Abstract
To reveal molecular drivers of glioma invasion, two distinct glioblastoma (GBM) cell phenotypes (invading cells and tumor core cells) were collected from 19 GBM specimens using laser capture microdissection. Isolated RNA underwent whole human genome expression profiling to identify differentially-expressed genes. Pathway enrichment analysis highlighted the bidirectional receptor/ligand tyrosine kinase system, EphB/ephrin-B, as the most tightly linked system to the invading cell phenotype. Clinical relevance of ephrin-B genes was confirmed in a clinically annotated expression data set of 195 brain biopsy specimens. Levels of ephrin-B1 and -B2 mRNA were significantly higher in GBM (n=82) than in normal brain (n=24). Kaplan-Meier analysis demonstrated ephrin-B2, but not ephrin-B1, expression levels were significantly associated with short term survival in malignant astrocytomas (n=97, p=0.016). In human brain tumor specimens, the production and phosphorylation of ephrin-B 2 were high in GBM. Immunohistochemistry demonstrated ephrin-B2 localization primarily in GBM cells but not in normal brain. A highly invasive glioma cell line, U87, expressed high levels of ephrin-B2 compared with relatively less invasive cell lines. Treatment with EphB2/Fc chimera further enhanced migration and invasion of U87 cells, whereas treatment with an ephrin-B2 blocking antibody significantly slowed migration and invasion. Forced expression of ephrin-B2 in the U251 cell line stimulated migration and invasion in vitro and ex vivo, concomitant with tyrosine phosphorylation of ephrin-B2. These results demonstrate that high expression of ephrin-B2 is a strong predictor of short term survival and that ephrin-B2 plays a critical role in glioma invasion rendering this signaling pathway as a potential therapeutic target.
Keywords: glioma, invasion, migration, ephrin, tyrosine kinase
Introduction
Gliomas are the most common primary tumors of the central nervous system, with glioblastomas (GBMs) as the most malignant entity. The poor prognosis of glioma patients is largely due to the highly invasive nature of these tumors. These invading cells are extremely resistant to radiation and chemotherapy, and currently there are no anti-invasive therapies available. Because local invasion of neoplastic cells into the surrounding brain is perhaps the most important aspect of the biology of gliomas precluding successful treatment, pharmacological inhibition of glioma cell migration and brain invasion is considered as a promising strategy for the treatment of GBM 1–5.
Microarray technology was used to explore the expression profile of migrating glioma cells. This led to the identification of genes overexpressed in invasive glioma cells. From these studies, we have recently identified a number of candidate genes overexpressed in invading glioma cells in vitro and in vivo with microarray analysis 6–8. These molecules may represent promising targets for the development of novel anti-invasive therapies.
The Eph receptors and their ephrin ligands control a diverse array of cell–cell interactions, mainly in the nervous systems. Upon cell-cell contact and ligand–receptor engagement, intracellular signaling is induced in a bidirectional fashion: ‘forward signaling’ starts in receptor-expressing cells, while ‘reverse signaling’ initiates in cells expressing the corresponding ligand. Signals generated by the engagement of ephrin ligands to Eph receptors generally result in repulsive responses. Eph receptors have been divided into an EphA subclass (9 members) and an EphB subclass (5 members) on the basis of sequence similarity and ligand affinity. Ephrin ligands have also been divided into two subclasses: glycosylphosphatidylinositol (GPI) linked ephrin-A’s (5 members) and transmembrane ephrin-B’s (3 members). Although promiscuity has been observed, generally the ephrin-A ligands bind preferentially to EphA receptors, while ephrin-B ligands bind preferentially to EphB receptors 9. This Eph/ephrin system has classically been characterized in normal tissues where it plays a role during embryonic development in cell migration, repulsion versus adhesion, and cell-cell communication. Recently, a role for the Eph/ephrin system, especially Eph forward signaling, has emerged in cancer, especially in the area of invasive behavior in numerous cancers 10, 11. In contrast, the role of ephrin reverse signaling in cancer cells is not as well characterized, although we have recently demonstrated the importance of not only EphB but also ephrin-B signaling in glioma cell invasion 12–14.
In this study, EphB/ephrin-B signaling was identified as the driver of glioma invasion in GBM biopsy specimens. We further characterize the role of ephrin-B ligands in invasive glioma cells, study molecular epidemiology of ephrin-B2 in GBMs, and demonstrate that the expression level is associated with poor survival in malignant astrocytomas. Phosphorylation of ephrin-B2 is correlated with migration and invasion, whereas blocking of ephrin-B2 activation inhibits glioma invasion. These results suggest a functional role for ephrin-B2 in the invasive behavior of malignant brain tumors.
Materials and methods
Clinical Samples and Histology
Under an institutional review board-approved protocol, fresh human brain tumor tissues were obtained from 34 patients who underwent therapeutic removal of astrocytic brain tumors. Non-neoplastic control brain tissues were identified from the margins of the tumors when possible. Histological diagnosis was made by standard light-microscopic evaluation of the sections stained with hematoxylin and eosin. The classification of human brain tumors used in this study is based on the revised World Health Organization criteria for tumors of the CNS 15. Twenty-six astrocytic tumors consisted of 3 diffuse astrocytomas, 4 anaplastic astrocytomas, and 19 GBMs. All tissue samples were obtained at primary resection, and none of the patients had undergone prior chemotherapy or radiation therapy.
Laser Capture Microdissection (LCM), Microarray and Pathway Enrichment Analysis
The transcriptional profile of invasive glioma cells and their stationary cognates isolated by LCM was assessed by whole human genome expression profiling as described previously 6. Briefly, modified hematoxylin and eosin staining of cryosectioned GBM specimens from 19 patients was carried out prior to microdissecting. 2500 invasive cells and 2500 stationary core cells were microdissected from each of the 19 GBM specimens using the Autopix instrument (Arcturus, Mountain View, CA). Extracted RNA was amplified in 2 rounds (RiboAmp HS, Arcturus) and Cy5-dye (Perkin-Elmer, Boston, MA) was incorporated in the second round of amplification. 2.5 µg Cy5-labeled amplified sample RNA was hybridized against 2 µg Cy3-labeled amplified universal reference RNA (Stratagene, La Jolla, CA) on a two-color whole human genome oligonucleotide microarray (Agilent, Santa Clara, CA). Gene-chips were scanned, feature extracted, and background noise subtracted, followed by Lowess normalization to compensate for known dye bias between Cy3 and Cy5 (see NCBI GEO Accession GSE12689). In order to identify significant differentially expressed genes between stationary (core) and invasive (rim) populations, a two-sample t-test was performed selecting genes with a P-value < 0.001. This list was further filtered for genes that showed ≥ ±2.5-fold differential expression in samples from invasive edge compared to core samples. Pathway enrichment analysis was performed on this list using MetaCore GeneGo software.
Gene Expression Profiling and Survival Analysis
Snap-frozen non-neoplastic brain specimens from epileptogenic patients (n = 24) and tumor specimens (n = 171) with clinical information were collected at Hermelin Brain Tumor Center, Henry Ford Hospital (Detroit, MI) under an institutional review board–approved protocol and de-identified for patient confidentiality. Clinical information was provided for all samples (29 astrocytomas, 82 GBMs, 49 oligodendrogliomas and 11 oligoastrocytomas).
Gene expression profiles of these brain specimens were captured using Affymetrix® U133 Plus2 GeneChips according to manufacturer’s protocol (Affymetrix, Santa Clara, CA). Expression profiling was performed by the Neuro-Oncology Branch at the National Cancer Institute, who kindly provided the exported text files (National Cancer Institute. 2005. REMBRANDT. <http://rembrandt.nci.nih.gov>. Accessed 2007 September 24). After exclusion of genes whose expression levels did not show variance across all samples by at least 30%, approximately 7,000 genes of high quality signal intensity remained.
Kaplan-Meier methods were used to compare median survival rates between high (≥ mean expression) and low (< mean expression) expressors of the target gene ephrin-B in malignant astrocytomas. Prognostic significance was assessed by Cox proportional hazard regression.
Antibodies and Reagents
Anti-phosphotyrosine monoclonal antibody was purchased from Cell Signaling Technology (Beverly, MA). Anti-ephrin-B2 polyclonal antibody, which recognizes the extracellular domain of ephrin-B2 for immunohistochemistry and migration assay, and EphB2/ Fc chimera were purchased from R&D systems (Minneapolis, MN). Anti-ephrin-B2 antibody for immunoblot analysis was purchased from Antibodies Incorporated (Davis, CA). α-tubulin monoclonal antibody was obtained from Oncogene Research (Boston, MA). Control mouse IgG and Fc fragment of mouse IgG were purchased from Sigma (St. Louis, MO) and Jackson ImmunoResearch (West Grove, PA), respectively.
Immunoprecipitation and Immunoblot Analysis
For immunoprecipitation, glioma tissue specimens or monolayers of cells were lysed on ice for 10 minutes in a buffer containing 10 mmol/L Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 150 mmol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L ethylenediamine tetraacetic acid, 2 mmol/L sodium vanadate, 10 µg/ml aprotinin, and 10 µg/ml leupeptin (Sigma, St. Louis, MO, USA) as previously described 13. Protein concentrations were determined using the bicinchoninic acid assay procedure (Pierce Chemical Co., Rockford, IL) with bovine serum albumin as a standard. Equivalent amounts of protein (300 µg) were precleared and immunoprecipitated from the lysates and washed with lysis buffer, followed by S1 buffer (10 mmol/L HEPES, pH 7.4, 0.15 mol/L NaCl, 2 mmol/L ethylenediamine tetraacetic acid, 1.5% Triton X-100, 0.5% deoxycholate, 0.2% sodium dodecyl sulfate). Samples were then resuspended in 2X sodium dodecyl sulfate-sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 25% glycerol) and denatured with 2-mercaptoethanol (Sigma), separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose (Invitrogen, Carlsbad, CA) by electroblotting. The nitrocellulose membrane was blocked with 5% bovine serum albumin in Tris-buffered saline, pH 8.0, with 0.1% Tween-20 before addition of primary antibody. Membranes were washed and then incubated with horseradish peroxidase-conjugated secondary antibody. Bound secondary antibodies were detected using a chemiluminescence system (NEN, Boston, MA). To detect the phosphorylation of ephrin-B2, U87 cells were stimulated with either EphB2/Fc for 10 min or treated with ephrin-B2 antibody for 30 min at 37°C then lysed.
Immunohistochemistry
Immunohistochemistry was performed using an avidin-biotin immunoperoxidase technique as previously described 13. Briefly, paraffin-embedded tissue blocks were sectioned (6 µm thick) onto slides and then deparaffinized. Sections were quenched with 3% hydrogen peroxide in methanol for 15 minutes, microwaved 5 minutes in H2O, and blocked for 1 hour with Tris-buffered saline (0.05 mol/L Tris-HCl, pH 7.6, 0.25 mol/L NaCl) containing 3% goat serum and 0.1% Triton X-100. Slides were incubated in rabbit anti-ephrin-B2 antisera or rabbit preimmune sera (1:100 dilution) overnight at 4°C. The secondary antibody (biotin-conjugated goat anti-rabbit IgG; Jackson Laboratories, Bar Harbor, ME) was applied at a 1:500 dilution in Tris-buffered saline containing 1% goat serum, followed by streptavidin-conjugated horseradish peroxidase (1:500 dilution; Amersham Biosciences, Piscataway, NJ). Sections were exposed to diaminobenzidine peroxidase substrate (Sigma) for 5 minutes and counterstained with Mayer’s hematoxylin.
Cell Culture
Human astrocytoma cell lines U87, SNB19, U251 and T98G (American Type Culture Collection, Manassas, VA) were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C.
Real-Time Quantitative RT (QRT)-PCR
QRT-PCR was carried out in a LightCycler (Roche Diagnostics, Indianapolis, IN) as described previously 12. PCR was performed with the following primers: ephrin-B2 (NM_004093): sense, 5'- GGAGGCACTCGCTGTTATCA -3'; antisense, 5'- CATCCAAAGCAGACCGACTCT -3' (amplicon size, 209 bp); histone H3.3 (NM_002107): sense, 5'-CCACTGAACTTCTGATTCGC-3'; antisense, 5'-GCGTGCTAGCTGGATGTCTT-3' (amplicon size, 215 bp). The nucleotide number and amplicon size for each primer are within parentheses. The LightCycler analysis software was used to analyze the PCR data, as described previously 12.
Migration Assays
Migration assays were performed using the microliter scale radial monolayer migration assay with a cell sedimentation manifold (CSM Inc., Phoenix, AZ) on 10-well slides coated with 10µg/ml laminin as described previously 12, 14. To investigate the influence of ephrin-B2 phosphorylation on glioma cell motility, U87 cells or U251 cells transfected with vector were seeded in the migration assay format and allowed to adhere. Media was then exchanged for serum free media containing 0.2–2 µg/ml of anti-ephrin-B2 antibody, recombinant EphB2/Fc chimera, or control mouse IgG and migration rate was evaluated for 24 h.
Cell Invasion Assay
Cell invasion assays were performed using Boyden chambers consisting of Transwells with precoated Matrigel membrane filter inserts in 24-well tissue culture plates (BD Biosciences Discovery Labware, Bedfold, MA) as described previously 12, 14. In certain experiments, ephrin-B2 antibody, EphB2/Fc, or control Fc fragment of mouse IgG was applied to the upper chamber.
Expression Plasmids and Cell Transfection
Expression plasmid for ephrin-B2 was constructed as follows. The cDNA fragment encoding ephrin-B2 was PCR-amplified using 293T cDNA as a template. The fragment was inserted into pEAK plasmid. Transient transfection was performed with U251 cells using Effectene (Qiagen, Valencia, CA), as recommended by the manufacturer’s protocol. U87 cells were co-transfected with ephrin-B2 and green fluorescence protein (GFP) using Effectene and were selected with 1.25 µg/ml puromycin for 48 h. This selection routinely resulted in 90% positive cells expressing GFP. Cells transfected with empty plasmid vector were used as controls.
Ex Vivo Invasion Assay on Rat Brain Slices
The ex vivo invasion assay into rat brain slices was carried out as described previously 12–14, 16. Approximately 1 × 105 glioma cells stably expressing green GFP were gently applied (0.5-µl transfer volume) to the putamen of the brain slice. Imaging of specimens was performed at ×10 magnification using a Macro-Fluorescent Imaging System (SZX12-RFL3; Olympus, Tempe, AZ) equipped with a GFP barrier filter (DP50; Olympus) at 0 and 72 h after seeding the cells. Glioma cell invasion into the rat brain slices was quantitated using a Laser scanning confocal microscope (Zeiss, Thornwood, NY) to observe GFP-labeled cells. The invasion rate was calculated as described previously 12–14, 16.
In Vivo Tumor Formation Assay
Following an institutional review board-approved protocol, intracranial transplantation of glioma cells into mice was done. Female nonobese diabetic/severe combined immunodeficiency disease (NOD/SCID) mice aged 7 wk were used. All mice were purchased from Charles River Laboratories, Osaka, Japan. Mice were anaesthetized with an intraperitoneal injection of pentobarbital (60–70 mg/kg body weight). A burr hole was placed 3 mm lateral to the bregma, and 1 × 105 U87 cells transfected with empty plasmid vector or ephrin-B2 vector in 2 µl PBS were stereotactically injected over 4 min at a depth of 3 mm below the dura mater. This procedure reproducibly results in tumor growth and obvious tumor-related symptoms about 3 wk after intracerebral injection. Four mice in each group were euthanized on day 21 after tumor injection. The brain tissue was embedded in paraffin, cut into 5 µm serial coronal sections and stained with haematoxylin and eosin.
Statistics
Statistical analyses were performed using the χ2 test, the two-tailed Mann-Whitney U test, and two-way ANOVA. P < 0.05 was considered significant.
Results
Ephrin-B Signaling Pathway Is Activated in Invasive Glioma Cells
Five hundred thirty two transcripts were identified to be significantly (P < 0.001) differentially expressed (≥ ±2.5-fold) between invasive and stationary glioma cells isolated by LCM from biopsy specimens derived from 19 human glioma patients. Gene enrichment analysis revealed the most highly significant enrichment for the EphB/ephrin-B signaling pathway (Table 1, P < 0.0001).
Table 1.
Signaling pathway associated with glioma invasion
| Name of signaling pathway | p value |
|---|---|
| EphB/ephrin-B receptor/ligand signaling | 0.00000159 |
| G Proteins/MAPK/ERK signaling | 0.00556 |
| PDGF/prostacyclin signaling | 0.00691 |
| cAMP signaling | 0.00863 |
| Rap2A signaling | 0.0155 |
MAPK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinase, PDGF: platelet-derived growth factor.
Ephrin-B2 mRNA Level Is High in GBMs and Is a Prognostic Marker in Malignant Astrocytomas
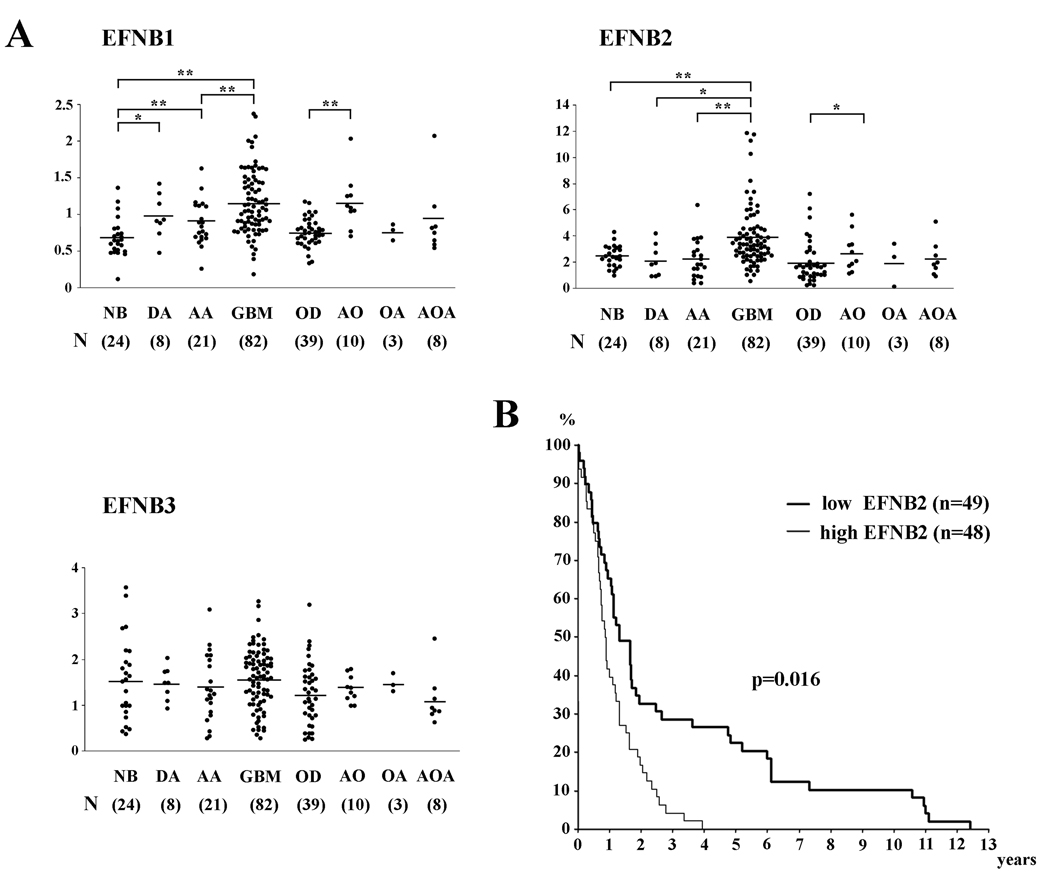
To evaluate a potential role of the EphB/ephrin-B signaling pathway for the malignant behavior of human gliomas, the expression levels of three members of the ephrin-B family were assessed on a human glioma tissue microarray. Whole genome expression profiling of a series of human brain tumor specimens derived from Henry Ford Hospital (Detroit, MI) was carried out at the National Cancer Institute and revealed ephrin-B1 and –B2 expression to be significantly higher in GBM (P < 0.01) than in anaplastic astrocytoma and normal brain specimens (Figure 1A). Ephrin-B1 and –B2 were also highly expressed in anaplastic oligodendroglioma compared with oligodendroglioma (P < 0.01, P < 0.05, respectively).
Figure 1. Analysis of ephrin-B expression in various human glial tumors.
(A) Expression levels of ephrin-B mRNA in nonneoplastic brain (NB), oligoastrocytoma (OL), anaplastic astrocytoma (AA), glioblastoma (GBM), oligodendroglioma (OD), anaplastic oligodendroglioma (AO), oligoastrocytoma (OA), and anaplastic oligoastrocytoma (AOA) were mined in an Affymetrix gene expression profile. *, P < 0.05; **, P < 0.01.
(B) Kaplan-Meier survival analysis of patients with malignant astrocytomas (n = 97) binned into high (≥median) and low (<median) expression of ephrin-B2.
Patients with high ephrin-B2 tumor levels (≥ median expression) had significantly shorter survival than patients with low ephrin-B2 tumor levels (< median expression, P = 0.016; Figure 1B). The one-year and two-year survival rates were 39.6 % and 16.7 % in the group showing high expression of ephrin-B2, as compared with 65.3 % and 32.7 % for patients with low expression of ephrin-B2, respectively. Other ephrin-B mRNA levels did not show correlation with survival (data not shown).
Overexpression and Phosphorylation of Ephrin-B2 in GBM
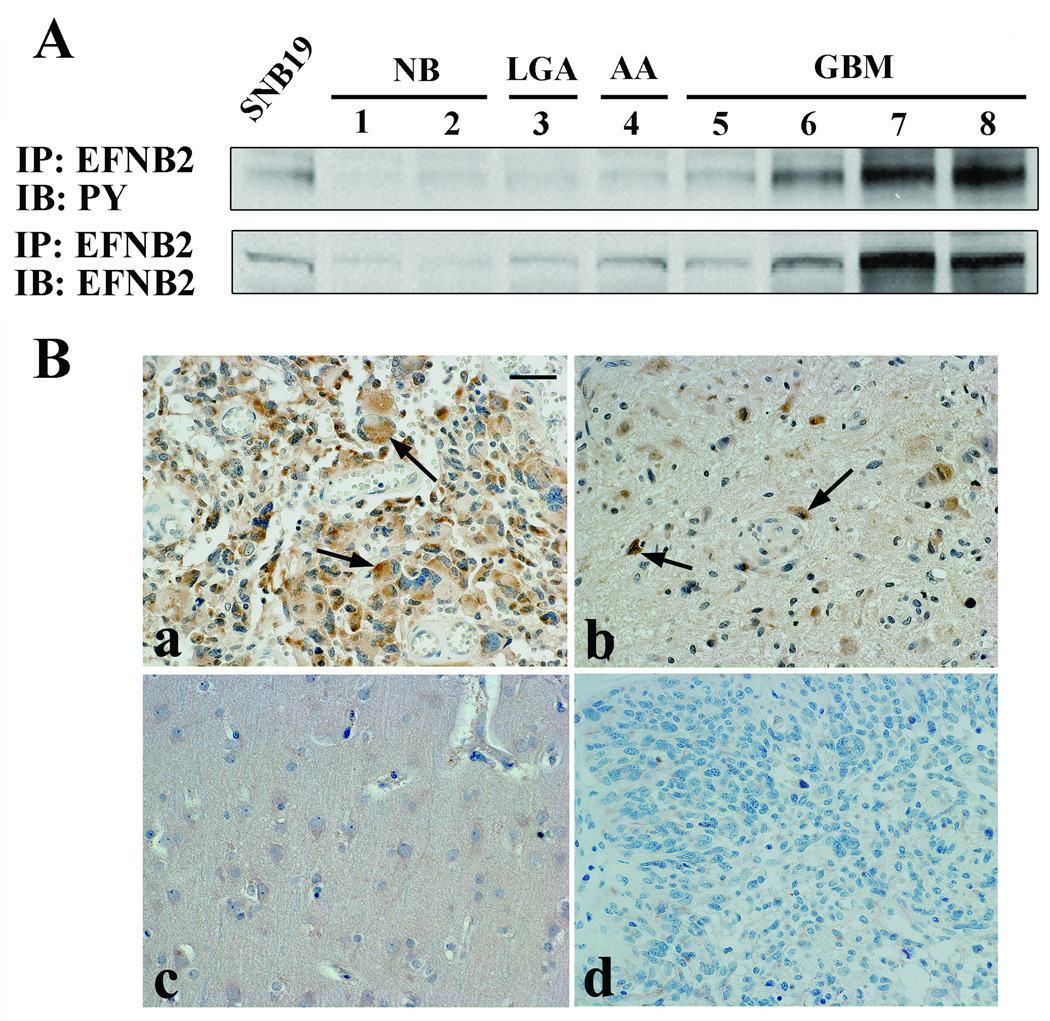
To assess the production level and phosphorylation level of ephrin-B2 in human gliomas, ephrin-B2 immunoprecipitation was conducted on surgical specimens as well as SNB19 cells as a control. Consistent with the microarray results, protein levels and tyrosine phosphorylation of ephrin-B2 were increased in GBM tissue relative to normal brain (Figure 2A).
Figure 2. The production, phosphorylation, and localization of ephrin-B2 in human glioma.
(A) Results of immunoprecipitation using total cell lysate. Equal amounts of cell lysates were immunoprecipitated (IP) with anti-ephrin-B2 antibody. The immunoprecipitates were probed by immunoblotting (IB) with the antibody indicated. PY, phosphotyrosine.
(B) Immunolocalization of ephrin-B2 in glioblastoma (GBM) tissues and normal brain tissues. Paraffin sections were immunostained with specific antibodies against ephrin-B2 (a, b and c) or nonimmune goat IgG (d). Note ephrin-B2 immunostaining in the GBM cells at the central region in the tumor (a, arrows), as well as in invading GBM cells at the tumor border (b, arrows) or reactive astrocytes, whereas no staining is observed in the normal brain with ephrin-B2 (c) or in GBM with nonimmune IgG (d). Hematoxylin counterstain; Bar = 100 µm.
Cells expressing ephrin-B2 in normal brain and GBM specimens were identified using immunohistochemistry. Ephrin-B2 was localized predominantly to neoplastic astrocytes of GBM specimens (8 of 10 cases; Figure 2Ba). Invading neoplastic cells also showed significant staining for ephrin-B2 (Figure 2Bb). Neoplastic astrocytes were identified by nuclear atypia in H&E-stained sections and were confirmed by immunopositivity for glial fibrillary acidic protein staining (data not shown). No staining was observed in normal brain (Figure 2Bc) or when the primary antibody was substituted for normal serum (Figure 2Bd). The immunohistochemistry results were consistent with that of microarray and immunoblot analysis.
Expression of Ephrin-B2 in Glioma Cell Lines
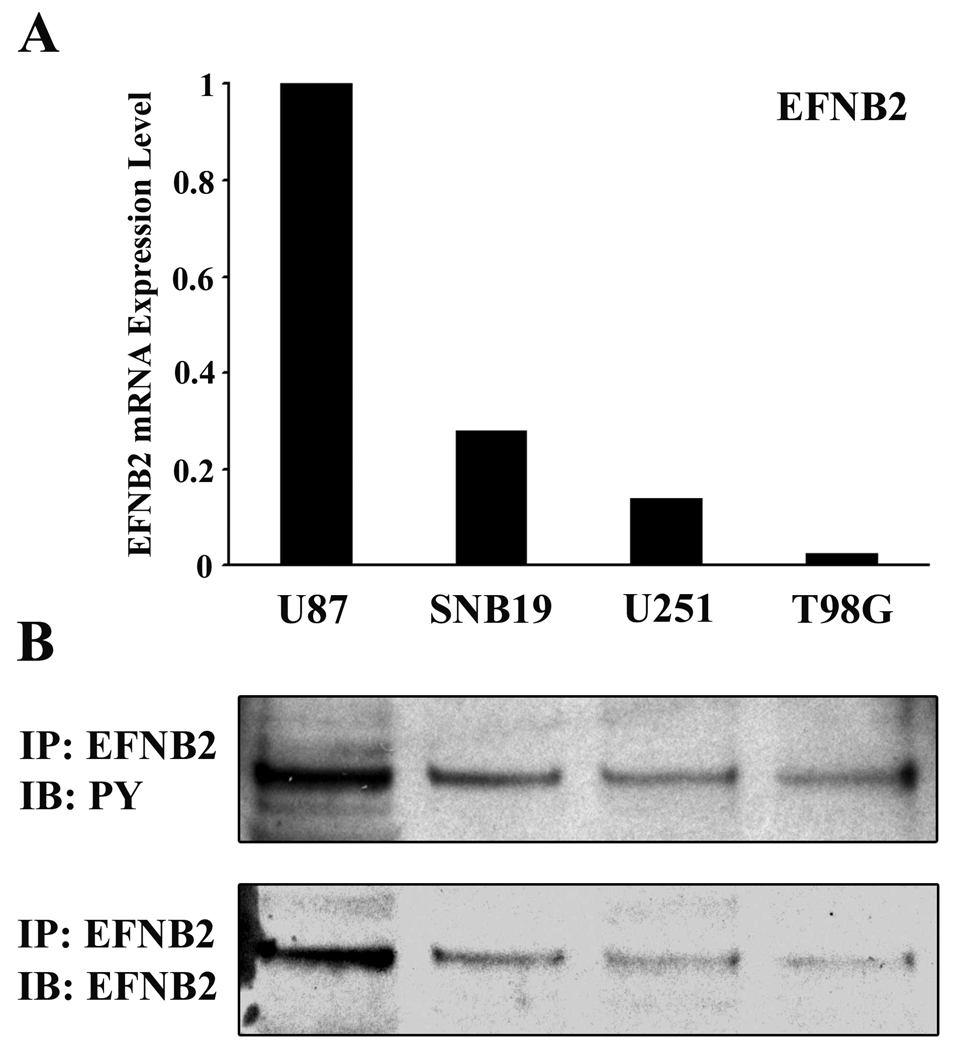
Expression of ephrin-B2 in human glioma cell lines was assessed by QRT-PCR using histone H3.3 mRNA as an internal quantitative reference. Levels of ephrin-B2 mRNA (ephrin-B2 mRNA/histone H3.3 mRNA ratios) were detected at various levels in all cell lines used (Figure 3A). To determine the endogenous levels of ephrin-B2 phosphorylation in glioma cells, we immunoblotted the ephrin-B2 immunoprecipitates with a specific monoclonal antibody directed against phospho-tyrosine residues. U87 glioma cells displayed the highest levels of ephrin-B2 protein (Figure 3B), which was consistent with QRT-PCR data. Tyrosine-phosphorylated ephrin-B2 was detected intensely in U87 cells, which has previously been demonstrated to be a highly migratory cell line 12. The level of phosphorylated ephrin-B2 in glioma cell lines appears to correlate with the migration rate (data not shown) 12, 14 but not proliferation rate of these tumor cells. 13.
Figure 3. Characterization of ephrin-B2 expression in human glioma cell lines.
(A) The relative mRNA expression levels of ephrin-B2 (target mRNA/histone H3.3 mRNA ratios) in the cells were analyzed by QRT-PCR. Each mRNA level is expressed as a proportion of the U87 mRNA level, which was given a value of 1.
(B) Immunoprecipitation using total cell lysate from each of the indicated cell lines was performed. Equal amounts of cell lysates were immunoprecipitated with anti-ephrin-B2 antibody. The immunoprecipitates were probed by immunoblotting with the antibody indicated. PY, phosphotyrosine.
Ephrin-B2 Phosphorylation Correlates with Migration and Invasion of U87 Cells
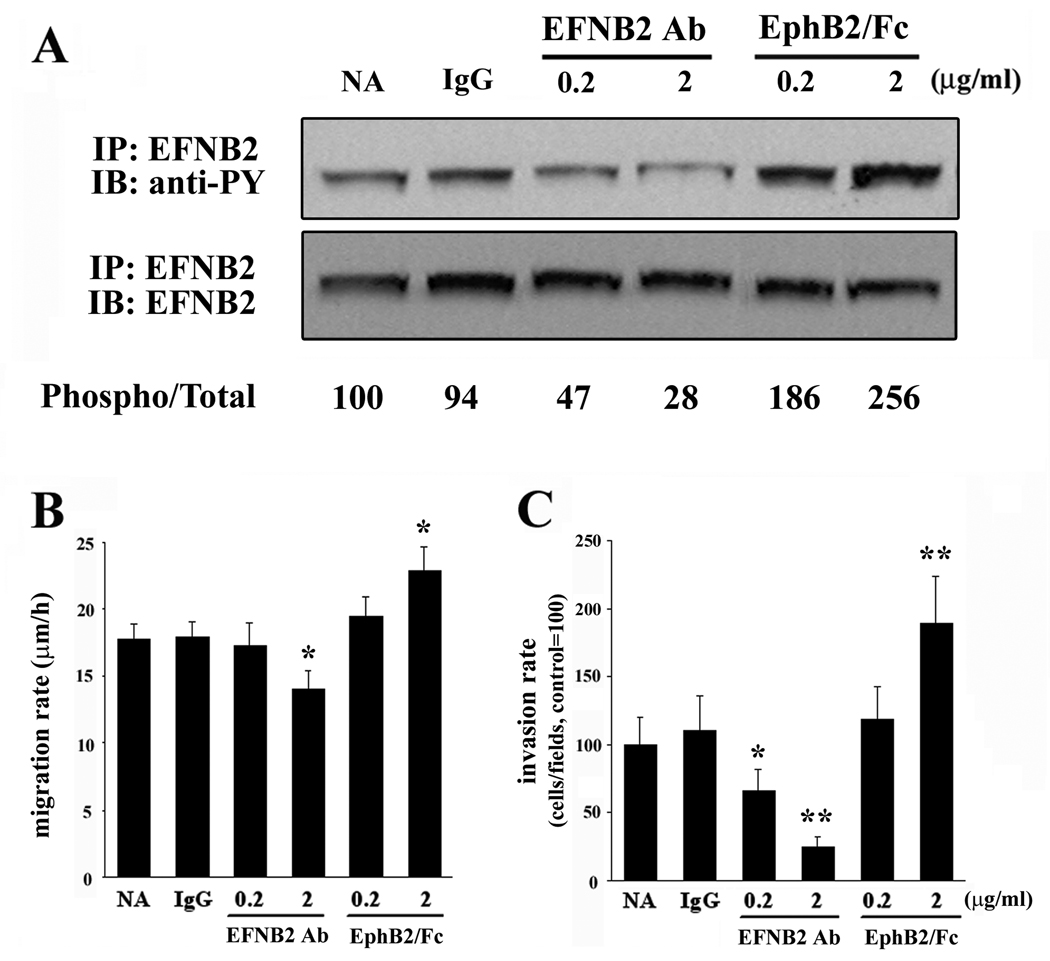
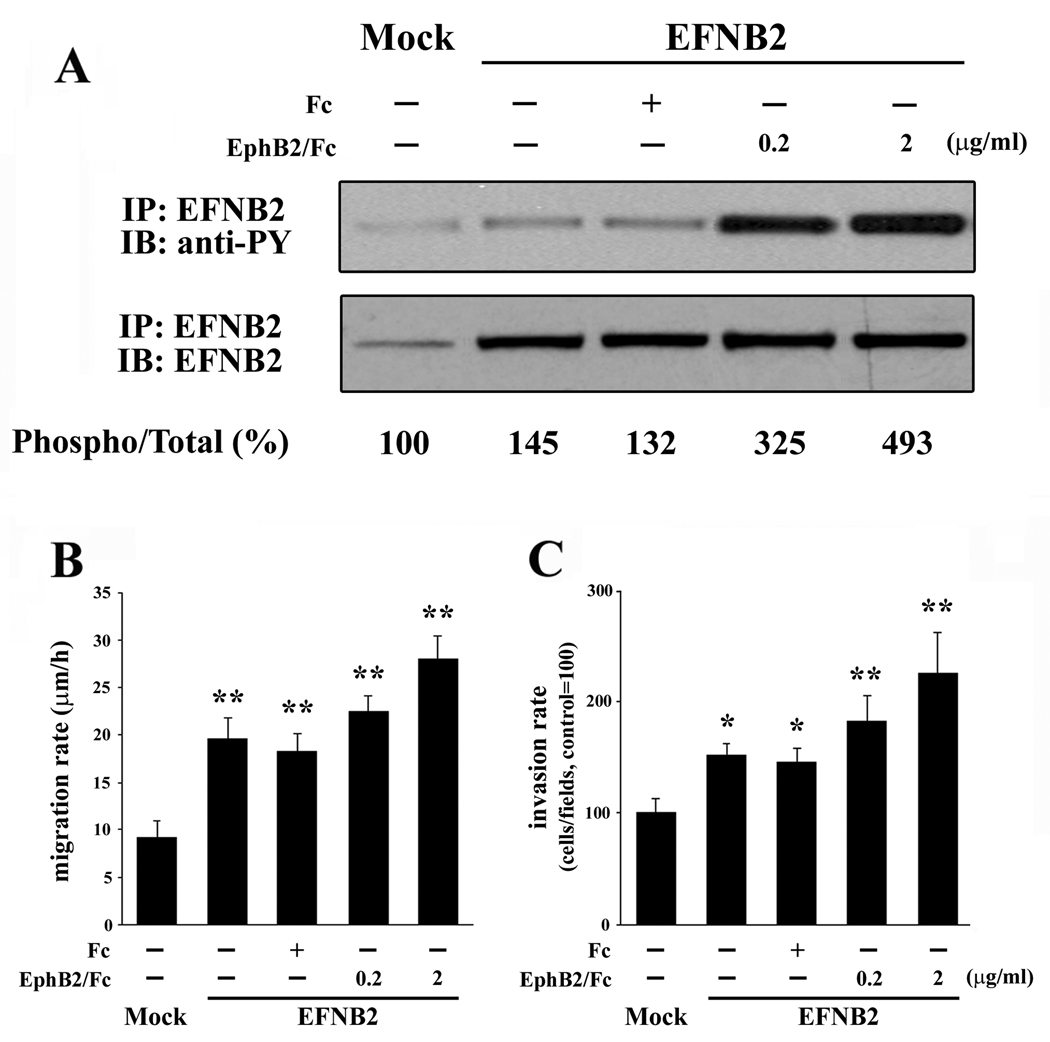
To investigate whether manipulation of ephrin-B2 phosphorylation influences glioma cell migration, we utilized a blocking antibody to ephrin-B2 to inhibit endogenous ephrin-B2 phosphorylation in U87. As shown in Figure 4A, phosphorylation of ephrin-B2 was inhibited by addition of ephrin-B2 antibody. Ephrin-B2 phosphorylation in U87 cells was induced in a dose dependent manner by addition of a recombinant EphB2 receptor (EphB2/Fc chimera) while treatment with control IgG was without effect.
Figure 4. The influence of ephrin-B2 activation on ephrin-B2 phosphorylation, migration, and invasion of U87 cells.
(A) Serum starved U87 cells on a plastic substrate were treated with control IgG (IgG), ephrin-B2 blocking antibody (EFNB2 Ab), or EphB2/Fc chimera (EphB2/Fc) at the indicated concentration for 10 min, after which immunoprecipitation of ephrin-B2 was performed. The numerical values indicate relative ratios as a percentage of the control (NA) for each band of phospho/total-ephrin-B2 after scanning and densitometric analysis using Gel Expert software by NucleoVision (NucleoTech, San Mateo, CA). Phosphorylation of ephrin-B2 in U87 is triggered by treatment with EphB2/Fc chimera. The ephrin-B2 blocking antibody suppressed the phosphorylation of ephrin-B2. NA, non addition.
(B) U87 cells were plated onto 10-well glass slides precoated with astrocytoma-derived extracellular matrix and then cultured in serum-free medium in the presence of control IgG (IgG), EphB2/Fc chimera (EphB2/Fc), or ephrin-B2 blocking antibody (EFNB2 Ab) at the indicated concentration after the attachment of the cells to the dishes. Cell migration was assessed over 24 h. Bars, SE.,*, P < 0.05 vs NA, IgG.
(C) U87 cells were treated as above and then applied to an invasion assay. Cells that invaded to the lower surface of the membrane were fixed and stained. Mean cell counts from at least six fields in each of four experiments are shown. Bars, SE., *, P < 0.05; **, P < 0.01 vs NA, IgG.
Migration assays and invasion studies were performed in the absence or presence of 0.2–2 µg/ml ephrin-B2 antibody, recombinant EphB2/Fc chimera, or control mouse IgG. The migration of U87 was significantly suppressed by addition of 2.0 µg/ml ephrin-B2 antibody (14.04 ±1.42 µm/h, P < 0.05) relative to addition of control IgG (17.99 ± 1.08 µm/h). EphB2/Fc chimera (2.0 µg/ml) effectively stimulated migration of U87 glioma cells (22.87 ± 1.78 µm/h, P < 0.05) approximately 1.27 fold relative to cells treated with control IgG (Figure 4B). Similar outcomes were obtained with SNB19 cells, which express ephrin-B2 (data not shown).
Invasion assay data also indicated that ephrin-B2 antibody inhibited the invasion of U87 cells (65 ± 16% of control, P < 0.05; and 25 ± 7% of control, P < 0.01) as concentrations of ephrin-B2 antibody increased (Figure 4C), whereas control IgG had no effect on U87 cell invasion. EphB2/Fc chimera (2.0 µg/ml) stimulated invasion of U87 cells (189 ± 35% of control, P < 0.01). Proliferation rate, however, is independent of ephrin-B2 phosphorylation levels in U87 cells (data not shown). Taken together, these data indicate that ephrin-B2 signaling is a positive regulator of glioma cell migration and invasion.
EphB2 Phosphorylation Promotes Cell Migration or lnvasion in U251 Cells
To further examine the functional role of ephrin-B2 in human gliomas, U251 cells transfected with ephrin-B2 or control vector (pEAK) constructs were evaluated in migration and invasion assays. Forced expression of the ephrin-B2 ligand in U251 cells results in phosphorylation of the transfected ligand (Figure 5A). These cells grew with comparable doubling times and morphology as the parental cells. In addition, overexpression of the ephrin-B2 ligand (Figure 5B) yielded an increased migration rate (19.59 ± 2.25 µm/h, P < 0.01) relative to pEAK transfected cells (9.14 ± 1.82 µm/h). The migration rate was enhanced by addition of EphB2/Fc chimera in the ephrin-B2 ransfectants (0.2 µg/ml; 22.49 ± 3.22 µm/h, 2.0 µg/ml; 28.02 ± 4.92 µm/h, P < 0.01), whereas addition of control Fc had no measurable effect (18.29 ± 3.78 µm/h) (Figure 5B).
Figure 5. Migration and invasion of U251 cells expressing ephrin-B2.
(A) Phosphorylation of ephrin-B2 in transiently transfected U251 cells. Ephrin-B2 was immunoprecipitated from cells transfected with pEAK (mock) or ephrin-B2 vector and treated with 0.2 or 2 µg/mL soluble EphB2/Fc chimera or control Fc for 10 minutes. The immunoprecipitates were probed by immunoblotting as indicated. Signals were quantified by densitometry using software by Nucleovision.
(B) Cells were plated onto 10-well glass slides precoated with astrocytoma-derived extracellular matrix, and then cultured in serum-free medium in the absence or presence of 0.2 or 2 µg/ml soluble EphB2/Fc after the attachment of the cells to the dishes. Cell migration was assessed over 24 h. Bars, SE. *, P < 0.05; **, P < 0.01 versus mock.
(C) Cells were treated as above and then applied to an invasion assay. Mean cell counts from at least six fields in each of four experiments are shown. Bars, SE. *, P < 0.05; **, P < 0.01 versus mock.
As shown in Figure 5C, cell invasion through membranes coated with Matrigel was increased in cells overexpressing ephrin-B2 (152 ± 10% of control, P < 0.05) and in cells treated with EphB2/Fc chimera (0.2 µg/ml; 183 ± 22% of control, 2.0 µg/ml; 225 ± 37% of control, P < 0.01). Additionally, no significant change was observed between ephrin-B2 transfection alone and ephrin-B2 transfection with the addition of control Fc (145 ± 13% of control), indicating that phosphorylation of ephrin-B2 seems to stimulate both migration and invasion.
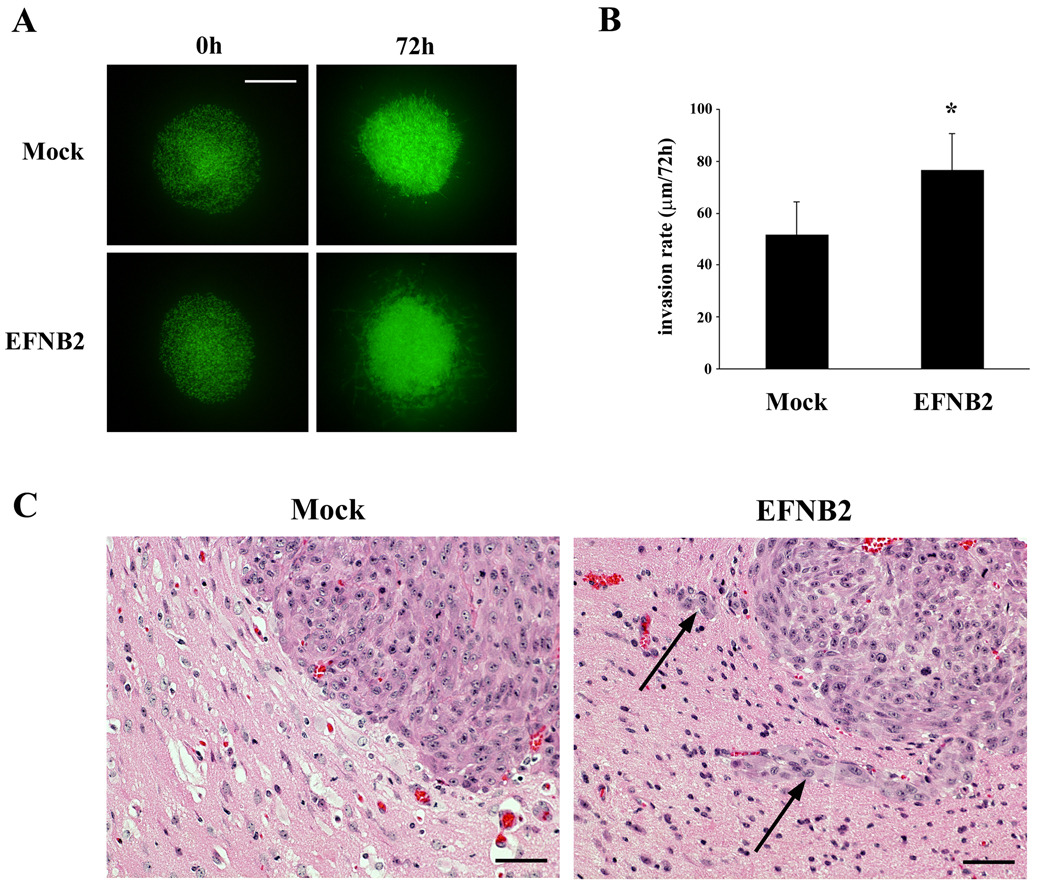
Ephrin-B2 promotes migration and invasion of glioma cells ex vivo
To evaluate the effects of ephrin-B2 on invasion through a more physiologically relevant matrix, U251 cells stably cotransfected with control or ephrin-B2 and GFP expression plasmids were examined for their growth and dispersion within an ex vivo organotypic rat brain slice. Overexpression of ephrin-B2 in U251-GFP cells increased phosphorylation of the transgene product (data not shown). In order to compare the growth of mock transfectants and ephrin-B2 stable transfectants, aggregations of each of these engineered cells were implanted in the putamen on contralateral sides of the same rat brain slice (Fig. 6A); images were taken at 0 h and 72 h after the implantation. The U251-ephrin-B2 cells displayed greater migration and invasion into the organotypic rat brain slice compared to the less invasive mock transfectant cells (Figure 6B). To quantify cell invasion, serial optical sections were obtained every 20 µm downward (z-axis) from the basal plane to the bottom using confocal microscopy. The U251-ephrin-B2 cells penetrated further into the brain slices (76.3 ± 14.2 µm/72 h) than the mock cells (51.7 ± 12.7 µm/72 h, P < 0.05) (Figure 6B). These data suggest that ephrin-B2 plays a role in invasion not only in vitro but also ex vivo.
Figure 6. Cell migration and invasion in organotypic rat brain slice and xenograft model.
(A) U251 cells co-transfected with GFP and pEAK or ephrin-B2 were transplanted bilaterally into the putamen of rat organotypic brain slices and observed at the indicated times. Bar = 500 µm.
(B) Invasion rates of U251 cells transfected with ephrin-B2 or pEAK were calculated from z-axis images collected by confocal laser scanning microscopy. The mean value of the invasion rate was obtained from six experiments. Bars, SE., *, P < 0.05.
(C) H&E images from representative brains of immunocompromised mice implanted with U87 cells transfected with empty plasmid vector (Mock) or ephrin-B2 vector (EFNB2) are displayed. Invading cells (arrows) were evident in EFNB2. Bar = 50 µm.
Overexpression of ephrin-B2 promotes glioma invasion in vivo
Furthermore, to evaluate whether altered ephrin-B2 receptor expression affects tumor invasion in vivo, we employed an orthotopic mouse xenograft model using U87 cells transfected with ephrin-B2 vector or empty plasmid vector. Invasive growth was observed at the border of the tumor in the animals that were transplanted with ephrin-B2 overexpressing cells, whereas invasion was not evident in the control group (Fig. 6C). These results demonstrate that ephrin-B2 signaling affects tumor invasion in intracerebrally implanted gliomas. No obvious change in glioma growth in vivo was observed, which is consistent with our in vitro data.
Discussion
We previously reported the analysis of the invasive transcriptome of in vitro and in situ human gliomas, which identified and validated target gene products linked to the invasion process 6–8. In an effort to further discover and validate mediators of invasion, we analyzed genes differentially expressed between invasive and stationary glioma cells in situ. Pathway enrichment analysis revealed strong EphB/ephrin-B signature coincident to the highly invasive behavior of human gliomas. Furthermore, we showed that levels of ephrin-B2 mRNA, protein, and the tyrosine-phosphorylated form of the protein are significantly higher in GBM tissue than in normal brain thereby substantiating this finding. Kaplan-Meier analysis showed that patients with high ephrin-B2 expression have significantly shorter median survival than those with low expression. This finding may be interpreted as a consequence of the increased invasiveness of tumors with higher ephrin-B2 expression, leading to a more unfavorable prognosis. Complementary results derived from the analysis of independent clinical sample sets examined by different methods, such as gene expression profiling, immunoblotting, and immunohistochemistry, support the importance of ephrin-B2 as a valuable biomarker for tumor progression, tumor invasion, and patient survival.
Other signaling pathways besides EphB/ephrin-B which were identified by pathway enrichment analysis as linked to invasion may represent additional intriguing targets for the treatment of glioma. We have recently demonstrated that MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) which is the 2nd most enriched pathway found in our analysis, is involved in the invasion phenotype of glioma 7. The 3rd candidate, PDGF (platelet-derived growth factor) is a growth factor family of ligand known to activate PI3K (phosphatidylinositol 3-kinase) and MAPK, which have been causally linked to glioma formation and invasion 17. Actually PDGF receptors are overexpressed in GBM 18. Recently the 4th candidate, cAMP has been reported to be involved in glioma invasion via the regulation of protease activity 19. The expression of the 5th candidate, Rap2A, which is a member of RAS oncogene family, has not been reported in glioma.
The highly migratory glioma cell line, U87, contained the highest level of total ephrin-B2 protein, as well as the highest concentration of tyrosine-phosphorylated ephrin-B2 protein, among four glioma cell lines. U87 migration and invasion was correlated with ephrin-B2 phosphorylation in vitro and in vivo. Forced expression of ephrin-B2 in U251, a poorly invasive glioma cell line 12 with low constitutive expression of ephrin-B2, transformed U251 into a more highly invasive phenotype as assessed by in vitro and ex vivo assays. These results support a role for ephrin-B2 in GBM invasion.
In our migration and invasion assay using U87, EphB2/Fc resulted in relatively small increases in migration and was somewhat independent of ephrin-B2 phosphorylation. We hypothesize that the less than proportional increase in migration relative to ephrin-B2 phosphorylation is due to phosphorylation of other ephrin-B's and de-phosphorylation of EphB's in a competitive manner upon treatment with EphB2/Fc. In fact, U87 cells express various types of EphB/ephrin-B at various levels (data not shown), and dephosphorylation of EphB2 in U87 suppresses migration and invasion 12.
Accumulating evidence suggests that EphB/ephrin-B signaling is associated with cancer progression 10, 11, 20, 21. Elevated expression and activity of EphB receptors have been correlated with the growth of solid tumors 21, 22. In addition, high expression of ephrin-B is associated with increased tumor growth, tumorigenicity, and metastasis 23–25. Despite recent progress, the current understanding of ephrin-B reverse signaling in cancer is still in its infancy. We recently reported that reverse signaling through ephrin-B3 promotes glioma cell migration 14. Thus, the present study in addition to our previous report demonstrates that ephrin-B ligands are involved in tumor invasion and malignant progression of human brain tumors.
Upon cell-cell contact, EphB receptor induces ephrin-B reverse signaling. EphB4 is known to interact only with ephrin-B2 among all ephrin-B ligands 26. Coexpression of both EphB4 and ephrin-B2 at high levels was described in melanoma 24, ovarian 27, head and neck 28, breast 29, esophagus 30, gastric 23, colorectal 31 and endometrial cancers 32, suggesting that EphB4 is a major receptor for ephrin-B2 in malignancies. EphB4 is also overexpressed in GBM compared with normal brain (our unpublished data). In addition, we previously showed that EphB2 is overexpressed in invading glioma cells 12. As suggested by our previous report, EphB2/Fc was utilized in our present study as a pathway agonist, phosphorylating both endogeneous and exogeneous ephrin-B2 effectively. Taken together these observations demonstrate that both EphB2 and EphB4 are receptors for ephrin-B2 in glioma.
Downstream signaling by ephrin-B2 in tumor cells is poorly understood, although it has been shown to activate small GTPases, such as Rac1 and RhoA 33. While activation or deactivation of small GTPases significantly impacts cell morphology, such an effect was not observed when manipulating ephrin-B2 in glioma cells, suggesting that small GTPases are not the main downstream effectors of ephrin-B2 signaling in glioma cells. Similarly, the PI3K/Akt pathway, which is a major signaling pathway for glioma invasion, does not seem to be involved downstream of ephrin-B2 as induction of Akt phosphorylation has not been observed in U87 cells stimulated by EphB2/Fc (data not shown). Recent investigations demonstrated that ephrin-B interacts with STAT3 (signal transducer and activator of transcription 3) transcription factor in a tyrosine phosphorylation-dependent manner, resulting in cancer cell invasion via enhanced transcriptional activation of STAT3 34, 35. Not surprisingly, aberrant expression and activation of STAT3 has been implicated in GBM pathology 36. Therefore, STAT3 may play a role in glioma invasion downstream of ephrin-B2. Another possibility is the involvement of matrix metalloproteinase (MMP). Recent investigation demonstrated that ephrin-B signaling induced the secretion of MMP, resulting in tumor invasion 37. In our experiments, low concentration of ephrin-B2 blocking antibody inhibited U87 invasion but not migration, suggesting matrix degrading enzymes such as MMP's are involved in ephrin-B2 signaling. Taken together, it is possible that STAT3 phosphorylation and/or MMP secretion induced by ephrin-B2 contributes at least in part to the behavioral changes of glioma cells that manipulate ephrin-B2 signaling. Analysis of ephrin-B2 signaling pathways in glioma cells is ongoing in our laboratory.
In conclusion, this study develops the biological significance of ephrin-B2 expression for tumor progression and brain invasion, resulting in poor prognosis. An understanding of the function and regulation of ephrin-B2 may lead to the development of effective therapies for patients with glioma.
Acknowledgement
We thank Drs. Hiroshi Sato and Hisashi Miyamori for making expression vector; Akiko Imamura and Joshua R. Niska for technical assistance; Dr. Jie Wu for assisting in organotypic brain slice culture; Ms. Emi Nambu and Ms. Natsuki Furuyama for performing xenograft model; and Dr. Nhan L. Tran, for his valuable discussion.
This work was supported by NIH grant NS042262 (M. E. Berens), Grant-in-Aid for Young Scientists (B-19790992 and A-21689038) from the Ministry of Education, Culture, Sports, Science and Technology and from the Japan Society for the Promotion of Science (M. Nakada), Foundation for Promotion of Cancer Research (M. Nakada), and Japan Brain Foundation (M. Nakada).
Abbreviations
- GBM
glioblastoma
- LCM
laser capture microdissection
- STAT3
signal transducer and activator of transcription 3
- MMP
matrix metalloproteinase
Footnotes
Novelty and impact of the paper
Pathway enrichment analysis using microarray data revealed the EphB/ephrin-receptor/ligand tyrosine kinase as the most tightly linked signal to the invading glioma cell phenotype. Among ephrin-B ligand family members, the overexpression of ephrin-B2 and its tyrosine phosphorylation was shown to be important in the invasive behavior of malignant astrocytic tumors.
Disclosure of Potential Conflict of Interest
M. E. Berens holds stock in the company that provided cell migration assay. The other authors disclosed no potential conflicts of interest.
REFERENCES
- 1.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 3.Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–478. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravarti A, Palanichamy K. Overcoming therapeutic resistance in malignant gliomas: current practices and future directions. Cancer Treat Res. 2008;139:173–189. [PubMed] [Google Scholar]
- 5.Drappatz J, Norden AD, Wen PY. Therapeutic strategies for inhibiting invasion in glioblastoma. Expert Rev Neurother. 2009;9:519–534. doi: 10.1586/ern.09.10. [DOI] [PubMed] [Google Scholar]
- 6.Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ, McDonough WS, Sloan A, Coons SW, Berens ME. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB, Beaudry CE, Henrichs AN, Anderson EM, Berens ME. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6:1212–1222. doi: 10.1158/1535-7163.MCT-06-0711. [DOI] [PubMed] [Google Scholar]
- 8.Demuth T, Rennert JL, Hoelzinger DB, Reavie LB, Nakada M, Beaudry C, Nakada S, Anderson EM, Henrichs AN, McDonough WS, Holz D, Joy A, et al. Glioma cells on the run - the migratory transcriptome of 10 human glioma cell lines. BMC Genomics. 2008;9:54. doi: 10.1186/1471-2164-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 10.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Campbell TN, Robbins SM. The Eph receptor/ephrin system: an emerging player in the invasion game. Curr Issues Mol Biol. 2008;10:61–66. [PubMed] [Google Scholar]
- 12.Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–3185. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- 13.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras Signaling Regulates Glioma Cell Adhesion, Growth, and Invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakada M, Drake KL, Nakada S, Niska JA, Berens ME. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–8500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- 15.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 26–9. [DOI] [PubMed] [Google Scholar]
- 16.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Fomchenko EI, Holland EC. Platelet-derived growth factor-mediated gliomagenesis and brain tumor recruitment. Neurosurg Clin N Am. 2007;18:39–58. doi: 10.1016/j.nec.2006.10.006. viii. [DOI] [PubMed] [Google Scholar]
- 18.Mischel PS, Cloughesy TF. Targeted molecular therapy of GBM. Brain Pathol. 2003;13:52–61. doi: 10.1111/j.1750-3639.2003.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JJ, Moreno MJ, Lam JC, Haqqani AS, Kelly JF. Identification of secreted proteins regulated by cAMP in glioblastoma cells using glycopeptide capture and label-free quantification. Proteomics. 2009;9:535–549. doi: 10.1002/pmic.200800257. [DOI] [PubMed] [Google Scholar]
- 20.Castano J, Davalos V, Schwartz S, Jr, Arango D. EPH receptors in cancer. Histol Histopathol. 2008;23:1011–1023. doi: 10.14670/HH-23.1011. [DOI] [PubMed] [Google Scholar]
- 21.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H, Kino I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54:3645–3650. [PubMed] [Google Scholar]
- 24.Vogt T, Stolz W, Welsh J, Jung B, Kerbel RS, Kobayashi H, Landthaler M, McClelland M. Overexpression of Lerk-5/Eplg5 messenger RNA: a novel marker for increased tumorigenicity and metastatic potential in human malignant melanomas. Clin Cancer Res. 1998;4:791–797. [PubMed] [Google Scholar]
- 25.Tang XX, Evans AE, Zhao H, Cnaan A, London W, Cohn SL, Brodeur GM, Ikegaki N. High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res. 1999;5:1491–1496. [PubMed] [Google Scholar]
- 26.Brambilla R, Br, uuml, ckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R. Similarities and Differences in the Way Transmembrane-Type Ligands Interact with the Elk Subclass of Eph Receptors. Mol Cell Neurosci. 1996;8:199–209. [PubMed] [Google Scholar]
- 27.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br J Cancer. 2008;98:845–851. doi: 10.1038/sj.bjc.6604216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yavrouian EJ, Sinha UK, Rice DH, Salam MT, Gill PS, Masood R. The significance of EphB4 and EphrinB2 expression and survival in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:985–991. doi: 10.1001/archotol.134.9.985. [DOI] [PubMed] [Google Scholar]
- 29.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachibana M, Tonomoto Y, Hyakudomi R, Hyakudomi M, Hattori S, Ueda S, Kinugasa S, Yoshimura H. Expression and prognostic significance of EFNB2 and EphB4 genes in patients with oesophageal squamous cell carcinoma. Dig Liver Dis. 2007;39:725–732. doi: 10.1016/j.dld.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan F, Bucana CD, Ellis LM. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer. 2002;94:934–939. doi: 10.1002/cncr.10122. [DOI] [PubMed] [Google Scholar]
- 32.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Overexpression of ephrinB2 and EphB4 in tumor advancement of uterine endometrial cancers. Ann Oncol. 2007;18:485–490. doi: 10.1093/annonc/mdl414. [DOI] [PubMed] [Google Scholar]
- 33.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 34.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13:1362–1366. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 35.Bong YS, Lee HS, Carim-Todd L, Mood K, Nishanian TG, Tessarollo L, Daar IO. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci U S A. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M, Sasaki K, Kamata R, Sakai R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J Cell Sci. 2007;120:2179–2189. doi: 10.1242/jcs.008607. [DOI] [PubMed] [Google Scholar]