Summary
Functional magnetic resonance imaging (fMRI) was employed to address the question whether medial temporal lobe activity prior to a stimulus event is predictive of whether the event will be successfully encoded in an incidental study task. Participants were scanned while making pleasantness judgments on words presented either in written or spoken form. A cue presented at a variable interval before the onset of each word signaled the modality of the upcoming item. Following the study phase, a surprise recognition memory test was administered that required items to be endorsed as ‘Remembered’, ‘Known’ or ‘New’. Activity in the medial temporal lobe, including the hippocampus, differed during the cue-item interval according to whether the item was later endorsed as Remembered rather than judged as Known or New. Thus, the level of hippocampal activity prior to the onset of an event predicts whether the event will be successfully encoded into episodic memory.
Keywords: Encoding, Hippocampus, MTL, fMRI, Episodic memory
Beginning with Brewer et al. (1998) and Wagner et al. (1998), numerous fMRI studies have investigated the neural correlates of human episodic memory encoding with the ‘subsequent memory procedure’. In this procedure, activity elicited by on a study trial is contrasted according to the response accorded the studied item on a later memory test (e.g. ‘remembered’ vs. ‘forgotten’). The overriding majority of these studies have focused on activity elicited by the study items. Together, the findings indicate that successful episodic encoding is often associated with enhanced study activity in the medial temporal lobe (MTL), including the hippocampus (e.g. Davachi et al., 2003; Ranganath et al., 2004; Kensinger and Schacter, 2006; see Eichenbaum et al., 2007 for review). Item-related activity is not, however, the only type of study activity to exhibit ‘subsequent memory effects’. Effects have also been reported for activity sustained across a series of study trials (Fernandez et al., 1999; Otten et al., 2002) and, the focus of the present study, for activity that precedes presentation of a study item.
The first reported study of ‘pre-stimulus subsequent memory effects’ (Otten et al., 2006) employed event-related potentials (ERPs). On each study trial, a cue instructed subjects to perform one of two tasks on the following visual word (experiment 1), or informed them whether the upcoming word would be visual or auditory (experiment 2). ERPs during the interval between the cue and the onset of semantically studied visual words were more negative-going for words that were later recollected than for words that were unrecollected or missed. In a subsequent study using fMRI (Adcock et al., 2006), subjects received a cue informing them whether accurate memory for the upcoming study item would be associated with high or low monetary reward. On ‘high-reward’ trials activity during the cue-item interval was enhanced for later recognized compared to missed items in several regions, including bilateral anterior hippocampus. In a third study (Mackiewicz et al., 2006), subjects were cued to anticipate the presentation of aversive or neutral pictures. Amygdala and anterior hippocampal activity in the cue-picture interval correlated across subjects with later recognition performance for the aversive pictures. Finally, in a recent magnetoencephalographic (MEG) study (Guderian et al., in press), it was reported that probability of later recall of words presented in a series of short study lists covaried with theta power in the 200ms time window immediately preceding each word.
The above findings indicate that the probability of successful episodic encoding is influenced by pre-stimulus neural activity. However, these findings provide little information about the regions that manifest pre-stimulus subsequent memory effects when encoding is incidental rather than intentional, and hence when – unlike in Adcock et al. (2006) and Guderian et al. (in press) – there is no incentive to learn upcoming study items or rehearse previously presented items. The findings of Otten et al. (2006) offer no information in this regard, and the findings of Mackiewicz et al. (2006) are restricted to aversive stimuli and subsequent memory effects identified by between-subject analyses. We address this question here with an fMRI study based on experiment 2 of Otten et al. (2006).
Eighteen participants (seven female; ages 18-25 years) contributed data to the study. All were right-handed, native English speakers with no self-reported history of neurological or psychiatric illness. Two additional participants were excluded due to excessive head movement or inadequate performance. Informed consent was obtained before participation in accordance with the requirements of the University of California-Irvine Institutional Review Board, which approved the experimental protocol. Participants were remunerated.
Experimental items were drawn from a pool of 411 pairs of written and spoken forms of words denoting everyday objects. Subject-specific study lists comprised a pseudo-random sequence of 252 critical items, comprising 126 written words and 126 spoken words, selected from separate visual and auditory word pools, and an additional 3 buffer items. Corresponding test lists consisted of 378 items, comprising a pseudo-random sequence of the 252 studied items and 126 new items (63 written, 63 spoken). The remaining items from the stimulus pool were used for study and test practice trials. Study and test lists were constrained such that no item type occurred more than three times in succession.
Participants were instructed and practiced on the study task prior to the experiment, which consisted of a single study-test cycle. Each study trial began with the presentation of a red fixation cross for 100 ms. This was followed by a pictorial cue (an eye or ear) for 500 ms, which signaled whether the upcoming study item would be visual or auditory. The cue was replaced by a white cross for the remainder of the cue-item interval. The interval varied randomly between 1500, 3000, and 4500ms. At the end of the interval, the study item was displayed visually for 600 ms, or was presented auditorily via MRI-compatible headphones (mean presentation duration of 689 ms [SD = 139]). The study item was followed by a white cross, which served as a response prompt. The duration of the cross varied randomly between 2500, 3500 and 4500 ms. Participants were instructed to judge whether each study item was ‘pleasant’ or ‘unpleasant’, depressing a corresponding response-button with the right index or middle finger. Participants were not informed their memory for the study items would be tested until the test phase. The study list was presented across three scanning sessions separated by approximately 3 min breaks.
Recognition memory for the study items was tested outside the scanner approximately 30 min later. Participants were first practiced with old items that had been presented in the practice study list. The test requirement was to indicate whether each item was i) remembered (R): specific details about the study presentation could be recalled, ii) known (K): the item had been presented in the study phase, but no specific details could be recollected, iii) new (N): the item was unstudied. Participants were instructed to respond ‘New’ if they were unsure about an item's study status. Old test items were presented in the same modality as at study, and new test items were randomly and evenly distributed between the two modalities. A pictorial cue signaling the modality of the upcoming test item appeared 500ms before the item was presented. The test was self-paced.
A Philips Achieva 3T MR scanner (Philips Medical Systems, Andover, MA), fitted with an 8-channel RF receiver head coil, was used to acquire T1–weighted anatomical volume images (MPRAGE, 256 × 238 matrix, 1mm3 voxels, sagittal acquisition) and T2*–weighted echo-planar images (EPIs) (80 × 80 matrix, 3mm × 3mm in-plane resolution, flip angle 70°, TE 30ms). EPIs were acquired using a sensitivity encoding (SENSE) reduction factor of 1.5. Each EPI volume comprised 30 3mm-thick axial slices acquired in an ascending order and separated by 1mm. Data were acquired during the study phase in three sessions comprising 307 volumes each, with a repetition time (TR) of 2s. Five additional volumes were collected at the beginning of each session and discarded to allow for T1 equilibration.
Data preprocessing and statistical analyses were performed with the Statistical Parametric Mapping software package (SPM 5, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk), implemented in MATLAB 7 (Mathworks, Natick, MA). For each subject, functional images were registered to the first image of each session and temporally realigned to the middle slice using sinc interpolation. The resulting data were normalized (Ashburner and Friston, 1999) to a standard EPI template based on the Montreal Neurological Institute (MNI) reference brain (Cocosco et al., 1997) and resampled into 3 mm3 voxels. Normalized images were smoothed with an 8 mm full-width half-maximum Gaussian kernel. The time series in each voxel were high-pass filtered to 1/128 Hz and scaled to a grand mean of 100 across voxels and volumes. T1-weighted anatomical images were co-registered to the mean EPI volume and normalized to a standard T1 template of the MNI brain.
Prior to model estimation, the imaging time-series were concatenated across sessions. Cue-related neural activity was modeled by boxcar functions that extended from cue onset until the onset of the study item. The predicted blood-oxygen-level dependent (BOLD) response was modeled by convolving these neural functions with a canonical hemodynamic response function (HRF) and its temporal and dispersion derivatives. Item-related activity was modeled by a delta function at stimulus onset convolved with a canonical HRF and its derivatives. In addition, six regressors were employed to model movement-related variance, and session-specific constant terms were employed to model differences in mean image intensity. In the first stage of data analysis, parameter estimates for events of interest were estimated for each subject using a General Linear Model incorporating the above-described regressors. In a second stage, linear contrasts of these subject-specific parameter estimates were computed, treating subjects as a random effect.
In order to identify brain activity in the medial temporal lobe, a group-based MTL mask was used, which was restricted to the hippocampus and surrounding MTL (Insausti et al., 1998). The mask was created by manually tracing the MTL on coronal slices of the across-subjects mean normalized anatomical image (MRIcroN: www.mricro.com) and then smoothing the result with an 8-mm FWHM Gaussian kernel. The mask was used in combination with small volume correction (Friston et al., 1994) to identify the effects within the boundary of the MTL. For primary contrasts, clusters were accepted as significant if the number of contiguous activated voxels (one-sided threshold at p < .0025) exceeded a corrected cluster-level threshold of p < .05 (corresponding to approximately 14 voxels in the present data). In addition, time-courses of identified effects were estimated with a finite-impulse response model applied to all voxels in a 5mm sphere centered on the voxel demonstrating the peak parameter estimate. No baseline adjustment was applied to the time-courses after estimation.
Study response times (RTs) were segregated by modality and later memory judgment. To parallel the principal fMRI analyses, an ANOVA (factors of modality and memory judgment) was conducted on the RTs associated with later R and K responses. This revealed main effects of modality (F[1,17] = 275.32, p < .001) and memory judgment (F[1,17] = 18.54, p < .001). The effects reflect slower judgments for auditory items (1286 ms for visual vs. 1566 ms for auditory), and for items later endorsed with a K rather than an R response (1483 ms vs. 1369 ms respectively). The interaction between the two factors was not significant (F < 1). Test performance (Table 1) was analyzed after transforming raw R and K rates according to the assumption that recollection and familiarity are independent (Yonelinas and Jacoby, 1995). Contrasts of the resulting recollection and familiarity estimates demonstrated a marked advantage for the visual study items in both cases (recollection: .51 vs. .19, t[17] = 9.97; familiarity: .64 vs. .33, t[17] = 9.49, both ps < .001).
Table 1.
Mean proportions of test judgments according to item modality (SD in parentheses).
| Studied items | New items | |||
|---|---|---|---|---|
| visual | auditory | visual | auditory | |
| Remember | .54 (.18) | .31 (.14) | .03 (.01) | .12 (.01) |
| Know | .34 (.17) | .34 (.15) | .09 (.02) | .15(.02) |
| New | .11 (.09) | .35 (.13) | .88 (.02) | .74 (.02) |
Turning to the fMRI results, eight subjects missed only a few (< 12) or none of the visually-presented items on the later memory test, precluding the analysis of study trials associated with these items. Therefore we focused on the analysis of subsequent memory contrasts for study trials associated with R versus K judgments on the later recognition task. Note that in the figure illustrating the findings from these analyses we also report the parameter estimates for auditory study items that went on to be missed. These estimates were statistically equivalent to those associated with K responses.
We first identified MTL pre-stimulus subsequent memory effects common to the two study modalities. We then searched for effects that were selective for one or the other study modality (cf., Otten et al., 2006). Finally, we searched for item-related subsequent memory effects, using procedures analogous to those used to identify the two classes of pre-stimulus effects described above. Neither task-independent nor task-selective item-related effects were identified by these latter analyses.
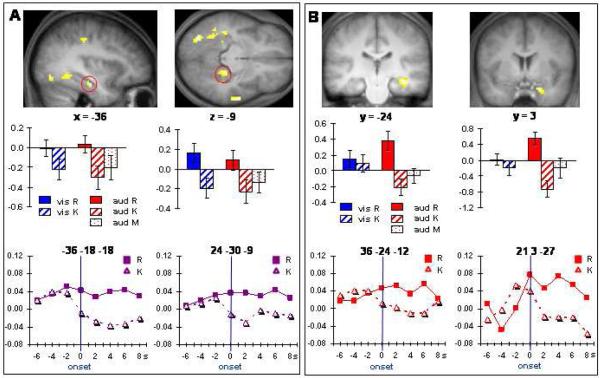
Modality-independent MTL pre-stimulus effects: Pre-stimulus effects common to the two modality conditions were identified by exclusively masking each side of the main effect of subsequent memory (R vs. K) with the F contrast (p < .1) of the interaction between modality and subsequent memory. No regions were identified where pre-stimulus activity was greater for K trials than for R trials. The reverse contrast revealed three MTL clusters (Table 2), two of which were in bilateral anterior hippocampus (Figure 1A).
Table 2.
MTL pre-stimulus subsequent memory effects
| Coordinates (x y z) | Z (# of voxels) | Region | BA | |||
|---|---|---|---|---|---|---|
| Modality-independent | ||||||
| −36 | −18 | −18 | 3.65 | 20 | L Anterior hippocampus | |
| 24 | −30 | −9 | 3.58 | 28 | R Posterior hippocampus | |
| −24 | −39 | 0 | 3.16 | 17 | L parahippocampal cortex | 27/36 |
| Auditorily-selective | ||||||
| 36 | −24 | −12 | 4.23 | 33 | R Hippocampus | |
| 21 | 3 | −27 | 4.09 | 24 | R Entorhinal cortex | 34 |
Coordinates and Z-values refer to the peak voxels of activated clusters. L, left; R, right; BA, Brodmann area (approximate).
Figure 1.
Regions demonstrating modality-independent (A) and auditorily-selective (B) pre-stimulus subsequent memory effects (p < .0025), overlaid on sections of the across-subjects mean T1-weighted structural image. Bar graphs show mean parameter estimates and standard errors (arbitrary units) of the peak voxel of the indicated effect. Time-courses of the independent and selective effects at the same loci are shown below. Time-courses were averaged across cue-item intervals, and aligned at the onset of the study item (0 s). Signal amplitude is in arbitrary units.
The time-courses associated with these effects (Figure 1A) indicated that they onset around the onset time of the study item, and thus before any possible item-related BOLD subsequent memory effect could have emerged. An ANOVA with factors of region (left hippocampus, right hippocampus), memory judgment (R, K) and time bin (0s, 2s, 4s) gave rise solely to a main effect of memory judgment (F[1,17] = 7.32, p = .01), indicating that activity within this time-range was reliably greater for trials associated with subsequent R than subsequent K judgments.
Modality-selective MTL pre-stimulus effects: Modality-selective effects were identified by exclusively masking each side of the modality-specific contrasts (p < .0025) by the alternate contrast (p < .05). No effects selective for the visual modality could be identified, and nor were there any regions where activity was greater for auditory items later receiving K vs. R judgments. As illustrated in Figure 1B, however, two MTL clusters – corresponding to right hippocampus and right entorhinal cortex - were identified where pre-stimulus activity was greater on trials associated with later R judgments. The corresponding time-courses are also shown in Figure 1B. As for the modality-independent effects, the modality-selective effects begin to diverge around the time of item onset. An ANOVA employing the same factors as in the prior time-course analysis revealed a main effect of memory judgment (F[1,17] = 6.02, p < .05). There were no main effects or interactions.
The present findings indicate that hippocampal pre-stimulus subsequent memory effects are not confined to intentional encoding tasks (cf., Adcock et al., 2006), but are also evident when encoding is incidental. The findings further extend previous results by demonstrating that these effects dissociate study trials not according to whether items are later recognized or missed, but whether recognition is accompanied or unaccompanied by a subjective sense of recollection (i.e. R vs. K judgments).
There is evidence from both lesion and functional neuroimaging studies that the hippocampus plays a more important role in recollection than in familiarity-driven recognition (Eichenbaum et al., 2007). The present finding that hippocampal pre-stimulus subsequent memory effects dissociate study trials containing items later endorsed as recollected versus familiar (or new) is consistent with this proposal (note that in the case of the auditory trials, hippocampal pre-stimulus effects were accompanied by effects in entorhinal cortex). This finding can however also be accommodated by the view that the hippocampus adds additional ‘strength’ to memory representations, and that the distinction between R and K judgments is better construed in terms of strong versus weak memory rather than recollection versus familiarity (Squire et al., 2007). The present findings are of interest regardless of how this issue is ultimately resolved. The findings indicate that the level of hippocampal BOLD activity just prior to the occurrence of a stimulus event predicts whether recognition of the event will be based on a memory signal that additionally supports the subjective experience of recollection. That is, hippocampally-mediated episodic encoding is potentiated by high levels of pre-stimulus activity.
What might be responsible for modulating the amount of pre-stimulus hippocampal activity? One obvious possibility is that activity is modulated by attentional or preparatory state. Notably, the study task required subjects to shift unpredictably between preparing for an upcoming visual or auditory study item. In both cases, preparation required a shift of attentional focus from the task cue to the upcoming item and the associated study task. In the auditory condition, moreover, it additionally necessitated a cross-modal attentional shift. We conjecture that pre-stimulus hippocampal activity was enhanced on those trials where processes set in train by the presentation of the cue culminated in a relatively optimal preparatory state. Consistent with this proposal, pre-stimulus subsequent memory effects were associated not only with increased likelihood of successful recollective encoding, but also with more efficient performance of the study task (RTs were some 100 ms faster for study items that went on to be recollected rather than judged familiar). A possible neurochemical mechanism underlying the above attentional shifts is suggested by the findings of Adcock et al. (2006), who reported that hippocampal pre-stimulus subsequent memory effects were accompanied by effects in the ventral tegmental area – the origin of the mesolimbic dopaminergic system. While Adcock et al. (2006) activated this system by the manipulation of the ‘reward value’ of the pre-stimulus cue (see Introduction), pre-stimulus effects have also been reported in this system for cues signaling the novelty of an upcoming study item (Wittmann et al., 2007). Thus, cue-driven activation of the mesolimbic dopaminergic system may play a role in the modulation of pre-stimulus hippocampal activity in circumstances other than reward anticipation.
The foregoing proposals may also account for the additional pre-stimulus effects – in right hippocampus and entorhinal cortex – that were associated with the auditory trials. As is evident from the behavioral data, study processing of auditory items was considerably more demanding than in the case of visual items. Auditory study trials likely required mobilization of additional attentional resources to both initiate a cross-modal attentional shift and to overcome the distracting effects of scanner noise, and thus placed an even greater premium on optimal pre-stimulus preparation.
Whereas we were able to identify robust pre-stimulus hippocampal subsequent memory effects, no item-related effects were evident. Item-related effects were identified, however, when the data were re-analyzed using a statistical model in which the pre-stimulus regressors were omitted. With this model, which corresponds to a ‘conventional’ subsequent memory analysis, both task-independent and auditorily-selective hippocampal subsequent memory effects were evident (independent effects: −32 −32 −15, Z = 3.98 and 33 −12 −18, Z = 3.49; auditory effects: 24, −12,−21, Z = 3.71). The significance of these findings is uncertain: on the one hand, these ‘item-related’ effects may merely reflect the propensity for item regressors to detect unmodeled pre-stimulus effects. This possibility was first proposed by Otten et al. (2006), who noted that conventional analyses of subsequent memory effects inevitably carry a risk of conflating pre-stimulus and item-related effects. On the other hand, the sensitivity of the model to item-related effects when the pre-stimulus regressors were omitted may be a consequence of the fact that the pre-stimulus and item-related regressors were partially collinear, and hence that a significant fraction of the subsequent memory effects elicited during study trials could not be uniquely attributed to either regressor. This issue cannot be resolved on the basis of the current data. Crucially, it does not detract from the principal findings of the study: the identification of robust pre-stimulus subsequent memory effects in the hippocampus in an incidental encoding task.
In conclusion, the present findings add substantially to prior observations that the likelihood that a stimulus event will be successfully encoded is associated not only with the pattern of neural activity elicited by the event itself, but also with the activity that immediately precedes the event. Important future challenges include identifying the boundary conditions for the emergence of pre-stimulus subsequent memory effects, for example, whether they occur only when it is necessary to shift attention on a trial-by-trial basis, and establishing the relationship of these effects to the activity elicited by the stimulus event.
Acknowledgments
This research was supported by the National Institute of Mental Health Grant MH074528.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RS, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S425. [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Brewer JG, Zhao Z, Glover GH, Gabrieli JDE. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: A functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the Significance of Focal Activations Using their Spatial Extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA. doi: 10.1073/pnas.0900289106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulous I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdale and hippocampus function in emotional memory. Proc Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RNA, Rugg MD. State-related and item-related neural correlates of successful memory encoding. Nat Neurosci. 2002;5:1339–1344. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom S, D' Esposito M. Dissociable correlates for familiarity and recollection within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen B, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan R, Duzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. J Mem Lang. 1995;34:622–643. [Google Scholar]