Abstract
In a previous study, it was shown that transgenic mice, designated G-NonP, forget the location of a water maze hidden platform when tested 7 days after the last training day (Holahan and Routtenberg, 2008). The memory loss in G-NonP mice might be related to altered hippocampal architecture suggested by the fact that in the rat, 7 days after water maze training, there is discernible mossy fiber (MF) growth (Holahan et al., 2006; Rekart et al., 2007). In the present report, we studied the distribution of the MF system within the hippocampus of naïve, untrained, G-NonP mouse. In WT mice, the MF projection was restricted to the stratum lucidum of CA3 with no detectable MF innervation in distal stratum oriens (dSO). In G-NonP mice, in contrast, there was an ectopic projection terminating in the CA3 dSO. Unexpectedly, there was nearly a complete loss of immunostaining for the axonal marker Tau1 in the G-NonP transgenic mice in the MF terminal fields indicating that transgenesis itself leads to off-target consequences (Routtenberg, 1996). Because transgenic mice overexpressing non-mutated, wild type GAP-43 do not show this ectopic growth (Rekart, et al., 2009) and the G-NonP mice overexpress a mutated form of GAP-43 precluding its phosphorylation by protein kinase C (PKC), the possibility exists that permanently dephosphorylated GAP-43 disrupts normal axonal fasciculation which gives rise to the ectopic growth into dSO.
Keywords: protein kinase C, mossy fibers, hippocampus, cytoskeleton, defasciculation, growth
Introduction
There is now abundant evidence that PKC-dependent phosphorylation of the growth-associated protein, GAP-43, plays a central role in the learning and memory process. Concerning GAP-43, it is neuron-specific (Alexander et al., 1988; Basi et al., 1987; Chan et al., 1986; Skene and Virag, 1989; Zuber et al., 1989) and null mutation leads to infant mortality pointing to a critical role for this PKC substrate in axonal pathfinding during development (Maier et al., 1999; Meiri et al., 1998; Strittmatter et al., 1995). In the adult, a direct relation exists between its phosphorylation state and enhancement of LTP (Gianotti et al., 1992; Lovinger et al., 1985; Lovinger and Routtenberg, 1988). In the step-down inhibitory avoidance task (Cammarota et al., 1997; Ehrlich et al., 1977; Ehrlich and Routtenberg, 1974) as well as in Pavlovian fear-conditioning (Young et al., 2002; 2000) an increase in GAP-43 phosphorylation after training has been demonstrated. Moreover, hemizygotic GAP-43 knockouts were significantly impaired in fear conditioning, confirming the central role of GAP-43 in learning and memory processes (Rekart et al., 2005).
Concerning PKC, Izquierdo et al., (2000) found a time-dependent blockade of 24-hr retention when PKC inhibitors were given up to 30 min after learning indicating that PKC was a key element in a learning process. A reduction in amygdaloid PKCβ, which preferentially phosphorylates GAP-43 (Sheu et al., 1990), impaired fear conditioning (Weeber et al., 2000) while such conditioning selectively activated the PKCβ isoform (Paratcha et al., 2000).
We have recently shown that in 3 different isoforms of transgenic mice overexpressing GAP-43 [phosphorylatable — G-Phos; unphosphorylatable — G-NonP; permanently pseudophosphorylated — G-Perm] only G-NonP transgenic mice were significantly impaired in a hidden platform retention test given 7 days later (Holahan and Routtenberg, 2008). During the retention test, the G-NonP mice showed little preference for the region where the platform was originally located spending significantly less time in the target annulus area, in dramatic contrast to the wild type (WT) as well as the other GAP-43 transgenic mice. It is important to note that G-NonP animals showed a learning curve over the 5 days of training that was similar to the WT and other transgenic mice.
As reported by Aigner, et al., (1995), transgenic mice overexpressing the 3 different forms of chicken GAP-43 all exhibited a pattern of zinc-positive mossy fiber (MF) staining that extended beyond the typical terminal zone of the stratum lucidum (SL) into the distal stratum oriens (dSO) of CA3. However, in a companion report (Rekart et al., 2009), it was found that G-Phos mice did not exhibit the ectopic growth reported by Aigner, et al., (1995). This discrepant finding prompted us to re-examine in the present report the MF system of the G-NonP mouse to determine whether MF growth was present in another GAP-43 transgenic mouse isoform. Such neuroanatomical remodeling resulting from the G-NonP mutation might then shed light on the impaired retention function seen in these animals.
Materials and Methods
Mice
Mice were generated as previously described (Aigner et al., 1995) and breeding was maintained at the Northwestern University animal facility. Transgenic mice (designated G-NonP by us, S42A by Aigner, et al., 1995) overexpressed a nonphosphorylatable form of chicken GAP-43 where alanine was substituted for serine 42 to prevent PKC from phosphorylating the transgenic GAP-43. Nontransgenic mice littermates were designated wild-type (WT) and used as controls. Mice (75 days old) were housed in groups of 3 to 5 per cage with free access to food and water. The temperature (22° C) and lighting (lights on: 0700 to 1900) of the animal housing unit were controlled. Animal care conformed to guidelines of the National Institute of Health Guide for the Care and Use of Animals and guidelines set by the Northwestern University Animal Care and Usage Committee.
Genotyping
Mice were discerned to be transgenic or WT using standard PCR primers that targeted the DNA sequence for chick GAP-43. Genomic DNA was extracted from the tail using Qiagen DNeasy Tissue Kit. DNA extract was mixed with 10 mM Tris·HCl and purity was determined by calculating the A260/A280 ratio. Only genomic DNA with purity levels greater than 1.8 were used for genotyping. Each PCR reaction tube contained 100 ng of genomic DNA, 5 μl PCR buffer, 1 μl dNTP, 1.5 units Taq polymerase (Promega), 25 pmoles of each primer (sequences: 5′ GACACGGGCTCAGAGCAG 3′ and 5′ TTCAGGCATTTTCTTGGTCC 3′) (Invitrogen), and brought up to a volume of 50 μl with autoclaved diH2O. The reaction tubes were placed in a thermalcycler (Techne Burlington, NJ) and underwent the following cycle: denature for 5 min at 94°C, 30 loops of 1 min at 94°C, 1 min at 55°C, 1 min at 72°C and a final extension for 5 min at 72°C. Ten μl of PCR product was mixed with 2 μl of loading dye and run on a 2% agarose gel with ethidium bromide.
Histology
Mice were given an overdose of sodium pentobarbital (Nembutal; 50 mg/kg) followed by transcardiac perfusion with 0.9% saline. Brains were removed and hemisected. The right hemisphere was placed into a 4% paraformaldehyde/ 0.1 M phosphate buffered saline solution and storing it overnight at 4°C. The left hemisphere from each animal was prepared for the Timm’s zinc stain (see Cantallops and Routtenberg, 1996 for procedural details) by placing it into 1% sodium sulphide at room temperature for 20 min then transferring it to a 3% glutaraldehyde/ 4% paraformaldehyde/ 0.1 M phosphate buffered saline solution and storing it overnight at 4°C. The following day the hemispheres were submerged in a 30% sucrose/ 0.1 M phosphate buffer solution.
Immunohistochemistry
Hemispheres were sectioned on a cryostat at 20 μm and collected in 0.1% sodium azide/ 0.1 M phosphate buffer. Sections were washed for 15 minutes in tris-buffered saline (TBS) and blocked in 1% normal goat serum for 1 hour at room temperature. Incubation in primary antibodies (GAP-43 antibody which recognizes transgenic chick and endogenous mouse GAP-43 antigens, 1:1000 Sigma; zinc transporter 1:250 gift from R. Palmiter see Wenzel et al., 1997, Tau1, 1:10,000 gift from L. Binder see Dotti et al., 1987; synaptophysin, 1:500 Abcam) occurred overnight at 4°C. The following day, tissue was washed in TBS followed by a 2-hour incubation in the secondary antibodies (1:500 goat anti-mouse 488 or 1:500 goat anti-rabbit 594; both from Molecular Probes). Tissue was given a final rinse in Tris buffer (pH 7.6), mounted on glass slides and coverslipped using VECTASHIELD Mounting Medium with DAPI (Vector labs).
In situ hybridization
The in situ hybridization procedure followed that as outlined (Wisden and Morris, 2002). Antisense and sense oligonucleotides for in situ hybridization were designed to be complementary to nucleotides 281-325 of the chicken cDNA as reported (Baizer et al., 1990). The sequences were: S:5′GATGCCCCCGCATCCGAGTCTGAGGCCGCCGACAAGAAGGACGAA 3′ & AS: 5′TTCGTCCTTCTTGTCGGCGGCCTCAGACTCGGATGCGGGGGCATC 3′. BLASTn searches confirmed the specificity of probes to transgenic GAP-43 and that probe sequences were not homologous to any known rodent genes. Desalted 50 nucleotide oligonucleotides were synthesized commercially (Invitrogen) and diluted to a concentration of 3 pmol/μL in diethylpyrocarbonate (DEPC)-treated water. Probes were radioactively labeled using terminal deoxynucleotidyl transferase and 35S-ATP. Before hybridization, sections were mounted onto slides and rinsed in 0.1 M TEA/0.25% acetic anhydride for 10 minutes, rinsed in 2×SSC, dehydrated through an ethanol series (70%, 95%, 100%) and allowed to dry. Radiolabeled probes were diluted to a concentration of 200,000 cpm/100 μL in 50% formamide/4×SSC/10% dextran sulfate and 100 μL/slide was applied to the sections and covered with parafilm. Sections were incubated for 16 hours at 42°C in a chamber humidified with 4×SSC and 50% formamide. After hybridization, parafilm coverslips were removed and slides were incubated in 1×SSC at room temperature for 10 minutes and then in 1×SSC at 55°C for 30 minutes. Sections were then rinsed in 0.1×SSC and dehydrated through an ethanol series (50%, 70%, 95%) and allowed to dry. Hybridized slides were apposed to Biomax MR film (Kodak) at room temperature for a minimum of 7 days.
Results
Transgenic in situ hybridization
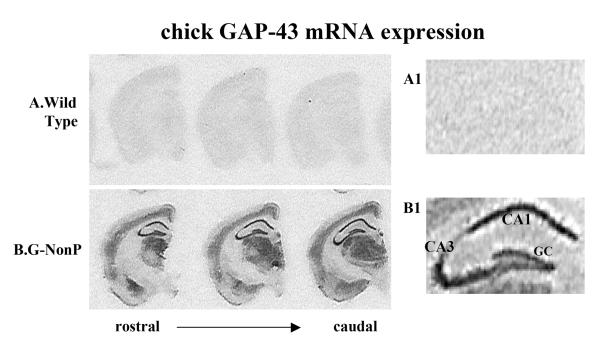
In adult WT mice, endogenous GAP-43 mRNA is essentially undetectable in granule cells of the dorsal hippocampus (Meberg and Routtenberg, 1991). In stark contrast, transgenic chicken GAP-43 mRNA expression in hippocampal granule cells occurred at high levels in the G-NonP adult mice (Fig 1B1). Transgenic GAP-43 mRNA signals in the WT mice (Fig 1A) were, as expected, at background levels indicating that our transgenic GAP-43 probe did not recognize endogenous mouse GAP-43 mRNA.
Figure 1.
Transgenic GAP-43 mRNA localization in A) the WT mice and B) G-NonP transgenic mice. Transgene GAP-43 mRNA signals in the WT mice were, as expected, at background levels. Of particular relevance to the mossy fiber axonal distribution found in the G-NonP mice, GAP-43 transgene mRNA occurred at high levels in the granule cells of the hippocampus (1B1).
Hippocampal MF pathways
Given the 7-day spatial memory retention deficit seen in G-NonP mice (Holahan and Routtenberg, 2008) along with the growth of rat MFs 7 days after spatial water maze training (Holahan et al., 2006; Ram¡rez-Amaya et al., 2001; Rekart et al., 2007) we thought to compare the distribution of the MF pathway in the G-NonP and WT mice. For this purpose we visualized the MFs using the Timm’s stain for heavy metals and the ZnT3 stain for the vesicle-associated zinc transporter protein present in abundance in the MFs (Wenzel et al., 1997; see our Fig 2).
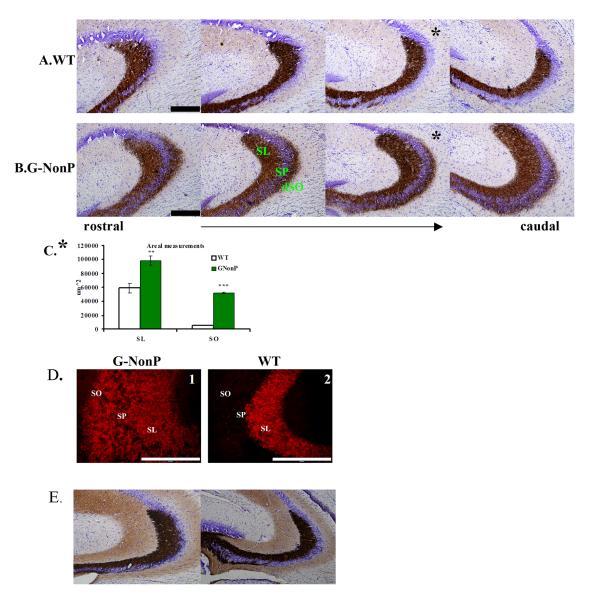
Figure 2.
Timm’s heavy metal staining from the dorsal hippocampus shows that G-NonP mice (B) have a larger mossy fiber-stained area in the stratum lucidum (SL) and distal stratum oriens (dSO) of CA3 than WT (A). This is observed throughout the entire rostral-caudal extent of the dorsal hippocampus. Cresyl violet counter stain shows pyramidal cell layer (SP). C. Quantification of the Timm’s staining in dorsal hippocampus at the mid-caudal level (asterisk) confirms that G-NonP mice have a larger Timm’s-stained area in SL and dSO compared to the WT group. Data are presented as the mean area of staining. Scale bar = 200 μm. ** p < 0.01, *** p < 0.001. D. Immunohistofluorescent labeling for the zinc transporter protein ZnT3 (red). Scale bar = 200 μm. E. Timm’s heavy metal staining from the dorsal hippocampus shows that G-Perm mice have no ectopic MF in dSO and thus a similar distribution of mossy fiber terminal fields as WT.
In the CA3 region, both stains revealed labeling that was highly concentrated in the stratum lucidum (SL), the terminal field of the zincergic MFs. In the WT mouse, the Timm’s (Fig 2A) and ZnT3 (Fig 3A) stains labeled a) the suprapyramidal mossy fiber field (SP-MF) that terminates exclusively in the SL on apical dendrites of CA3 pyramidal cells and b) the infra/intrapyramidal mossy fiber field (IIP-MF) that appears mainly restricted to the most proximal aspects of the CA3 stratum oriens (SO) on the basal dendrites.
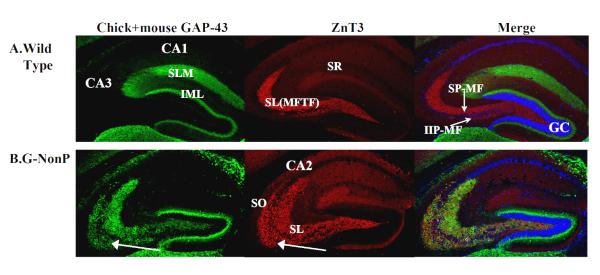
Figure 3.
Immunohistochemical localization of the endogenous (mouse) and transgenic (chicken) GAP-43 protein, the ZnT3 protein and their merged images. A) WT mice. Staining in the WT mouse revealed no traces of GAP-43 staining in the mossy fiber pathways as labeled with the ZnT3 antibody. B) G-NonP mice. There was a striking pattern of elevated GAP-43 staining in the CA3 SL and dSO region of the G-NonP mice which appeared to overlap with the ZnT3 staining. This points to an ectopic distribution of GAP-43 in the G-NonP mice located in the mossy fiber axonal terminals. SLM: stratum lacunosum moleculare, IML: inner molecular layer of the granule cells (GC), SR: stratum radiatum, SP-MF, supra-pyramidal mossy fiber pathway, IIP-MF: infra/intra-pyramidal mossy fiber pathway.
In striking contrast to the distribution of MF pathways in WT mice, the G-NonP group showed Timm’s and ZnT3 staining ectopically distributed in the most distal aspects of SO (dSO; Fig 2B and 2D and Fig 3B). Not only was there a discernible staining in dSO, but the SL showed a larger Timm’s- and ZnT3-stained area in G-NonP than WT mice [compare images in Fig 2A and 2B and area quantification in 2C; two-way ANOVA (group by region): region F(1,1) = 92.25, p < 0.01, group F(1,4) = 98.40, p < 0.01, interaction F(1,4) < 1.0; Fisher’s post-hoc tests, p < 0.01 G-NonP vs WT both SL and SO areas) at all rostral-caudal levels (as shown in Figs 2A and B)].
G-Perm transgenic mice overexpressing permanently pseudophosphorylated GAP-43 did not demonstrate ectopic distribution of MFs in dSO in contrast to G-NonP animals (Figure 2E). This indicates that mutation of GAP-43 itself is not a prerequisite for ectopic MF distribution. Moreover, in a recent confocal study of G-Phos mice, no MF ectopic distribution was observed (Rekart et al., 2009) indicating that overexpression of GAP-43 per se does not drive such aberrant MF distribution.
GAP-43 protein labeling
Because of the high levels of transgenic GAP-43 mRNA expression in the hippocampus, especially the granule cells, we wished to examine the MF distribution of the GAP-43 protein using immunohistochemical localization of the transgenic chick and endogenous mouse GAP-43 antigens. Staining in the WT mouse (Fig 3A) revealed no traces of GAP-43 immunoreactivity in the MF pathways, which were identified by the ZnT3 antibody (Fig 3A). In direct contrast, in the G-NonP mice, there was a striking pattern of elevated GAP-43 staining in both in the CA3 SL and dSO regions (Fig 3B). This GAP-43 positive staining in the SL and dSO in the G-NonP mice appeared to overlap with the ZnT3 staining (Fig 3B).
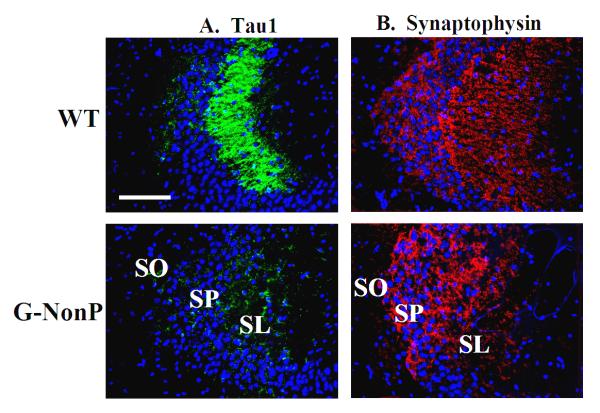
To characterize further the ectopic MF distribution in G-NonP mice, we studied the distribution of both the microtubule-associated protein Tau1, which identifies axons (Dotti et al., 1987), as well as synaptophysin, to locate presynaptic terminal distribution. Unexpectedly there was a dramatic reduction of Tau1 staining in the SL CA3 region in the G-NonP group, a virtual complete absence, which was reduced to background levels as compared to the WT group (Fig 4A). In contrast, levels of synaptophysin staining were comparable between WTs and G-NonP transgenics (Fig 4B).
Figure 4.
Immunohistofluorescent labeling for the axon-specific microtubule associated protein Tau1 (A) and the presynaptic terminal marker, synaptophysin (B) using DAPI (blue) counterstain to indicate nuclei. Images are from the dorsal hippocampus of a G-NonP and WT mouse. Note the complete absence of Tau1 staining in the SL of the G-NonP mouse as compared to the high level of staining in the same region of the WT mouse. Note that the synaptophysin staining in the G-NonP mouse was comparable to that seen in WT. Scale bar = 200 μm.
Discussion
The differential memory effects of targeting the PKC site on GAP-43 (Holahan and Routtenberg, 2008) may have a potential structural correlate in the distribution of mossy fibers (MFs) studied here. As shown previously, overexpression of the nonphosphorylatable GAP-43 protein (G-NonP) has a profound, but selective, impact on the transgenic G-NonP mouse’s ability to remember. This contrasts with transgenic mice that overexpress native GAP-43 or permanently phosphorylated GAP-43. In parallel with these behavioral data, the present results, along with Rekart et al. (2009), indicate that ectopic distribution of MFs into dSO is only observed in GAP-43 mice overexpressing a non-phosphorylatable GAP-43.
These findings are consistent with evidence that posttranslational modification of GAP-43 regulates pathfinding during axonal elongation (for reviews see Caroni, 1997; Caroni, 2001; Dent and Meiri, 1992) as motile growth cones contain lower levels of phosphorylated GAP-43 than stationary growth cones (Dent and Meiri, 1992). Phosphorylated GAP-43 sequesters actin and inhibits the polymerization-depolymerization cycle. This stabilizes actin filaments and thereby, axonal motility (He et al., 1997).
As first noted by Rosenthal, et al.(Rosenthal et al., 1987) GAP-43 mRNA expression is undetectable in adult rat granule cells and thus GAP-43 protein is not detected in the adult mouse MF system. It is, however, present in developing postnatal and post-epileptic rat granule cells (Cantallops and Routtenberg, 1996; Meberg et al., 1993) as well as in mice that overexpress GAP-43 driven by the Thy-1 promoter (Holahan et al., 2007). Therefore, the presence of G-NonP GAP-43 protein in granule cell axons would be capable of facilitating MF outgrowth well beyond the critical developmental time point when MF outgrowth would normally end.
The present findings may be relevant to a growing list of gene targeting studies that show ectopic distribution of hippocampal MFs. Such studies indicate that modification of genes, including GAP-43, that are involved in regulating MF axonal development can alter its targeting. Both knockout (Cremer et al., 1997) and conditional ablation (Bukalo et al., 2004) of NCAM leads to a pattern of MF axonal innervation into dSO similar to that observed in the present report. Given the documented interactions between GAP-43 and membrane-associated lipid ‘rafts’ (Aarts et al., 1999), overexpression of nonphosphorylatable GAP-43 may perturb the ability of adhesion molecules to exert their presynaptic, stabilizing effect (He and Meiri, 2002; Meiri et al., 1998).
The importance of the phosphorylation status of GAP-43 in normal axonal pathfinding is evident in GAP-43 heterozygote knockout mice that were missing their hippocampal commisure and corpus callosum and only only 1% GAP-43 was available for phosphorylation (Shen et al., 2002); heterozygotic mice with approximately 10% GAP-43 available for phosphorylation possessed a hippocampal commisure and corpus callosum that was, however, smaller than normal.
In addition to impaired axonal fasciculation arising from dephospho-GAP-43, the inability to phosphorylate GAP-43 has been shown to impair neuronal polarity in cerebellar granule neurons and alter the point of emergence of axons from the cell body (Mishra et al., 2008). This in effect would lead to axons emerging from inappropriate locations on the cell body and thus be unable to follow the paths set forth by previous axons. These two processes, axonal defasciculation and neuronal polarity disruption, could in combination cooperate in driving the ectopically-located MF terminals.
In the present report, the reduction of Tau1 staining in dSO of CA3 may provide clues as to the mechanism underlying the impaired spatial processing ability seen in our G-NonP mice. Because the dSO field lacks this critical presynaptic microtubule-associated protein, it seems possible that this projection is functionally impaired and thus disrupts MF:CA3 synapse communication.
The combination of ectopic growth of MFs and the presence of mutated GAP-43 in those MF terminals regulating spatial memory, may contribute to the memory deficits we have previously reported. Moreover, the memory deficit might also arise as a consequence of attenuation of expression of Tau1. It is therefore attractive to think, given the elevated levels of GAP-43 seen in AD patients (Rekart et al., 2004), that the memory deficit seen in our Tau1 impoverished G-NonP mice may be of relevance to the memory deficit seen in Alzheimer patients with well-described ‘tauopathies’ (Binder et al., 2005).
Acknowledgements
This work was supported by a Postdoctoral Traineeship to MRH (NIA AG20506), research grant NIH MH65436 to AR and an Individual Discovery Grant from the National Sciences and Engineering Research Council of Canada to MRH. We are grateful to Dr. Pico Caroni of the Friedrich Miescher Institute for providing the transgenic mice. During the preparation of this manuscript, AR was a Distinguished Visiting Professor at the National University of Singapore, Yong Loo Lin School of Medicine.
References
- Aarts LH, Verkade P, van Dalen JJ, van Rozen AJ, Gispen WH, Schrama LH, Schotman P. B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol Cell Neurosci. 1999;14(2):85–97. doi: 10.1006/mcne.1999.0775. [DOI] [PubMed] [Google Scholar]
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Alexander KA, Wakim BT, Doyle GS, Walsh KA, Storm DR. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. Journal of Biological Chemistry. 1988;263(16):7544–7549. [PubMed] [Google Scholar]
- Baizer L, Alkan S, Stocker K, Ciment G. Chicken growth-associated protein (GAP)-43: primary structure and regulated expression of mRNA during embryogenesis. Brain Res Mol Brain Res. 1990;7(1):61–8. doi: 10.1016/0169-328x(90)90074-n. [DOI] [PubMed] [Google Scholar]
- Basi GS, Jacobson RD, Virag I, Schilling J, Skene JH. Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell. 1987;49(6):785–791. doi: 10.1016/0092-8674(87)90616-7. [DOI] [PubMed] [Google Scholar]
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta. 2005;1739(2-3):216–23. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT, Schweizer M, Dityatev A, Schachner M. Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci. 2004;24(7):1565–77. doi: 10.1523/JNEUROSCI.3298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Paratcha G, de Stein M Levi, Bernabeu R, Izquierdo I, Medina JH. B-50/ GAP-43 phosphorylation and PKC activity are increased in rat hippocampal synaptosomal membranes after an inhibitory avoidance training. Neurochemistry Research. 1997;22(4):499–505. doi: 10.1023/a:1027324214060. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Routtenberg A. Rapid induction by kainic acid of both axonal growth and F1/ GAP-43 protein in the adult rat hippocampal granule cells. The Journal of Comparative Neurology. 1996;366:303–319. doi: 10.1002/(SICI)1096-9861(19960304)366:2<303::AID-CNE9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Caroni P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays. 1997;19(9):767–775. doi: 10.1002/bies.950190906. [DOI] [PubMed] [Google Scholar]
- Caroni P. Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. The EMBO Journal. 2001;20(16):4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Murakami K, Routtenberg A. Phosphoprotein F1: purification and characterization of a brain kinase C substrate related to plasticity. The Journal of Neuroscience. 1986;6:3618–3627. doi: 10.1523/JNEUROSCI.06-12-03618.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci. 1997;8(5):323–35. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- Dent EW, Meiri KF. GAP-43 phosphorylation is dynamically regulated in individual growth cones. J Neurobiol. 1992;23(8):1037–53. doi: 10.1002/neu.480230809. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Banker GA, Binder LI. The expression and distribution of the microtubule-associated proteins tau and microtubule-associated protein 2 in hippocampal neurons in the rat in situ and in cell culture. Neuroscience. 1987;23(1):121–30. doi: 10.1016/0306-4522(87)90276-4. [DOI] [PubMed] [Google Scholar]
- Ehrlich YH, Rabjohns RR, Routtenberg A. Experiential input alters the phosphorylation of specific proteins in brain membranes. Pharmacology, Biochemistry, and Behavior. 1977;6(2):169–174. doi: 10.1016/0091-3057(77)90068-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich YH, Routtenberg A. Cyclic AMP regulates phosphorylation of three protein components of rat cerebral cortex membranes for thirty minutes. FEBS Letters. 1974;45(1):237–243. doi: 10.1016/0014-5793(74)80852-5. [DOI] [PubMed] [Google Scholar]
- Gianotti C, Nunzi MG, Gispen WH, Corradetti R. Phosphorylation of the presynaptic protein B-50 (GAP-43) is increased during electrically induced long-term potentiation. Neuron. 1992;8:843–848. doi: 10.1016/0896-6273(92)90198-m. [DOI] [PubMed] [Google Scholar]
- He Q, Dent EW, Meiri KF. Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. The Journal of Neuroscience. 1997;17(10):3515–3524. doi: 10.1523/JNEUROSCI.17-10-03515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Meiri KF. Isolation and characterization of detergent-resistant microdomains responsive to NCAM-mediated signaling from growth cones. Mol Cell Neurosci. 2002;19(1):18–31. doi: 10.1006/mcne.2001.1060. [DOI] [PubMed] [Google Scholar]
- Holahan M, Routtenberg A. The protein kinase C phosphorylation site on GAP-43 differentially regulates information storage. Hippocampus. 2008;18(11):1099–102. doi: 10.1002/hipo.20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Honegger KS, Tabatadze N, Routtenberg A. GAP-43 gene expression regulates information storage. Learn Mem. 2007;14(6):407–15. doi: 10.1101/lm.581907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Rekart JL, Sandoval J, Routtenberg A. Spatial learning induces presynaptic structural remodeling in the hippocampal mossy fiber system of two rat strains. Hippocampus. 2006;16(6):560–70. doi: 10.1002/hipo.20185. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Vianna M, Barros DM, Mello e Souza T, Ardenghi P, Sant’Anna, Rodrigues C, Medina JH, Izquierdo I. Short- and long-term memory are differentially affected by metabolic inhibitors given into hippocampus and entorhinal cortex. Neurobiology of Learning and Memory. 2000;73(2):141–149. doi: 10.1006/nlme.1999.3925. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Akers RF, Nelson RB, Barnes CA, McNaughton BL, Routtenberg A. A selective increase in phosporylation of protein F1, a protein kinase C substrate, directly related to three day growth of long term synaptic enhancement. Brain Res. 1985;343(1):137–43. doi: 10.1016/0006-8993(85)91167-9. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Routtenberg A. Synapse-specific protein kinase C activation enhances maintenance of long-term potentiation in rat hippocampus. Journal of Physiology (London) 1988;400:321–333. doi: 10.1113/jphysiol.1988.sp017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci U S A. 1999;96(16):9397–402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Gall CM, Routtenberg A. Induction of protein F1 gene expression in hippocampal granule cells after seizures. Molecular Brain Research. 1993;17:295–299. doi: 10.1016/0169-328x(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Routtenberg A. Selective expression of protein F1/(GAP-43) mRNA in pyramidal but not granule cells of the hippocampus. Neuroscience. 1991;45(3):721–733. doi: 10.1016/0306-4522(91)90284-u. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Saffell JL, Walsh FS, Doherty P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J Neurosci. 1998;18(24):10429–37. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Gupta SK, Meiri KF, Fong M, Thostrup P, Juncker D, Mani S. GAP-43 is key to mitotic spindle control and centrosome-based polarization in neurons. Cell Cycle. 2008;7(3):348–57. doi: 10.4161/cc.7.3.5235. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Furman M, Bevilaqua L, Cammarota M, Vianna M, de Stein ML, Izquierdo I, Medina JH. Involvement of hippocampal PKCá1 isoform in the early phase of memory formation of an inhibitory avoidance learning. Brain Research. 2000;855(2):199–205. doi: 10.1016/s0006-8993(99)02323-9. [DOI] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Balderas I, Sandoval J, Escobar ML, Bermudez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. The Journal of Neuroscience. 2001;21(18):7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekart JL, Meiri K, Routtenberg A. Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus. 2005;15(1):1–7. doi: 10.1002/hipo.20045. [DOI] [PubMed] [Google Scholar]
- Rekart JL, Quinn B, Mesulam MM, Routtenberg A. Increased brain growth protein in a hippocampal subfield of Alzheimer’s patients. Neuroscience. 2004;126(3):579–84. doi: 10.1016/j.neuroscience.2004.03.060. [DOI] [PubMed] [Google Scholar]
- Rekart JL, Routtenberg A. Two Populations of Hippocampal Mossy Fibers in the Transgenic GAP-43 Mouse. Submitted.
- Rekart JL, Sandoval CJ, Bermudez-Rattoni F, Routtenberg A. Remodeling of hippocampal mossy fibers is selectively induced seven days after the acquisition of a spatial but not a cued reference memory task. Learn Mem. 2007;14(6):416–21. doi: 10.1101/lm.516507. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Chan SY, Henzel W, Haskell C, Kuang W-J, Chen E, Wilcox JN, Ullrich A, Goeddel DV, Routtenberg A. Primary structure and mRNA localization of protein F1, a growth-related protein kinase C substrate associated with synaptic plasticity. The EMBO Journal. 1987;6(12):3641–3646. doi: 10.1002/j.1460-2075.1987.tb02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A. Reverse piedpiperase: is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19(11):471–2. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22(1):239–47. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu FS, Marais RM, Parker PJ, Bazan NG, Routtenberg A. Neuron-specific protein F1/GAP-43 shows substrate specificity for the beta subtype of protein kinase C. Biochem Biophys Res Commun. 1990;171(3):1236–43. doi: 10.1016/0006-291x(90)90818-8. [DOI] [PubMed] [Google Scholar]
- Skene JH, Virag I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. Journal of Cell Biology. 1989;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80(3):445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. The Journal of Neuroscience. 2000;2016(16):5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94(23):12676–81. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with oligonucleotide probes. Int Rev Neurobiol. 2002;47:3–59. doi: 10.1016/s0074-7742(02)47051-1. [DOI] [PubMed] [Google Scholar]
- Young E, Cesna T, Meiri KF, Perrone-Bizzozero N. Changes in protein kinase C (PKC) activity, isoenzyme translocation, and GAP-43 phosphorylation in the rat hippocampal formation after a single-trial contextual fear conditioning paradigm. Hippocampus. 2002;12:457–464. doi: 10.1002/hipo.10015. [DOI] [PubMed] [Google Scholar]
- Young EA, Owen EA, Meiri KF, Wehner JM. Alterations in hippocampal GAP-43 phosphorylation and protein level following contextual fear conditioning. Brain Research. 2000;860:95–103. doi: 10.1016/s0006-8993(00)02021-7. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Strittmatter SM, Fishman MC. A membrane-targeting signal in the amino acid terminus of the neuronal protein GAP-43. Nature. 1989;341(6240):345–348. doi: 10.1038/341345a0. [DOI] [PubMed] [Google Scholar]