Abstract
Calpain 10 has been localized to the mitochondria and is a key mediator of Ca2+ induced mitochondrial dysfunction. A peptide screen followed by a series of modifications identified the homodisulfide form of CYGAK (CYGAK)2 as an inhibitor of calpain 10, while showing no inhibitory activity against calpain 1. Methylation or truncation of the N-terminal cysteine significantly reduced the inhibitory activity of (CYGAK)2 and inhibition was reversed by reducing agents, suggesting that CYGAK forms a disulfide with a cysteine near the active site. Data suggests CYGAK may be a P’ calpain inhibitor and may achieve its specificity through this mechanism. CYGAK inhibited calpain activity in intact mitochondria, renal cells, and hepatocytes, prevented Ca2+ induced cleavage of NDUFV2, and blocked Ca2+ induced state III dysfunction. (CYGAK)2 is the first P’ specific calpain inhibitor and will be a valuable tool to prevent Ca2+ induced mitochondrial dysfunction and explore the function of calpain 10.
Keywords: Calpain 10, mitochondria, inhibitor, mitochondrial dysfunction, kidney
Introduction
Calpains are a family of cysteine proteases first described and purified by Dayton et al in 1976 1, 2. The calpains are conserved among eukaryotes with various calpain genes expressed in fungi, C. elegans, Drosophila, and mammals 3. Within the vertebrate family, there are 14 large subunit calpain isoforms and a 28 kDa small calpain subunit 3, some of which are ubiquitously expressed 4 and others display a more tissue-specific distribution 5-7. The seemingly ubiquitous calpain family members consist of calpains 1, 2, 4, 5, 7, 9, and 10 3, 8-12. The calpain family also can be divided into what are termed typical and atypical calpains 3. The typical calpains contain a penta-EF hand Ca2+ binding motif in domain IV and are comprised of calpains 1, 2, 3, 8, 9, 11, 12, 13, and 14. The atypical calpains lack a penta-EF hand domain and consist of calpains 5, 6, 7, 10, and 15 (SOLH) 13-15.
Calpains have a number of physiological roles, including cytoskeleton remodeling, cell migration, embryonic development, and platelet function 16-19. Calpains also play pathological roles in acute organ failure (e.g. liver, kidney, spinal cord injury, and stroke) and Alzhemeimer’s disease as a mediator of apoptotic and necrotic cell death 20.
Calpain 10 has come to the forefront of calpain research because it has been identified as a diabetes mellitus type-2 susceptibility gene 21-26. It may play a role in insulin secretion and trafficking as part of its regulation of cytoskeletal changes 22, 27. More recently, we identified calpain 10 as the predominant mitochondrial calpain 28. Over-expression of calpain caused mitochondrial dysfunction while calpain 10 depletion caused epithelial cell death (28, unpublished observations). Furthermore, mitochondrial calpain 10 mediates Ca2+-induced electron transport chain dysfunction through the cleavage of NDUFV2 and ND6 and inhibition of complex 1 activity 28, 29. Development of effective and specific inhibitors of calpain isoforms may therefore be useful as therapeutics for a number of calpainopathies.
Calpain inhibitors found in nature were discovered and isolated from the Streptomyces species. Examples of this generation of inhibitors include leupeptin 30 and antipain 31. However, these peptide aldehydes lack cell permeability and/or selectivity for calpains over other protease systems. Subsequent investigation of these peptidyl aldehydes resulted in improved inhibitor potency through structure-activity relationship analysis. Cell permeability and stability were enhanced by the addition of hydrophobic N-terminal capping groups 31. The inhibitor derivatives generated by these efforts include calpeptin 32, MDL 28170 (1) 33, and calpain inhibitors I and II (Ac-Leu-Leu-Nle-H and Ac-Leu-Leu-Met-H) 34.
Most investigators have endeavored to design peptide mimetics containing chemical ‘warheads’ that replace the scissile amide bond with an electron deficient center that can either bind reversibly or irreversibly with the thiolate moiety of the active site cysteine 31. Electrophilic carbonyl-containing functional groups that have been used as chemical ‘warheads’ include α-diketones 35, α-keto phosphorous compounds 36, aldehydes 32-34, 37-39, and α-keto carbonyl compounds such as α-ketoacids, α-ketoamides, and α-ketoesters 35, 40-42. It was later discovered that irreversible inhibitors could be produced by replacing the scissile bond of calpain substrates with methylsulfonium salts 43-45, disulfide linkages 46, epoxysuccinates and their derivatives, vinyl sulfones 47, and ketomethylenes 45, 48-52. Of note, a number of ‘non-warhead’ peptide biphenyl hybrid compounds have been synthesized, the most potent of which exhibits an IC50 of 87 pM 53, 54
A good example of nonpeptide calpain inhibitors is the α-mercaptoacrylates 31 described by Wang et al in 1996 55. The most potent α-mercaptoacrylates, PD150606 (2) and PD151746 (3), were not only cell permeable, but were 600-fold more selective for calpain over cathepsin B 55. This selectivity is thought to be derived from the novel binding site of the α-mercaptoacrylates as compared to active-site calpain inhibitors.
Ultimately, all of the calpain inhibitors identified to date suffer from lack of potency, specificity between calpains and other cysteine proteases, specificity among calpain family members and/or lack of cell permeability. The lack of selectivity among calpain isoforms has made it difficult to determine which specific isoforms, such as mitochondrial calpain 10, are responsible for various physiological and pathological responses. Thus, we have endeavored to develop an inhibitor that is both potent and selective for calpain 10. Peptide screening identified a mitochondrial calpain 10 inhibitor, which was then characterized and refined and examined for its ability to inhibit Ca2+-induced mitochondrial dysfunction in renal cortical mitochondria.
Materials and Methods
Materials
Unless stated otherwise, all chemical reagents were obtained from Sigma-Aldrich, St. Louis, MO. Female New Zealand white rabbits (1.5-2.0 kg) were purchased from Myrtle’s Rabbitry, Thompson Station, TN. Percoll® was obtained from Amersham Biosciences, Piscataway, NJ. The calpain substrate, Suc-Leu-Leu-Val-Tyr-AMC (sLLVY-AMC), was purchased from Bachem, King of Prussia, PA. Calpeptin and purified calpain 2 from porcine kidney were obtained from EMD Biosciences, La Jolla, CA. All peptides were purchased as custom syntheses from Anaspec, San Jose, CA or synthesized in our laboratory
Mitochondrial Isolation, Purification and Subfractionation
Kidney mitochondria were isolated as previously described28 and suspended in 130 mM KCl, 9 mM Tris-PO4, 4 mM Tris-HCl, 1 mM EGTA, (pH 7.4) with the addition of 5 mM pyruvate and 5 mM malate (Buffer A). Crude rabbit renal cortical mitochondria were layered onto a Percoll®/sucrose gradient and centrifuged at 20,000 g for 20 minutes. Following purification, mitochondria were sub-fractionated as described previously28. Purified matrix fraction was stored at −80°C until use.
Matrix Calpain 10 and Calpain 1 Activities
Calpain activity was determined by spectrofluorometrically using the calpain substrate sLLVY-AMC (50μM.) as previously described28. Mitochondrial matrix or respiring mitochondria were incubated with various concentrations of inhibitors and calpain 10 activity was measured under linear conditions as a function of AMC hydrolysis. Calpain 1 activity was determined as described above using purified calpain 1. In experiments reflecting calpain activity in the presence or absence of Ca2+, either EGTA (1mM) or Ca2+ (1μM) was added to activity buffer prior to the addition of mitochondrial matrix. Matrix was then added to buffer followed by the addition of inhibitor and substrate.
Measurement of Cellular Calpain
Calpain activity was measured spectrofluorometrically using the calpain substrate, SLLVY-AMC, as previously described 28, 56. Cellular suspensions (both NRK-52E cells and hepatocytes) were diluted in an isotonic calpain activity buffer (150 mM KCL, 20 mM HEPES, pH 7.4) and assayed for sLLVY-AMC (100 μM) cleavage in a 96-well plate (5×104 cells/well) for 20 minutes. In all cases, activity was measured under linear conditions as a function of sLLVY-AMC hydrolysis using excitation and emission wavelengths of 355 nm and 444 nm respectively.
Mitochondrial Respiration
Oxygen consumption was monitored using a YSI Model 5300 Biological Oxygen Monitor (YSI Incorporated, Yellow Springs, OH). RCM were suspended at approximately 1.3 mg protein/ml in Buffer A. The respiration chamber was maintained at 37° C and stirred magnetically. After the basal rate (state 4) of oxygen consumption was measured, ADP was added to a final concentration of 1 mM to measure state 3 respiration. For mitochondrial protection experiments, respiration measurements were performed following 30 minutes of incubation with calpain inhibitors and a five min exposure to 1 μM Ca2+ or diluent.
Immunoblotting
Mitochondria were boiled for 10 minutes and then subjected to SDS/PAGE and transferred to nitrocellulose membranes. Membranes were incubated with a primary antibody to NDUFV2 (A generous gift from Dr. Yamaguchi, The Scripps Research Institute, La Jolla, CA). Antibody incubation was followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Santa Cruz, 1:1000 dilution). Immunoreactive protein was visualized by enhanced chemiluminescence (Pierce) and imaged using an Alpha Innotech imaging station.
Peptide Synthesis
Peptides were synthesized manually using FMOC amino acids and HCTU coupling. Rink amide MBHA resins (0.1 mmol, 139 mg) were swollen in NMP for two hours and then treated with 20% (v/v) piperidine in NMP (2 min × 5) and washed with NMP (1 min, 5 times). Amino acids were coupled following activation with HCTU (0.5 mmol, 206 mg) and DIEA (0.2 mL). Kaiser tests were performed to verify the completion of coupling to resins and subsequent amino acids respectively. FMOC protecting groups were removed with 20% (v/v) piperidine followed by washing with NMP. The peptides were cleaved with a TFA cocktail (TFA (80%, v/v), H2O (5%, v/v), phenol (5%, w/v), triisopropyl silane (5%, v/v), anisole (5%, v/v)), for 2 h and were then precipitated into cold methyl tert-butyl ether. The suspension was kept at −81 °C for 30 min and centrifuged (~ 1000 g, 10 min). After decantation, the residue was dried and purified by reversed phase HPLC using a Waters C18 radial compression column with a H2O/CH3CN in 0.1% TFA gradient. The MW of the peptides were confirmed by MALDI mass spectrometry.
Renal Cells and Rat Hepatocytes
Normal rat kidney (NRK-52E) epithelial cells were cultured as previously described 57 in DMEM (high glucose) supplemented with 10% fetal calf serum. Cells were dislodged by gently scraping and pipetting to produce a homogenous suspension of cells. Following preparation of cell suspensions, cell viability was determined via trypan blue exclusion and only preparations with greater than 90% viability were used for analysis. Suspensions of rat hepatocytes were prepared from the liver of male Sprague-Dawley rats (200-250 g) by collagenase perfusion as previously described 58. Only hepatocyte preparations yielding greater than 90% viability, as measured by the trypan blue exclusion assay, were used for experiments. The cells were suspended in Waymouth’s MB-752/1 medium containing 10% fetal calf serum, 100 nM insulin, and 100 nM dexamethasone.
Statistics
NRK-52E cells from one passage, hepatocytes from one rat and RCM isolated from one rabbit represent one experiment (n=1). All data are expressed as means ± SE.
Results
Because Calpain 10 is a mitochondrial calpain and is responsible for mitochondrial calpain-like activity in RCM 28, mitochondrial matrix was utilized as the source of calpain 10.
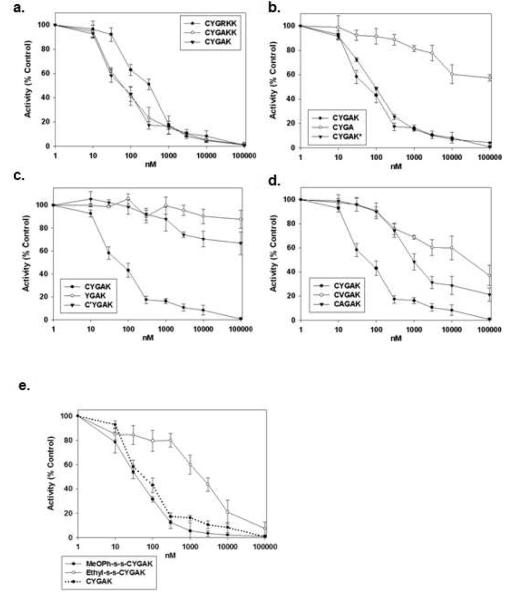
An initial peptide screen identified the 6-mer CYGRKK as a potential calpain 10 inhibitor. To determine the amino acids that are critical for the calpain 10 inhibitory activity of CYGRKK, several modification schemes were utilized. To evaluate the role of the positively charged arginine, it was replaced with the inert amino acid alanine and incubated with mitochondrial matrix in the presence of the calpain substrate SLLVY-AMC. The potency of CYGAKK was greater than that of CYGRKK (IC50 of 90 nM versus 290 nM) (Fig 2a, table 1). Truncation of the C-terminal lysine, (i.e. CYGAK), had no significant effect when compared to CYGAKK (Fig. 1a, table 1); however, further truncation of the lysine (i.e. CYGA) resulted in complete loss of inhibitory activity (Fig. 1b, table 1). To determine if the positive charge on the lysine of CYGAK is required for inhibitory activity, the lysine was acetylated (CYGAK*). Surprisingly, the observed efficacy and potency of CYGAK* was no different than that of CYGAK, suggesting that the structural characteristics of the lysine side chain are necessary and not the positive charge. To evaluate the role of the tyrosine in CYGAK, tyrosine was substituted with either valine (CVGAK) or alanine (CAGAK). Substitution with either valine or alanine significantly reduced the inhibitory activity of CYGAK, suggesting that tyrosine is critical for calpain inhibition (Fig. 1d, table 1).
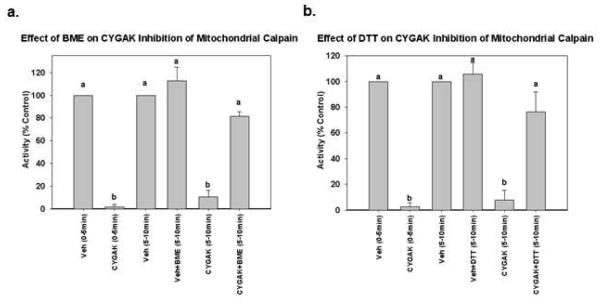
Figure 2. Reducing agents reverse CYGAK-induced inhibition of mitochondrial calpain 10 activity.
Matrix calpain 10 activity was measured for 5 min in the presence and absence of CYGAK for 5 min. After 5 min the reducing agent BME (a) or DTT (b) was added and calpain activity was monitored for an additional 5 min. Data represent the means and SE (n ≥ 3). Different subscripts are statistically different from each other; p<0.05.
Table 1. Potency of inhibitors of mitochondrial calpain 10.
The IC50 values of peptides that inhibit calpain 10 activity in mitochondrial matrix fractions using sLLVY-AMC. Data represent the means and SE’s of at least 3 separate experiments. Different subscripts represent IC50’s that are statistically different from each other; p<0.05.
| Mean IC50 (nmol) |
|
|---|---|
| CYGRKK | 290 ± 20 a |
| CYGRK | 240 ± 70 a |
| CYGAK | 100 ± 10 b |
| CYGA | > 100μM |
| YGAK | > 100μM |
| CYGAK | > 100μM |
| CVGAK | >40μM |
| CAGAK | 2000 ± 140 c |
| MeOPh-s-s-CYGAK | 60 ± 10 d |
| Ethyl-s-s-CYGAK | 2000 ± 130 c |
| CYGAK* | 90 ± 10 b |
| Calpeptin | 4 ± 1 e |
Figure 1. Optimization of the mitochondrial calpain 10 inhibitor.
The effect of increasing concentrations of peptides on mitochondrial matrix calpain 10 activity using sLLVY-AMC as a substrate. Data represent the means and SE (n ≥ 3).
We hypothesized that the N-terminal cysteine of the CYGAK was forming a sulfhydryl bond with the cysteine in or near the active site of mitochondrial calpain 10. The cysteine was removed (YGAK) or methylated (C’YGAK) and compared to CYGAK. Truncation of CYGAK to YGAK or methylation of the CYGAK cysteine (C’YGAK) completely abolished inhibitory activity, suggesting that the free sulfhydryl of cysteine in CYGAK is necessary for calpain inhibition (Fig. 1c, table 1).
Because the N-terminal cysteine is necessary for the inhibitory activity of CYGAK (Fig.1c), we determined if the activity of CYGAK was due to the formation of a mixed disulfide with cyteine in or near the active site of calpain 10. To accomplish this, mitochondrial matrix was pre-incubated for 15 min with CYGAK (1 μM) and calpain activity was then measured for 5 min. As expected, CYGAK pretreatment inhibited greater than 90% of calpain activity. The reducing agent β-mercaptoethanol (BME) (1 mM) or ditiothreitol (DTT) (1 mM) was added to matrix containing CYGAK for 5 min and calpain activity was again monitored for an additional 5 min. BME or DTT completely reversed the inhibitory activity of CYGAK (Fig. 2).
It was observed that CYGAK in aqueous solution readily forms a disulfide homodimer (CYGAK)2. To determine whether mixed disulfides could be effective calpain 10 inhibitors, disulfides of CYGAK and methoxythiophenol (MeOPhs-sCYGAK) or ethanethiol (Ets-sCYGAK) were prepared. The MeOPhs-sCYGAK mixed disulfide was more potent than CYGAK2 (IC50 of 56 nM versus 96 nM) whereas Ets-sCYGAK was significantly less potent (IC50 of 2000 nM) (Fig. 1e, table 1), which reflects the differences in the thiol pKa values (ethanethiol = 10.61, methoxythiophenol = 6.8).
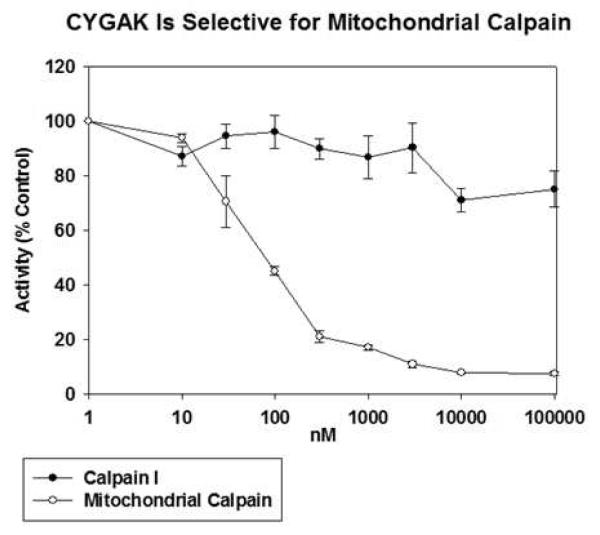
Because most pharmacological inhibitors of calpain have historically shown minimal selectivity among calpain isoforms 41, 42, CYGAK2 was tested against mitochondrial calpain 10 and purified calpain I. Although CYGAK2 inhibited calpain 10, it had no inhibitory effect on calpain 1 at concentrations as high as 100 μM (Fig.3), identifying CYGAK2 as a selective inhibitor of calpain 10.
Figure 3. CYGAK is selective for mitochondrial calpain 10.
Increasing concentrations of CYGAK were added to matrix calpain 10 or purified calpain 1 and sLLVY-AMC determined hydrolysis determined after 30 min. Data represent the means and SE (n ≥ 3).
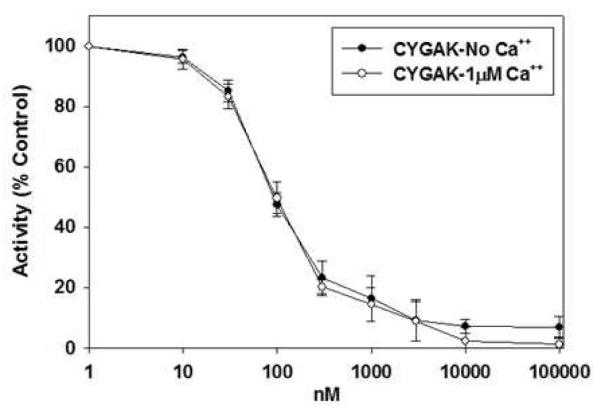
Because Ca2+ increases the activity of most calpains, the inhibitory activity of CYGAK2 was evaluated in the presence and absence of 6 mM Ca2+. No changes in the inhibitory potency or efficacy of CYGAK2 were observed in the presence or absence of Ca2+ (Fig. 4), suggesting that the binding and activity of CYGAK2 to calpain 10 is Ca2+ independent.
Figure 4. Inhibitory activity of CYGAK to calpain 10 is independent of Ca2+.
Inhibitory activity of CYGAK dimer was measured in the presence of EGTA or in the presence of 1μM Ca++. Data represent the means and SE (n ≥ 3).
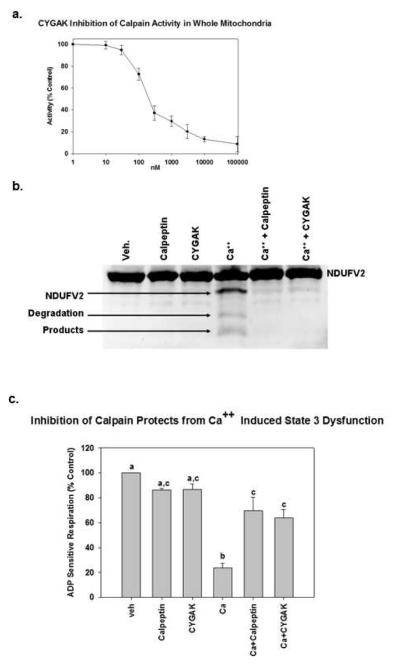
To determine if CYGAK2 is effective in whole mitochondria, isolated mitochondria were incubated with CYGAK2 and calpain 10 activity was measured over time. CYGAK2 was effective at inhibiting calpain 10 activity in whole mitochondrial with an IC50 of 200 nM (Fig. 5a), demonstrating that CYGAK2 crossed mitochondrial membranes with an approximate two-fold loss of potency (100 vs. 200 nM).
Figure 5. CYGAK inhibition of calpain activity in intact mitochondria prevents Ca++ induced cleavage of complex I proteins and state 3 dysfunction.
Isolated RRC mitochondria were pretreated for 15 min with DMSO or the calpain inhibitors calpeptin (10μM) or CYGAK (10μM) and exposed to Ca2+ for 5 min. Mitochondrial damage was then assessed by measuring cleavage of the complex I subunit NDUFV2 by immunoblot (b) or by measuring state 3 respiration (c). Data represent the means and SE (N ≥ 3). Different subscripts are statistically different from each other; p<0.05.
We have shown previously that mitochondrial matrix calpain 10 was responsible for Ca2+-induced cleavage of complex 1 proteins ND6 and NDUFV2, and inhibition of state 3 respiration 28. Because CYGAK2 was effective at inhibiting calpain activity in whole mitochondria, we sought to determine if CYGAK2 was capable of blocking Ca2+ induced mitochondrial dysfunction. Isolated mitochondria were preincubated with calpeptin (10 μM) or CYGAK2 (10 μM) and then treated with Ca2+ (1 μM) for 5 min. Pretreatment with both calpeptin and CYGAK2 blocked the Ca2+-induced cleavage of the complex I subunit NDUFV2 (Fig. 5b), and it also blocked Ca2+ induced attenuation of state 3 respiration (Fig. 5c).
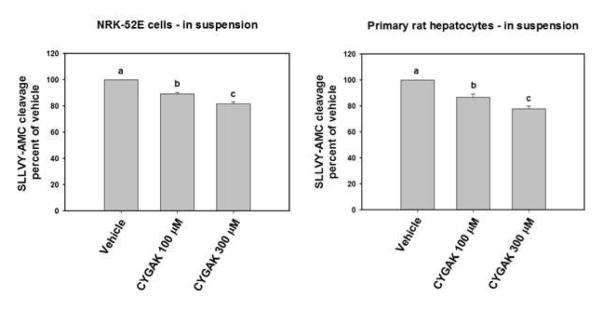
To evaluate the effectiveness of the calpain 10 specific inhibitor CYGAK2 in cellular systems, SLLVY-AMC cleavage was monitored in suspensions of NRK-52E cells and hepatocytes. The calpain 10 inhibitor, CYGAK2, inhibited cleavage of the substrate at 100 μM and 300 μM in both NRK-52E cells (10 and 18%) and hepatocytes (14 and 22%) (figure 6).
Figure 6. CYGAK inhibits calpain 10 activity in NRK52-E cells and isolated rat hepatocytes.
NRK52-E cells and rat hepatocyte suspensions were incubated with SLLVY-AMC (100 μM) and either vehicle control or CYGAK at indicated concentrations for 20 min. Activity was measured under linear conditions and presented as percent of vehicle. Data represent the means and SE (n ≥ 3). Different lettered bars indicate statistically different means (p<0.05.
Discussion
To elucidate the pharmacophore in the initial inhibitor of mitochondrial calpain 10, CYGRKK, a series of substitutions were performed. Substitution of an alanine for arginine of CYGRKK significantly improved the potency of the inhibitor, demonstrating the arginine was non-essential for recognition by calpain 10. Additional experiments revealed that 1) the C-terminal lysine of CYGAKK was not needed to inhibit calpain 10, 2) the C-terminal lysine of CYGAK was needed to inhibit calpain 10 but the ε-amino group was not, 3) that the loss of tyrosine markedly decreased the inhibitory potency of CYGAK, and 4) that the loss of cysteine from CYGAK resulted in no inhibitory activity. Finally, methylation of the sulfhydryl group on cysteine in CYGAK completely blocked inhibition of calpain 10 by CYGAK. Thus, CYGAK2 is an optimized inhibitor of mitochondrial calpain 10 with an IC50 of approximately 100 nM. To confirm the selectivity of CYGAK2, CYGAK2 did not inhibit the ubiquitously expressed calpain 1 at concentrations up to 100 μM, 1,000 times greater that the IC50 for calpain 10. To confirm that CYGAK2 could cross mitochondrial membranes, it was shown that CYGAK2 inhibits calpain 10 activity in charged mitochondria with an IC50 of approximately 200 nM. In summary, CYGAK2 is a potent and selective inhibitor of mitochondrial calpain 10 and is capable of crossing mitochondrial membranes. Because most of the calpain family members have not been characterized biochemically, we can not confirm that CYGAK2, only inhibits calpain 10.
As stated above, removal of the cysteine from CYGAK or methylation of the sulfhydryl group, completely blocked the calpain 10 inhibitory activity of CYGAK, suggesting that the sulfhydryl group in the cysteine of CYGAK was necessary to inhibit calpain 10. Calpain 10 activity was restored by the reducing agents dithiothhreitol and β-mercaptoethanol, suggesting that the calpain inhibitory activity of CYGAK is through the formation of a mixed disulfide with a cysteine in or near the active site. While this is the first report using the reactive sulfhydryl of cysteine as a warhead for a calpain inhibitor, it is not surprising that a peptide that effects the sulfhydryl group in or near the active site of calpain 10 would be an effective inhibitor as sulfydryl modifying agents have been shown in the past to reduce the activity of calpains 62-64. Interestingly, the addition of a reducing agent to calpain 10, does not significantly effect the activity of the enzyme. In contrast, the addition of similar concentrations of a reducing agent to calpain I resulted in an 11 fold increase in cleavage of sLLVY-AMC (data not shown), suggesting that the activity of these enzymes is differentially regulated by the oxidation state of the cysteines.
During our studies we determined that CYGAK readily forms a disulfide in solution. Thus, in all the above experiments, the calpain 10 inhibitors, except those lacking cysteine or a free-sulfhydryl group, were presumably disulfides. The thiol exchange reaction between a critical thiolate and peptide disulfide would be considerably faster than oxygen mediated oxidation of peptide thiol bound to the calpain. To test this mechanism, two mixed disulfides, MeOPhs-sCYGAK and Ets-SCYGAK, were synthesized. Both mixed disulfides showed significant inhibitory activity, which demonstrates that thiol exchange can occur. These observations also demonstrate that a single CYGAK monomer is sufficient for recognition. Indeed, the MeOPhs-sCYGAK peptide was nearly twice as potent as CYGAK dimer. It is notable that the MeOPhs-sCYGAK peptide is about 100-fold more potent than the Ets-sCYGAK peptide. Based on the differences in pKa, the p-methoxythiophenol should be a susbstantially better leaving group than ethanethiol and CYGAK. We conclude that the mechanism for inhibition involves attack of a calpain thiolate on the cysteine sulfur and subsequent loss of the second thiol. The observation that the mixed disulfides did not inhibit calpain 1 suggests that only when bound to calpain 10 does the reactive thiolate have the appropriate distance and geometry to affect a nucleophilic substitution on a critical disulfide, which could be highly beneficial for specificity.
The peptide sLLVY-AMC and other calpain inhibitors based on the xLLxx motif such as ALLN, ALLM, and calpeptin are all recognized by calpains on the P-side of the scissile bond as demonstrated by the C-terminal liberation of AMC from sLLVY-AMC 59. A P-side inhibitor, calpeptin (Z-Leu-Nle-CHO), is capable of inhibiting typical calpains with low nanomolar IC50s (~40 nM, calpain I, ~60 nM calpain 2) (data not shown). In our previous studies we demonstrated that calpeptin inhibited mitochondrial calpain 10 with an IC50 of 4 nM 28 when using sLLVY-AMC as a substrate. These results support the idea that the P-side of calpains 1, 2, and 10 have similar structural features. Tompa et al. reported that calpain substrates have preferred amino acids in the P2, P1, P1′ and P2′ positions. For example, lysine, tyrosine and arginine are preferred in P1 and leucine, threonine, and valine at P2. In contrast, serine, threonine, and alanine are preferred in P1’ and proline is preferred in P2′ 60. Currier et al., designed a calpain 1 substrate with an optimal cleavage motif, P3 – PLFAER – P3′ 61. Based on these studies, we would predict that tyrosine of CYGAK would be recognized at P1 and that glycine, alanine and lysine would occupy P1′, P2′ and P3′, respectively. Because MeOPhs-sCYGAK also potently inhibits calpain 10 acitivty, it is likely that the thiol exchange reaction is occurring with the CYGAK moiety situated primarily on the P’ side of the active site in the canonical peptide orientation that allows for N-terminal thiol exchange to occur. A P’ inhibitor is novel in that most peptide inhibitors of proteases achieve their specificity by binding to the P sites. In the proposed alignment, the cysteine would align with the P2 site and would inhibit calpain 10 through the interaction with a cysteine in or near the active site of calpain 10. Targeting P’ interactions may therefore be an attractive target for achieving isoform specificity among other calpain inhibitors.
Arrington et al., identified mitochondrial calpain 10 as an important mediator of Ca2+-induced mitochondrial dysfunction 28. Inhibition of mitochondrial calpain 10 with calpeptin blocked the Ca2+-induced cleavage of complex I subunits and Ca2+-induced state III dysfunction 28. Similar to what is seen with calpeptin, pretreatment of mitochondria with CYGAK2 also protected mitochondria from Ca2+-induced state 3 dysfunction and prevented the Ca2+-induced cleavage of the complex I NDUFV2 subunit. An inhibitor such as CYGAK2 that blocks Ca2+-induced mitochondrial dysfunction in the setting of ischemia-reperfusion, drug or toxicant injury may have therapeutic potential. Furthermore, an isoform specific inhibitor of calpain 10 will limit unwanted toxicities secondary to the inhibition of typical calpains.
Additionally, we have shown that the novel calpain 10 specific inhibitor is effective at inhibiting the cleavage of the calpain substrate (sLLVY-AMC) in two cellular models. However, it should be noted that the concentrations required are 100-fold higher than that needed for isolated mitochondria, suggesting that this inhibitor does not readily cross the plasma membrane. Future studies will be directed towards increasing the cell permeability of CYGAK2.
Supplementary Material
Non-Standard Abbreviations
- (RCM)
Renal Cortical Mitochondria
- (MeOPhs-sCYGAK)
disulfide of CYGAK-methoxythiophenol
- (Ets-sCYGAK)
disulfide of CYGAK-ethanethiol
- (sLLVY-AMC)
Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin
- (DTT)
Dithiothreitol
- (BME)
β-Mercaptoethanol
Footnotes
**Analysis of compound purity is provided as supplemental material and is available on through the Journal of Medicinal Chemistry Website.**
References
- 1.Dayton WR, Goll DE, Zeece MG, Robson RM, Reville WJ. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976;15:2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- 2.Dayton WR, Reville WJ, Goll DE, Stromer MH. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976;15:2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- 3.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 4.Thompson VF, Goll DE. Purification of m-calpain, m-calpain, and calpastatin from animal tissues. Humana. 2000;Vol. 144:14. [PubMed] [Google Scholar]
- 5.Sorimachi H, Ishiura S, Suzuki K. A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca(2+)-binding domain. J Biol Chem. 1993;268:19476–19482. [PubMed] [Google Scholar]
- 6.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem J. 1997;328(Pt 3):721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorimachi H, Toyama-Sorimachi N, Saido TC, Kawasaki H, Sugita H, Miyasaka M, Arahata K, Ishiura S, Suzuki K. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J Biol Chem. 1993;268:10593–10605. [PubMed] [Google Scholar]
- 8.Futai E, Kubo T, Sorimachi H, Suzuki K, Maeda T. Molecular cloning of PalBH, a mammalian homologue of the Aspergillus atypical calpain PalB. Biochim Biophys Acta. 2001;1517:316–319. doi: 10.1016/s0167-4781(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Tomioka S, Kinbara K, Masumoto H, Jeong SY, Sorimachi H, Ishiura S, Suzuki K. Characterization of a human digestive tract-specific calpain, nCL-4, expressed in the baculovirus system. Arch Biochem Biophys. 1999;362:22–31. doi: 10.1006/abbi.1998.1021. [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Li L, Cohen SN. Antisense RNA-mediated deficiency of the calpain protease, nCL-4, in NIH3T3 cells is associated with neoplastic transformation and tumorigenesis. J Biol Chem. 2000;275:31093–31098. doi: 10.1074/jbc.M005451200. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Fukiage C, Kim YH, Duncan MK, Reed NA, Shih M, Azuma M, Shearer TR. Characterization and expression of calpain 10. A novel ubiquitous calpain with nuclear localization. J Biol Chem. 2001;276:28525–28531. doi: 10.1074/jbc.M100603200. [DOI] [PubMed] [Google Scholar]
- 12.Matena K, Boehm T, Dear N. Genomic organization of mouse Capn5 and Capn6 genes confirms that they are a distinct calpain subfamily. Genomics. 1998;48:117–120. doi: 10.1006/geno.1997.5133. [DOI] [PubMed] [Google Scholar]
- 13.Kamei M, Webb GC, Young IG, Campbell HD. SOLH, a human homologue of the Drosophila melanogaster small optic lobes gene is a member of the calpain and zinc-finger gene families and maps to human chromosome 16p13.3 near CATM (cataract with microphthalmia) Genomics. 1998;51:197–206. doi: 10.1006/geno.1998.5395. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–8. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 15.Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005;352:2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 16.Raynaud F, Jond-Necand C, Marcilhac A, Furst D, Benyamin Y. Calpain 1-gamma filamin interaction in muscle cells: a possible in situ regulation by PKC-alpha. Int J Biochem Cell Biol. 2006;38:404–413. doi: 10.1016/j.biocel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol Cell Biol. 2001;21:2213–2220. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HC, Wang CJ, Chou CL, Lin SM, Huang CD, Lin TY, Wang CH, Lin HC, Yu CT, Kuo HP, Liu CY. Tumor necrosis factor-alpha induces caspase-independent cell death in human neutrophils via reactive oxidants and associated with calpain activity. J Biomed Sci. 2006;13:261–273. doi: 10.1007/s11373-005-9052-8. [DOI] [PubMed] [Google Scholar]
- 20.Bertipaglia I, Carafoli E. Calpains and human disease. Subcell Biochem. 2007;45:29–53. doi: 10.1007/978-1-4020-6191-2_2. [DOI] [PubMed] [Google Scholar]
- 21.Harris F, Chatfield L, Singh J, Phoenix DA. Role of calpains in diabetes mellitus: a mini review. Mol Cell Biochem. 2004;261:161–167. doi: 10.1023/b:mcbi.0000028751.10560.dc. [DOI] [PubMed] [Google Scholar]
- 22.Paul DS, Harmon AW, Winston CP, Patel YM. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem J. 2003;376:625–632. doi: 10.1042/BJ20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YP, Sreenan S, Pan CY, Currie KP, Bindokas VP, Horikawa Y, Lee JP, Ostrega D, Ahmed N, Baldwin AC, Cox NJ, Fox AP, Miller RJ, Bell GI, Polonsky KS. A 48-hour exposure of pancreatic islets to calpain inhibitors impairs mitochondrial fuel metabolism and the exocytosis of insulin. Metabolism. 2003;52:528–534. doi: 10.1053/meta.2003.50091. [DOI] [PubMed] [Google Scholar]
- 25.Baier LJ, Permana PA, Yang X, Pratley RE, Hanson RL, Shen GQ, Mott D, Knowler WC, Cox NJ, Horikawa Y, Oda N, Bell GI, Bogardus C. A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J Clin Invest. 2000;106:R69–73. doi: 10.1172/JCI10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanis CL, Boerwinkle E, Chakraborty R, Ellsworth DL, Concannon P, Stirling B, Morrison VA, Wapelhorst B, Spielman RS, Gogolin-Ewens KJ, Shepard JM, Williams SR, Risch N, Hinds D, Iwasaki N, Ogata M, Omori Y, Petzold C, Rietzch H, Schroder HE, Schulze J, Cox NJ, Menzel S, Boriraj VV, Chen X, Lim LR, Lindner T, Mereu LE, Wang YQ, Xiang K, Yamagata K, Yang Y, Bell GI. A genome-wide search for human non-insulin-dependent (type 2) diabetes genes reveals a major susceptibility locus on chromosome 2. Nat Genet. 1996;13:161–166. doi: 10.1038/ng0696-161. [DOI] [PubMed] [Google Scholar]
- 27.Marshall C, Hitman GA, Partridge CJ, Clark A, Ma H, Shearer TR, Turner MD. Evidence that an isoform of calpain-10 is a regulator of exocytosis in pancreatic beta-cells. Mol Endocrinol. 2005;19:213–224. doi: 10.1210/me.2004-0064. [DOI] [PubMed] [Google Scholar]
- 28.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–1171. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 29.Giguere CJ, Covington MD, Schnellmann RG. Mitochondrial calpain 10 activity and expression in the kidney of multiple species. Biochem Biophys Res Commun. 2008;366:258–262. doi: 10.1016/j.bbrc.2007.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoyagi T, Umezawa K. Cold Spring Harbor; Cold Spring Harbor: 1975. p. 25. [Google Scholar]
- 31.Donkor IO. A survey of calpain inhibitors. Curr Med Chem. 2000;7:1171–1188. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 32.Yano Y, Shiba E, Kambayashi J, Sakon M, Kawasaki T, Fujitani K, Kang J, Mori T. The effects of calpeptin (a calpain specific inhibitor) on agonist induced microparticle formation from the platelet plasma membrane. Thromb Res. 1993;71:385–396. doi: 10.1016/0049-3848(93)90163-i. [DOI] [PubMed] [Google Scholar]
- 33.Mehdi S, Angelastro MR, Wiseman JS, Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988;157:1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T, Kishi M, Saito M, Tanaka T, Higuchi N, Kominami E, Katunuma N, Murachi T. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- 35.Angelastro MR, Mehdi S, Burkhart JP, Peet NP, Bey P. Alpha-diketone and alpha-keto ester derivatives of N-protected amino acids and peptides as novel inhibitors of cysteine and serine proteinases. J Med Chem. 1990;33:11–13. doi: 10.1021/jm00163a002. [DOI] [PubMed] [Google Scholar]
- 36.Tao M, Bihovsky R, Wells GJ, Mallamo JP. Novel peptidyl phosphorus derivatives as inhibitors of human calpain I. J Med Chem. 1998;41:3912–3916. doi: 10.1021/jm980325e. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee S, Gu ZQ, Dunn D, Tao M, Josef K, Tripathy R, Bihovsky R, Senadhi SE, O’Kane TM, McKenna BA, Mallya S, Ator MA, Bozyczko-Coyne D, Siman R, Mallamo JP. D-amino acid containing, high-affinity inhibitors of recombinant human calpain I. J Med Chem. 1998;41:2663–2666. doi: 10.1021/jm980035y. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee S, Iqbal M, Mallya S, Senadhi SE, O’Kane TM, McKenna BA, Bozyczko-Coyne D, Kauer JC, Siman R, Mallamo JP. Exploration of the importance of the P2-P3-NHCO-moiety in a potent di- or tripeptide inhibitor of calpain I: insights into the development of nonpeptidic inhibitors of calpain I. Bioorg Med Chem. 1998;6:509–522. doi: 10.1016/s0968-0896(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 39.Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- 40.Harbeson SL, Abelleira SM, Akiyama A, Barrett R, 3rd, Carroll RM, Straub JA, Tkacz JN, Wu C, Musso GF. Stereospecific synthesis of peptidyl alpha-keto amides as inhibitors of calpain. J Med Chem. 1994;37:2918–2929. doi: 10.1021/jm00044a013. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Ortega-Vilain AC, Patil GS, Chu DL, Foreman JE, Eveleth DD, Powers JC. Novel peptidyl alpha-keto amide inhibitors of calpains and other cysteine proteases. J Med Chem. 1996;39:4089–4098. doi: 10.1021/jm950541c. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Patil GS, Golubski ZE, Hori H, Tehrani K, Foreman JE, Eveleth DD, Bartus RT, Powers JC. Peptide alpha-keto ester, alpha-keto amide, and alpha-keto acid inhibitors of calpains and other cysteine proteases. J Med Chem. 1993;36:3472–3480. doi: 10.1021/jm00074a031. [DOI] [PubMed] [Google Scholar]
- 43.Pliura DH, Bonaventura BJ, Smith RA, Coles PJ, Krantz A. Comparative behaviour of calpain and cathepsin B toward peptidyl acyloxymethyl ketones, sulphonium methyl ketones and other potential inhibitors of cysteine proteinases. Biochem J. 1992;288(Pt 3):759–762. doi: 10.1042/bj2880759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw E. Peptidyl sulfonium salts. A new class of protease inhibitors. J Biol Chem. 1988;263:2768–2772. [PubMed] [Google Scholar]
- 45.Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- 46.Puri RN, Matsueda R, Umeyama H, Bradford HN, Colman RW. Modulation of thrombin-induced platelet aggregation by inhibition of calpain by a synthetic peptide derived from the thiol-protease inhibitory sequence of kininogens and S-(3-nitro-2-pyridinesulfenyl)-cysteine. Eur J Biochem. 1993;214:233–241. doi: 10.1111/j.1432-1033.1993.tb17916.x. [DOI] [PubMed] [Google Scholar]
- 47.Palmer JT, Rasnick D, Klaus JL, Bromme D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J Med Chem. 1995;38:3193–6. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- 48.Anagli J, Hagmann J, Shaw E. Investigation of the role of calpain as a stimulus-response mediator in human platelets using new synthetic inhibitors. Biochem J. 1991;274(Pt 2):497–502. doi: 10.1042/bj2740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatterjee S, Ator MA, Bozyczko-Coyne D, Josef K, Wells G, Tripathy R, Iqbal M, Bihovsky R, Senadhi SE, Mallya S, O’Kane TM, McKenna BA, Siman R, Mallamo JP. Synthesis and biological activity of a series of potent fluoromethyl ketone inhibitors of recombinant human calpain I. J Med Chem. 1997;40:3820–3828. doi: 10.1021/jm970197e. [DOI] [PubMed] [Google Scholar]
- 50.Dolle RE, Singh J, Whipple D, Osifo IK, Speier G, Graybill TL, Gregory JS, Harris AL, Helaszek CT, Miller RE, et al. Aspartyl alpha-((diphenylphosphinyl)oxy)methyl ketones as novel inhibitors of interleukin-1 beta converting enzyme. Utility of the diphenylphosphinic acid leaving group for the inhibition of cysteine proteases. J Med Chem. 1995;38:220–222. doi: 10.1021/jm00002a002. [DOI] [PubMed] [Google Scholar]
- 51.Krantz A, Copp LJ, Coles PJ, Smith RA, Heard SB. Peptidyl (acyloxy)methyl ketones and the quiescent affinity label concept: the departing group as a variable structural element in the design of inactivators of cysteine proteinases. Biochemistry. 1991;30:4678–4687. doi: 10.1021/bi00233a007. [DOI] [PubMed] [Google Scholar]
- 52.Peet NP, Burkhart JP, Angelastro MR, Giroux EL, Mehdi S, Bey P, Kolb M, Neises B, Schirlin D. Synthesis of peptidyl fluoromethyl ketones and peptidyl alpha-keto esters as inhibitors of porcine pancreatic elastase, human neutrophil elastase, and rat and human neutrophil cathepsin G. J Med Chem. 1990;33:394–407. doi: 10.1021/jm00163a063. [DOI] [PubMed] [Google Scholar]
- 53.Montero A, Albericio F, Royo M, Herradon B. Solid-phase combinatorial synthesis of peptide-biphenyl hybrids as calpain inhibitors. Org Lett. 2004;6:4089–4092. doi: 10.1021/ol048216j. [DOI] [PubMed] [Google Scholar]
- 54.Montero A, Alonso M, Benito E, Chana A, Mann E, Navas JM, Herradon B. Studies on aromatic compounds: inhibition of calpain I by biphenyl derivatives and peptide-biphenyl hybrids. Bioorg Med Chem Lett. 2004;14:2753–2757. doi: 10.1016/j.bmcl.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 55.Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadreza I, Probert AW, Jr., Marcoux FW, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins JL, Fergus A, Lee KS, Lunney EA, Hays SJ, Yuen P. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atsma DE, Bastiaanse EM, Jerzewski A, Van der Valk LJ, Van der Laarse A. Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res. 1995;76:1071–1078. doi: 10.1161/01.res.76.6.1071. [DOI] [PubMed] [Google Scholar]
- 57.Covington MD, Bayless KJ, Burghardt RC, Davis GE, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells: evidence for a role of metalloproteinases. Am J Physiol Renal Physiol. 2005;289:F280–288. doi: 10.1152/ajprenal.00351.2004. [DOI] [PubMed] [Google Scholar]
- 58.Gores GJ, Nieminen AL, Fleishman KE, Dawson TL, Herman B, Lemasters JJ. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988;255:C315–322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- 59.Guttmann RP, Sokol S, Baker DL, Simpkins KL, Dong Y, Lynch DR. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J Pharmacol Exp Ther. 2002;302:1023–1030. doi: 10.1124/jpet.102.036962. [DOI] [PubMed] [Google Scholar]
- 60.Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, Hudecz F, Friedrich P. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- 61.Cuerrier D, Moldoveanu T, Davies PL. Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J Biol Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- 62.Malik MN, Fenko MD, Iqbal K, Wisniewski HM. Purification and characterization of two forms of Ca2+-activated neutral protease from calf brain. J Biol Chem. 1983;258:8955–8962. [PubMed] [Google Scholar]
- 63.Malik MN, Fenko MD, Wisniewski HM. Purification and partial characterization of two forms of Ca2+-activated neutral protease from calf brain synaptosomes and spinal cord. Neurochem Res. 1984;9:233–240. doi: 10.1007/BF00964171. [DOI] [PubMed] [Google Scholar]
- 64.Yoshihara Y, Ueda H, Imajoh S, Takagi H, Satoh M. Calcium-activated neutral protease (CANP), a putative processing enzyme of the neuropeptide, kyotorphin, in the brain. Biochem Biophys Res Commun. 1988;155:546–553. doi: 10.1016/s0006-291x(88)80529-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.