Abstract
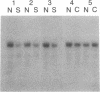
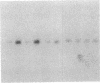
In situ hybridization histochemistry in combination with RNA blot techniques was used to study the regulation of opioid gene expression in rat hippocampus. By use of a prodynorphin cDNA probe, a strong hybridization signal was identified in the granule cell layer of the hippocampus. However, experiments using a proenkephalin cDNA probe revealed that the content of proenkephalin mRNA was considerably lower than that of prodynorphin mRNA. Following five brief trains of high-frequency electrical stimulation to the dentate gyrus, the proenkephalin mRNA content of the granule cells, measured 22 hr later, was substantially increased on the stimulated side. In contrast, levels of prodynorphin mRNA were markedly decreased ipsilateral to the stimulation site. These results were confirmed by RNA blot analysis of extracted mRNA. The decrease in prodynorphin mRNA content first became apparent between 4 and 7 hr after the end of stimulation. Distinct mechanisms, therefore, regulate the expression of proenkephalin mRNA and prodynorphin mRNA in rat hippocampus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Douglas R. M., Errington M. L., Lynch M. A. Correlation between long-term potentiation and release of endogenous amino acids from dentate gyrus of anaesthetized rats. J Physiol. 1986 Aug;377:391–408. doi: 10.1113/jphysiol.1986.sp016193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes A., Bradley P. B. The effects of kappa opioid agonists in the rat hippocampal slice. Neuropeptides. 1984 Dec;5(1-3):261–264. doi: 10.1016/0143-4179(84)90077-5. [DOI] [PubMed] [Google Scholar]
- Chavkin C., Shoemaker W. J., McGinty J. F., Bayon A., Bloom F. E. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci. 1985 Mar;5(3):808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Friedman W. J., Markey K. A., Black I. B. Depolarizing stimuli increase tyrosine hydroxylase in the mouse locus coeruleus in culture. Brain Res. 1986 Aug 6;379(2):216–222. doi: 10.1016/0006-8993(86)90774-2. [DOI] [PubMed] [Google Scholar]
- Feasey K. J., Lynch M. A., Bliss T. V. Long-term potentiation is associated with an increase in calcium-dependent, potassium-stimulated release of [14C]glutamate from hippocampal slices: an ex vivo study in the rat. Brain Res. 1986 Jan 29;364(1):39–44. doi: 10.1016/0006-8993(86)90985-6. [DOI] [PubMed] [Google Scholar]
- Gall C., Brecha N., Karten H. J., Chang K. J. Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol. 1981 May 10;198(2):335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- Gramsch C., Höllt V., Mehraein P., Pasi A., Herz A. Regional distribution of methionine-enkephalin- and beta-endorphin-like immunoreactivity in human brain and pituitary. Brain Res. 1979 Aug 3;171(2):261–270. doi: 10.1016/0006-8993(79)90332-9. [DOI] [PubMed] [Google Scholar]
- Harlan R. E., Shivers B. D., Romano G. J., Howells R. D., Pfaff D. W. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987 Apr 8;258(2):159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- Henriksen S. J., Chouvet G., Bloom F. E. In vivo cellular responses to electrophoretically applied dynorphin in the rat hippocampus. Life Sci. 1982 Oct 18;31(16-17):1785–1788. doi: 10.1016/0024-3205(82)90210-7. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllt V., Haarmann I., Bovermann K., Jerlicz M., Herz A. Dynorphin-related immunoreactive peptides in rat brain and pituitary. Neurosci Lett. 1980 Jun;18(2):149–153. doi: 10.1016/0304-3940(80)90318-3. [DOI] [PubMed] [Google Scholar]
- Iadarola M. J., Shin C., McNamara J. O., Yang H. Y. Changes in dynorphin, enkephalin and cholecystokinin content of hippocampus and substantia nigra after amygdala kindling. Brain Res. 1986 Feb 12;365(1):185–191. doi: 10.1016/0006-8993(86)90738-9. [DOI] [PubMed] [Google Scholar]
- Ishida I., Deguchi T. Effect of depolarizing agents on choline acetyltransferase and acetylcholinesterase activities in primary cell cultures of spinal cord. J Neurosci. 1983 Sep;3(9):1818–1823. doi: 10.1523/JNEUROSCI.03-09-01818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamatsu T., Obie J., Grimes L., McGinty J. F., Yoshikawa K., Sabol S., Hong J. S. Kainic acid alters the metabolism of Met5-enkephalin and the level of dynorphin A in the rat hippocampus. J Neurosci. 1986 Oct;6(10):3094–3102. doi: 10.1523/JNEUROSCI.06-10-03094.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley N., Loeffler J. P., Pittius C. W., Höllt V. Proenkephalin A gene expression in bovine adrenal chromaffin cells is regulated by changes in electrical activity. EMBO J. 1986 May;5(5):967–970. doi: 10.1002/j.1460-2075.1986.tb04310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGamma E. F., White J. D., Adler J. E., Krause J. E., McKelvy J. F., Black I. B. Depolarization regulates adrenal preproenkephalin mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8252–8255. doi: 10.1073/pnas.82.23.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J. P., Kley N., Pittius C. W., Almeida O. F., Höllt V. In vivo and in vitro studies of GABAergic inhibition of prolactin biosynthesis. Neuroendocrinology. 1986;43(4):504–510. doi: 10.1159/000124574. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Jensen R. A., McGaugh J. L., Davila K., Oliver M. W. Effects of enkephalin, morphine, and naloxone on the electrical activity of the in vitro hippocampal slice preparation. Exp Neurol. 1981 Mar;71(3):527–540. doi: 10.1016/0014-4886(81)90030-3. [DOI] [PubMed] [Google Scholar]
- McGinty J. F., Henriksen S. J., Goldstein A., Terenius L., Bloom F. E. Dynorphin is contained within hippocampal mossy fibers: immunochemical alterations after kainic acid administration and colchicine-induced neurotoxicity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):589–593. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B. J., Haarmann I., Kempter B., Höllt V., Herz A. Localization of prodynorphin messenger rna in rat brain by in situ hybridization using a synthetic oligonucleotide probe. Neurosci Lett. 1986 Aug 15;69(1):104–108. doi: 10.1016/0304-3940(86)90423-4. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Haarmann I., Kempter B., Höllt V., Herz A. Localization of prodynorphin messenger rna in rat brain by in situ hybridization using a synthetic oligonucleotide probe. Neurosci Lett. 1986 Aug 15;69(1):104–108. doi: 10.1016/0304-3940(86)90423-4. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Moneta M. E., ten Bruggencate G., Höllt V. Levels of prodynorphin mRNA in rat dentate gyrus are decreased during hippocampal kindling. Neurosci Lett. 1987 Oct 5;80(3):298–302. doi: 10.1016/0304-3940(87)90471-x. [DOI] [PubMed] [Google Scholar]
- Naranjo J. R., Iadarola M. J., Costa E. Changes in the dynamic state of brain proenkephalin-derived peptides during amygdaloid kindling. J Neurosci Res. 1986;16(1):75–87. doi: 10.1002/jnr.490160108. [DOI] [PubMed] [Google Scholar]
- Naranjo J. R., Mocchetti I., Schwartz J. P., Costa E. Permissive effect of dexamethasone on the increase of proenkephalin mRNA induced by depolarization of chromaffin cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1513–1517. doi: 10.1073/pnas.83.5.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittius C. W., Kley N., Loeffler J. P., Höllt V. Proenkephalin B messenger RNA in porcine tissues: characterization, quantification, and correlation with opioid peptides. J Neurochem. 1987 Feb;48(2):586–592. doi: 10.1111/j.1471-4159.1987.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Kley N., Loeffler J. P., Höllt V. Quantitation of proenkephalin A messenger RNA in bovine brain, pituitary and adrenal medulla: correlation between mRNA and peptide levels. EMBO J. 1985 May;4(5):1257–1260. doi: 10.1002/j.1460-2075.1985.tb03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. E., Eiden L. E., Affolter H. U. Elevated potassium stimulates enkephalin biosynthesis in bovine chromaffin cells. Neuropeptides. 1985 Dec;6(6):543–552. doi: 10.1016/0143-4179(85)90117-9. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Vidal C., Maier R., Zieglgänsberger W. Effects of dynorphin A (1-17), dynorphin A (1-13) and D-ala2-D-leu5-enkephalin on the excitability of pyramidal cells in CA1 and CA2 of the rat hippocampus in vitro. Neuropeptides. 1984 Dec;5(1-3):237–240. doi: 10.1016/0143-4179(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Vindrola O., Briones R., Asai M., Fernández-Guardiola A. Amygdaloid kindling enhances the enkephalin content in the rat brain. Neurosci Lett. 1981 Jan 1;21(1):39–43. doi: 10.1016/0304-3940(81)90054-9. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Moises H. C., Coy D. H., Baldrighi G., Akil H. Nonopiate effects of dynorphin and des-Tyr-dynorphin. Science. 1982 Dec 10;218(4577):1136–1138. doi: 10.1126/science.6128791. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Barchas J. D., Li C. H. beta-Lipotropin: localization of cells and axons in rat brain by immunocytochemistry. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5155–5158. doi: 10.1073/pnas.74.11.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. J., Khachaturian H., Akil H., Coy D. H., Goldstein A. Comparison of the distribution of dynorphin systems and enkephalin systems in brain. Science. 1982 Dec 10;218(4577):1134–1136. doi: 10.1126/science.6128790. [DOI] [PubMed] [Google Scholar]
- White J. D., Gall C. M., McKelvy J. F. Enkephalin biosynthesis and enkephalin gene expression are increased in hippocampal mossy fibers following a unilateral lesion of the hilus. J Neurosci. 1987 Mar;7(3):753–759. doi: 10.1523/JNEUROSCI.07-03-00753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Zieglgänsberger W., French E. D., Siggins G. R., Bloom F. E. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979 Jul 27;205(4404):415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]