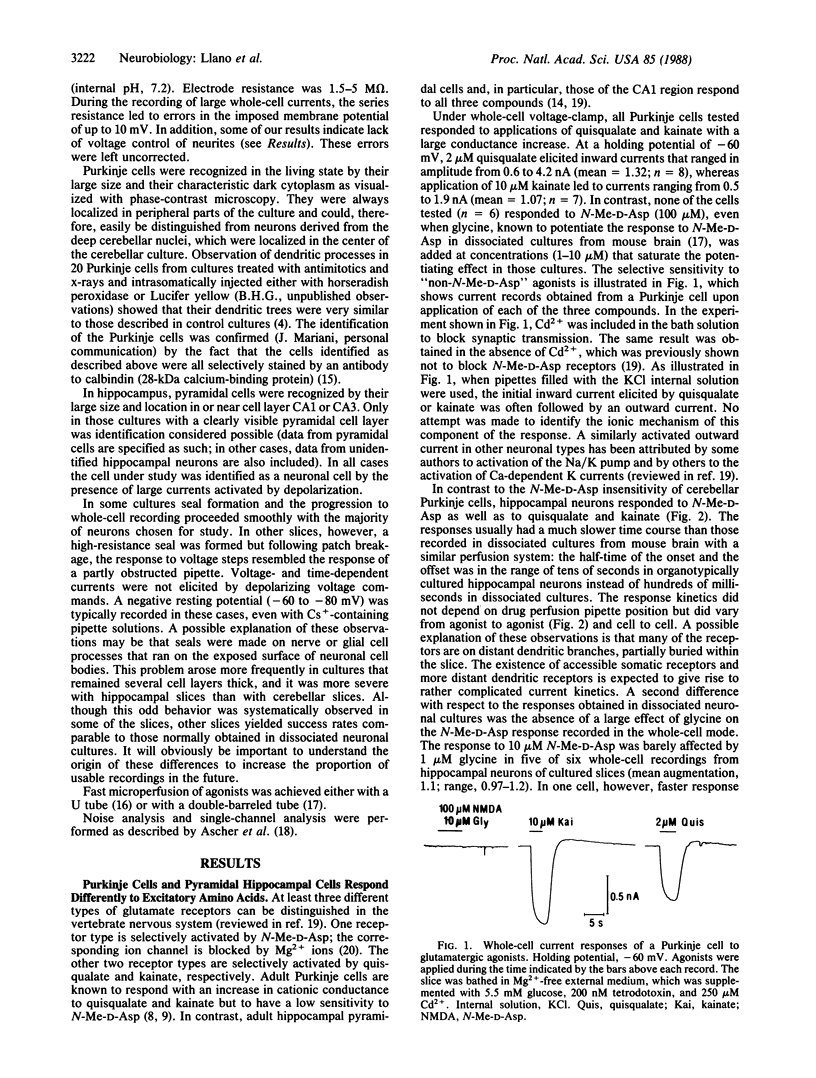
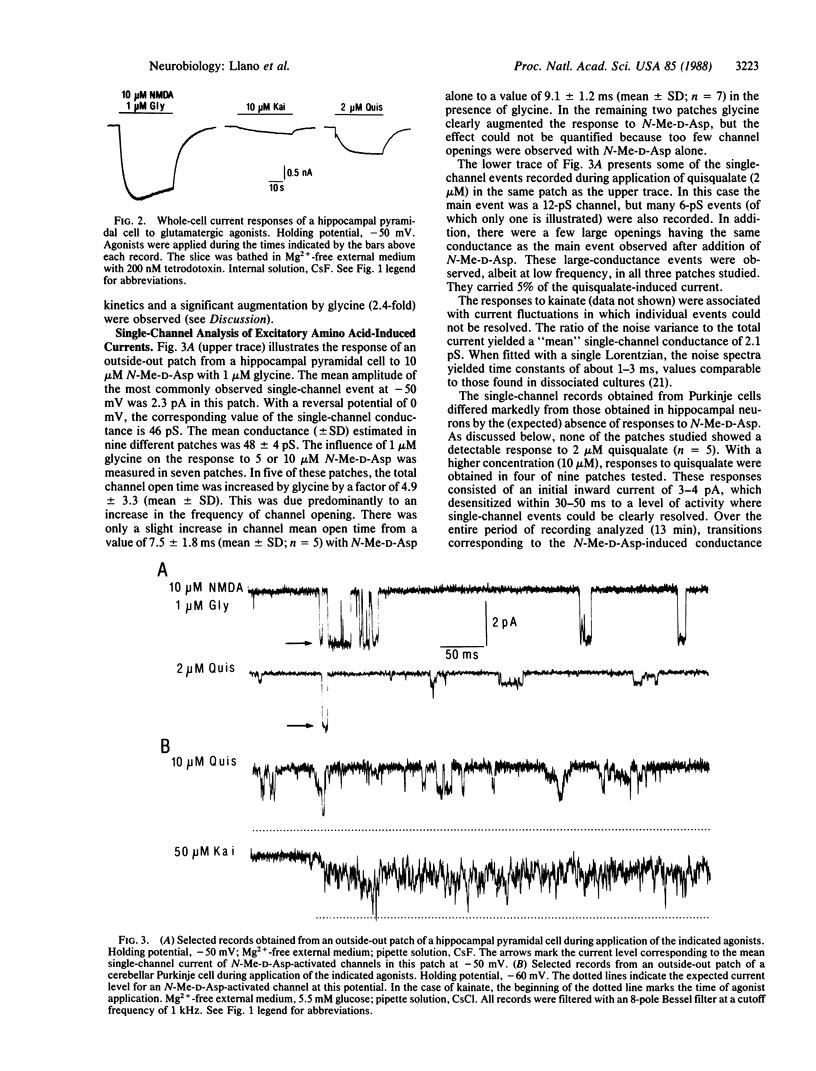
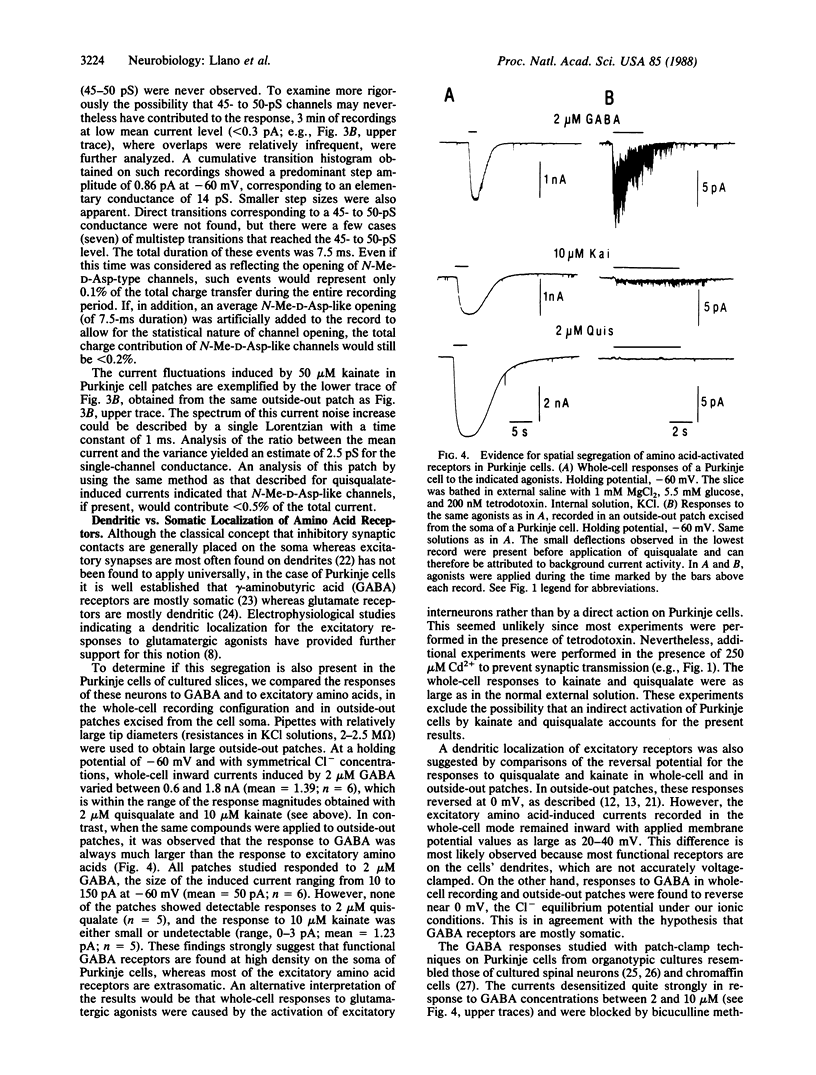
Patch-clamp recording techniques were used to study the properties of amino acid-activated channels in cultured "organotypic" slices from rat cerebellum and hippocampus. Hippocampal pyramidal cells responded to the three main glutamatergic agonists, N-methyl-D-aspartate (N-Me-D-Asp), quisqualate, and kainate, whereas Purkinje cells responded only to quisqualate and kainate. Analysis of single-channel events recorded in outside-out patches from hippocampal neurons showed large conductance events (50 pS), which occurred more frequently in the presence of glycine. These events could be produced by N-Me-D-Asp and also, at low frequency, by quisqualate. On the other hand, 50-pS events were never observed in Purkinje neurons. This supports the hypothesis that N-Me-D-Asp and "non-N-Me-D-Asp" receptors are distinct molecular entities. Comparison of whole-cell and outside-out patch recordings from Purkinje cells revealed a clear spatial segregation of gamma-aminobutyric acid (GABA) and glutamate receptors: although GABA receptors are found at high density in somatic membrane, quisqualate and kainate receptors are mostly extrasomatic. The results show that organotypic slice cultures are amenable to patch-clamp methods. They also show that, in these cultures, amino acids receptors have specific distribution patterns according to cell type and to region within a cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Autoradiographic localization of gamma-aminobutyric acid receptors in the rat central nervous system by using [3H]muscimol. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1024–1028. doi: 10.1073/pnas.75.2.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dupont J. L., Gardette R. Voltage clamp analysis of the effect of excitatory amino acids and derivatives on Purkinje cell dendrites in rat cerebellar slices maintained in vitro. Brain Res. 1983 Nov 21;279(1-2):311–315. doi: 10.1016/0006-8993(83)90200-7. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dupont J. L., Gardette R., Crepel F. Postnatal development of the chemosensitivity of rat cerebellar Purkinje cells to excitatory amino acids. An in vitro study. Brain Res. 1987 Jul;431(1):59–68. doi: 10.1016/0165-3806(87)90195-7. [DOI] [PubMed] [Google Scholar]
- Gahwiler B. H., Brown D. A. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985 Feb 14;313(6003):577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Hajós F. Amino acid neurotoxicity: relationship to neuronal depolarization in rat cerebellar slices. Neuroscience. 1986 Jun;18(2):449–460. doi: 10.1016/0306-4522(86)90165-x. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Olson J. M., Penney J. B., Jr, Young A. B. Autoradiographic characterization of N-methyl-D-aspartate-, quisqualate- and kainate-sensitive glutamate binding sites. J Pharmacol Exp Ther. 1985 Apr;233(1):254–263. [PubMed] [Google Scholar]
- Gähwiler B. H. Development of the hippocampus in vitro: cell types, synapses and receptors. Neuroscience. 1984 Apr;11(4):751–760. doi: 10.1016/0306-4522(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981 Dec;4(4):329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H. Slice cultures of cerebellar, hippocampal and hypothalamic tissue. Experientia. 1984 Mar 15;40(3):235–243. doi: 10.1007/BF01947561. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jande S. S., Maler L., Lawson D. E. Immunohistochemical mapping of vitamin D-dependent calcium-binding protein in brain. Nature. 1981 Dec 24;294(5843):765–767. doi: 10.1038/294765a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]



