Abstract
This study explored the effects of propofol on c-Fos and Egr-1 in neuroblastoma (N2A) cells. We demonstrate that propofol induced the expression of c-Fos and Egr-1 within 30 and 60 min of exposure time. At 16.8 µM concentration, propofol induced a 6 and 2.5-fold expression of c-Fos and Egr-1, respectively. However, at concentrations above 100 µM, propofol failed to induce expression of c-Fos or Egr-1. Propofol-induced c-Fos and Egr-1 transcription was unaffected by bicuculline, a γ-aminobutyric acid-A receptor antagonist, but was abolished by PD98059, a mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Our study shows that clinically relevant concentrations of propofol induce c-Fos and Egr-1 expression through an extracellular signal-regulated kinase mediated and γ-aminobutyric acid-A independent pathway.
Keywords: γ-aminobutyric acid, extracellular signal-regulated kinase, immediate early genes, propofol
Introduction
Propofol is a commonly used intravenous agent for the induction and maintenance of general anesthesia used on millions of patients worldwide. It is thought to act primarily through the hyperpotentiation of the γ-aminobutyric acid (GABA)-A receptor [1,2]. Although the GABA-A dependent mechanisms are well established, there is a growing interest in elucidating secondary mechanisms that might have long-lasting side effects [3,4]. For example, Pang et al. [5] reported a dose-dependent effect of propofol administration on rat memory, whereas Veselis and coworkers [6] suggested potential long-term memory effects because of changes in cerebral blood flow. The underlying mechanism for these effects is unclear but may be related to long-term changes in the transcriptional state of the neuronal cells.
Several groups have demonstrated that the expression of rapidly inducible genes known as immediate-early genes (IEGs) play a critical role in long-term potentiation and memory consolidation [7]. In particular, transcription factors, such as c-Fos, Egr-1, Nurr1, and Arc have been implicated in learning, memory, and long-term potentiation of GABA-A receptor [8,9]. Anesthetic agents such as midazolam and thiopental were shown to induce the transcription of c-Fos, JunB, and Egr-1, through a GABA-A independent pathway [10,11]. However, the same studies showed that high concentrations of propofol did not affect the expression of c-Fos, JunB, or Egr-1 in culture [10,11]. In contrast, Hamaya et al. [12] reported that propofol increases the expression of c-Fos and Jun B in the rat brain; whereas Kubota and coworkers [13] showed that propofol regulates the expression of c-Fos in brain slices. Therefore, the interaction between propofol and these immediate early transcription factors is still under debate.
In this study, we investigated the ability of propofol to induce the transcription of c-Fos and Egr-1 in nerve growth factor (NGF) differentiated mouse neuroblastoma (N2A) cells [14]. Using this system we demonstrate a time and dose-dependent transcription of c-Fos and Egr-1. Remarkably, although 16.8 µM of propofol, corresponding to plasma concentrations in general anesthesia, induced a 6 and 2.5-fold transcription of c-Fos and Egr-1, respectively, higher concentrations failed to induce any transcriptional changes. The induction of c-Fos and Egr-1 was GABA-A independent but relied on the mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinase (ERK) pathway. These results suggest a parallel pathway of action with an unclear role in the activity of general anesthetics.
Methods
Materials
Fetal bovine serum, phosphate-buffered saline, Dulbecco’s modified Eagle medium, penicillin, streptomycin, and trypsin–EDTA were obtained from Invitrogen Life Technologies (Carlsbad, California, USA). Propofol was purchased from AstraZeneca (Wilmington, Delaware, USA). 7S NGF, intralipid, GABA-A receptor agonists (GABA), GABA-A receptor antagonists (bicuculline) and PD98059 were purchased from Sigma (Sigma, St Louis, Missouri, USA). Live/Dead viability assay kit was purchased from Molecular Probes (Eugene, Oregon, USA).
Cell culture and treatment
N2A cells (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 2% penicillin and streptomycin (Invitrogen Life Technologies) in a humidified incubator at 37°C and 5% carbon dioxide. Cells were seeded at a density of 5000 cells/cm2 on six-well plates in culture media supplemented with 10 nM of NGF to induce neuronal differentiation. Following 4 days of NGF stimulation, more than 95% of the cells appeared to be morphologically differentiated with neurites at least twice the length of the cell diameter. At this stage N2A cells were left untreated (negative control), treated with intralipid (vehicle control), or treated with increasing concentrations of propofol ranging from 5.6 to 112.2 µM. The transcription of c-Fos and Egr-1 genes was measured at several time intervals posttreatment (Fig. 1a).
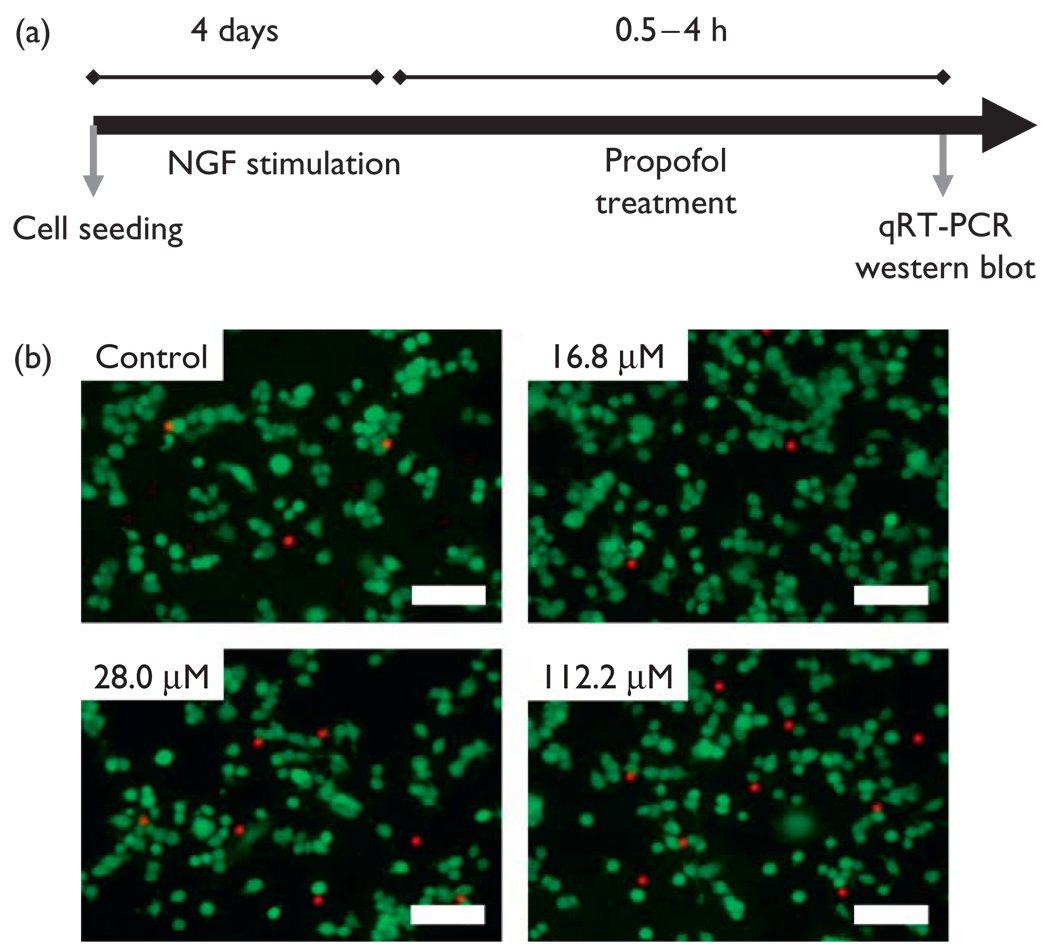
Fig. 1.
(a) Schematic representation of the experimental design.
(b) Fluorescence live/dead staining of N2A cells following 4 h stimulation with vehicle control or propofol at doses of 16.8, 28.0, and 112.2 µM. Scale bar= 100 µm. Cells were stained using a fluorescent viability assay in which live cells are stained green because of esterase activity, whereas the nucleus of dead cells is labeled red because of loss of membrane integrity. NGF, nerve growth factor; qRT-PCR, quantified using quantitative reverse transcription-PCR.
Live/Dead assay
N2A cells were treated with increasing concentrations of propofol for 4 h and then washed with phosphate-buffered saline. The cells were stained using a fluorescent Live/Dead viability assay in which the cytoplasm of live cells accumulates green fluorescent calcein because of esterase activity, whereas the nucleus of dead cells is labeled red by ethidium homodimer because of loss of membrane integrity. Cells were quantified using Image J (NIH, Bethesda, Maryland, USA).
Real-time quantitative reverse transcriptase
Analysis of c-Fos and Egr-1 transcription was carried out using Mx3000P QPCR system (Stratagene, La Jolla, California, USA). RNA from the cells was extracted and purified using a Qiagen’s Nucleospin RNA II kit (Valencia, California, USA) and quantified using Nanodrop ND-1000 (Wilmington, Delaware, USA). Total mRNA (100 ng) was reverse transcribed to cDNA using a Superscript Platinum Two-Step quantitative reverse transcription-PCR kit (Invitrogen Life Technologies) and amplified in a Perkin Etus Thermal Cycler 480 (Applied Biosystems Inc., Foster City, California, USA). Reported values were normalized to the internal standard actin. The primer sequences were as follows: c-Fos, (forward) 5′-GAAGGAACCAGACAGGTCCA, (reverse) 5′-TCACCCTGCCTCTTCTCAAT; Egr-1, (forward) 5′-AGCGAACAACCCTATGAGCA, (reverse) 5′-TCGTTTGGCTGGGATAACTC; Actin, (forward) 5′-GTCGTACCACTGGCATTGTG, (reverse) 5′-CTCTCAGCTGTGGTGGTGAA.
Western blot
Cultured N2A cells treated with propofol were incubated with lysis buffer containing 2% sodium dodecyl sulfate, 62.5 mM Tris–Cl (pH 6.8), 10% glycerol, 0.02% bromophenol blue, 50 mM dithiothreitol on ice for 5 min. Collected cell lysates were boiled for 5 min and separated on a 10% gradient acrylamide gel, and transferred electrophoretically to polyvinyl difluoride membranes. Nonspecific binding of antibody was blocked with 5% nonfat dry milk. Immunodetection was performed with c-Fos, Egr-1, phospho-specific ERK1/2, and ERK1/2 primary antibodies (1 : 2000) followed by incubation with the peroxidase-conjugated secondary antibodies, and the blots were developed using an enhanced chemiluminescence method. β-tubulin served as an internal protein control (1 : 2000). All antibodies were purchased from Cell Signaling Technologies (Danvers, Massachusetts, USA).
Statistics
Data were presented as means ± SD. Pair-wise comparisons were performed using Student’s t-test where appropriate. P values less than 0.05 were considered statistically significant.
Results
To evaluate whether N2A cells suffer damage after propofol treatment, we quantified cellular viability. NGF differentiated N2A cells were treated with increasing concentrations of propofol and stained using a fluorescent Live/Dead assay (Fig. 1b). Even at concentrations as high as 112.2 µM, cell viability was 87 ± 9% and not statistically different from control (P = 0.15, N = 3). Therefore, our results show that propofol did not affect cellular viability.
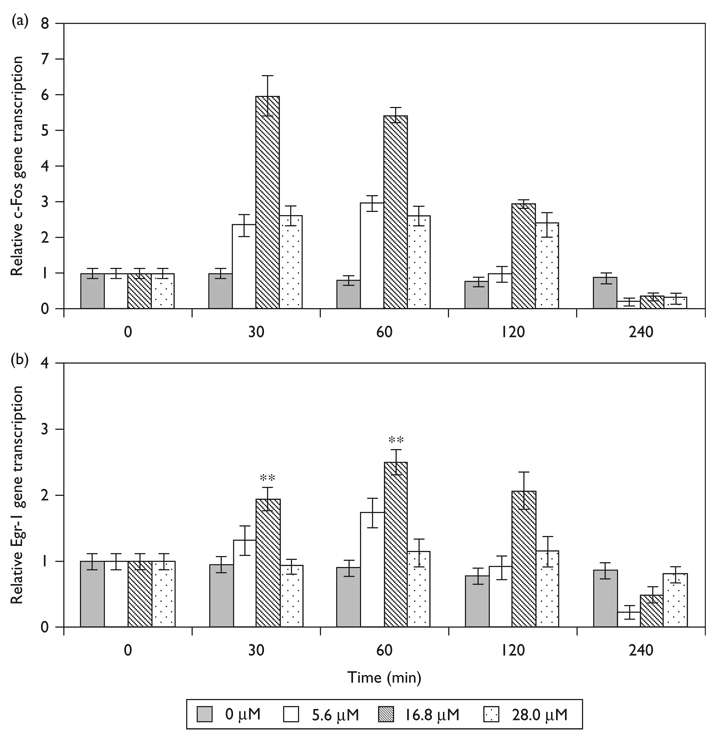
To investigate the effect of propofol on the transcription of c-Fos and Egr-1, we exposed N2A cells to varying concentrations of propofol at increasing time intervals. The transcription of c-Fos (Fig. 2a) and Egr-1 (Fig. 2b) peaked between 30 and 60 min of exposure and rapidly returned to control levels. Maximal induction of c-Fos and Egr-1 occurred following 30 min stimulation with 16.8 µM propofol, resulting in 6.0 ± 0.5 (P = 0.008, N = 3) and 2.5 ± 0.2 fold (P = 0.010, N = 3) increase in transcription, respectively. Surprisingly, both lower and higher doses of propofol resulted in a significant, but submaximal induction of c-Fos transcription 2.4 ± 0.3 (P = 0.028, N = 3) and 2.6 ± 0.3 (P = 0.021, N = 3) fold change for 5.6 and 28.0 µM concentration respectively. Similarly, both lower and higher doses of propofol resulted in a submaximal induction of Egr-1 transcription, 1.7 ± 0.2 (P = 0.021, N = 3) and 1.1 ± 0.2 (P = 0.072, N = 3) fold change for 5.6 and 28.0 µM concentration, respectively.
Fig. 2.
Time and dose-dependent propofol induced (a) c-Fos and (b) Egr-1 transcription in N2A cells. Transcription of c-Fos and Egr-1 was quantified using quantitative reverse transcription-PCR. Gene transcription is normalized to actin and nontreated controls. Data is presented as mean ± SD from three independent experiments. **P<0.01 versus nontreated control.
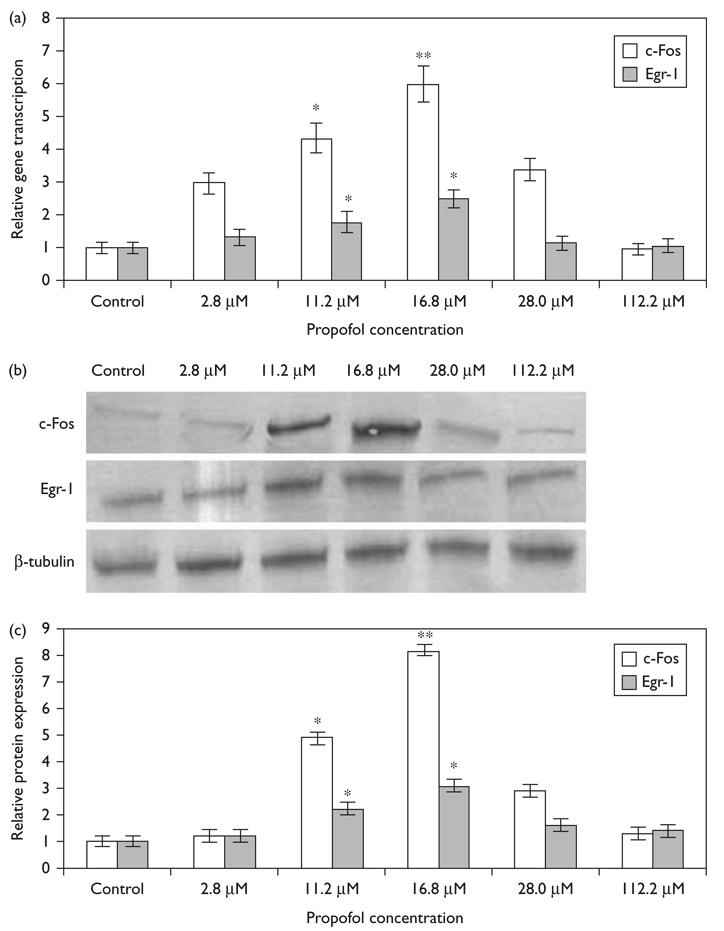
To further probe the dose-dependence of c-Fos and Egr-1 transcription on propofol concentration, we exposed N2A cells to increasing concentrations of propofol for 60 min. Figure 3a shows that at clinically relevant concentrations (16.8 µM) propofol caused a 6-fold induction in c-Fos transcription and a 2.5-fold induction in the transcription of Egr-1. However, at a dose of 112.2 µM, propofol did not significantly alter c-Fos (0.96 ± 0.2; P = 0.194, N = 3) or Egr-1 (1.07 ± 0.17; P = 0.156, N = 3) gene expression compared with control. Figure 3b and c show that c-Fos and Egr-1 protein expression follows a similar trend to the gene transcription and 16.8 µM propofol caused an 8-fold increase in c-Fos expression and a 3-fold increase in Egr-1 expression.
Fig. 3.
Dose-dependant expression of c-Fos and Egr-1 by propofol following 60 min stimulation. (a) The transcription of c-Fos and Egr-1 was quantified using quantitative reverse transcription-PCR. Gene transcription is normalized to actin and to nontreated controls. Data is presented as mean ± SD from three independent experiments. *P<0.05; **P<0.01 versus nontreated control. (b) Representative immunoblots of c-Fos and Egr-1 protein expression following propofol stimulation. (c) Quantification of c-Fos and Egr-1 protein expression induced by propofol. The amount of proteins is expressed as ratio to control. *P<0.05, **P<0.01 versus nontreated control.
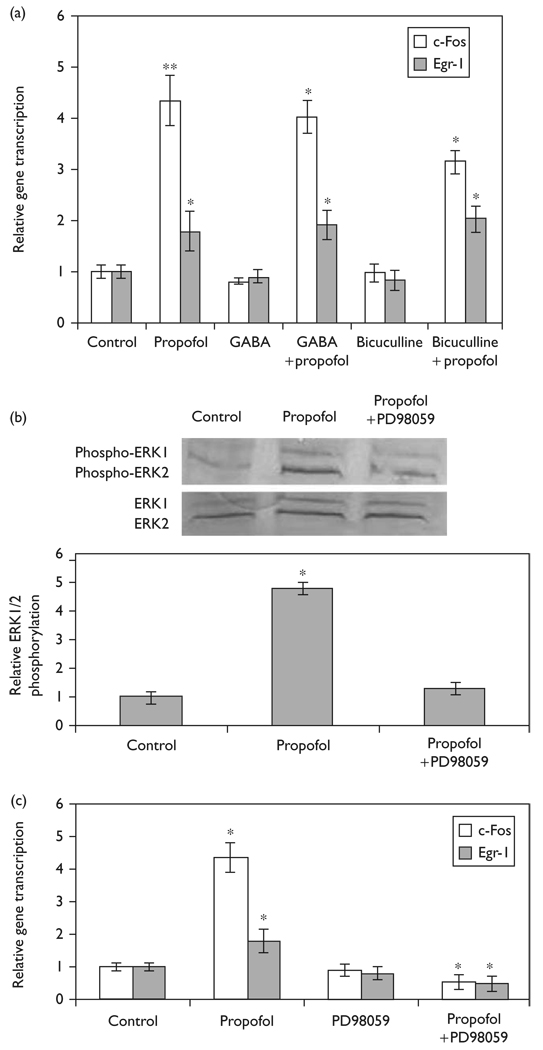
To evaluate the role of the GABA pathway in the induction of c-Fos and Egr-1 expression we exposed cells pretreated with GABA-A receptor agonists or antagonists to propofol (Fig. 4a and b). Pretreatment of N2A cells with 100 µM GABA-A receptor agonist, GABA, for 60 min did not significantly change the transcription of c-Fos (0.81 ± 0.1; P = 0.055, N = 3) or Egr-1 (0.90 ± 0.12; P = 0.060, N = 3). The expression of c-Fos and Egr-1 in GABA pretreated cells exposed to 16.8 µM propofol was not significantly different (P = 0.281, N = 3), than cells treated with propofol alone. N2A cells pretreated with 100 µM GABA-A receptor antagonist, bicuculline, for 60 min did not induce the transcription of c-Fos (0.98 ± 0.18; P = 0.167, N = 3) or Egr-1 (0.83 ± 0.18; P = 0.124, N = 3) compared with control. When N2A cells pretreated with bicuculline were exposed to propofol, it induced c-Fos (3.25 ± 0.23; P = 0.135, N = 3) and Egr-1 (2.03 ± 0.26; P = 0.158, N = 3) gene transcription, which was not statistically different compared with propofol treatment alone.
Fig. 4.
Elucidating the mechanism of propofol-induced c-Fos and Egr-1 expression in N2A cells. The transcription of c-Fos and Egr-1 was assessed using quantitative reverse transcription-PCR. (a) N2A cells in the presence and absence of GABA or bicuculline pretreatment were stimulated with propofol (16.8 µM) for 60 min. Data is presented as mean ± SD from three independent experiments. *P<0.05; **P<0.01 versus nontreated control. (b) Representative immunoblots of ERK1/2 phosphorylation and total ERK1/2 following propofol stimulation and quantification of ERK1/2 phosphorylation expressed as ratio to control. *P<0.05 versus nontreated control. (c) N2A cells with or without pretreatment of 50 µM PD98059 were stimulated with 16.8 µM propofol for 60 min and the expression of c-Fos and Egr-1 was measured using quantitative reverse transcription-PCR. Data is presented as mean ± SD from three independent experiments. *P<0.05 versus propofol treatment.
To assess the involvement of the MAPK/ERK pathway in propofol induction of c-Fos and Egr-1, we quantified ERK phosphorylation by immunoblot analysis (Fig. 4b). We show that 16.8 µM of propofol induced a 4.8 ± 0.22 fold increase in ERK1/2 phosphorylation. The addition of PD98059, an inhibitor of MAPK/ERK pathway blocked this increase in phosphorylated ERK. Figure 4c shows that pretreatment of N2A cells with 50 µM of PD98059 did not induce c-Fos (0.91 ± 0.19; P = 0.183, N = 3) or Egr-1 (0.82 ± 0.20; P = 0.176, N = 3) gene transcription compared with control. However, stimulation of PD98059 pretreated N2A cells with 16.8 µM of propofol resulted in a significantly lower c-Fos (0.55 ± 0.22; P = 0.034, N = 3) and Egr-1 (0.50 ± 0.24; P = 0.027, N = 3) gene transcription compared with propofol treatment alone, demonstrating the role of ERK in propofol induction of the IEGs, c-Fos and Egr-1.
Discussion
Previous work indicated that general anesthetics could potentially decrease cell viability, impair DNA integrity, and provoke stress-induced apoptosis in several neuronal cell systems [15,16]. Such cell damage could introduce confounding results to our study because of the induction of stress and apoptotic genes. Our results show that propofol did not affect cellular viability even at high concentrations of the drug, suggesting that the observed gene induction was not caused because of cellular damage or stress.
The data shows a time and dose-dependent induction of c-Fos and Egr-1 expression in N2A cells following exposure to propofol. These results are especially interesting as previous reports suggested that propofol did not affect the expression of c-Fos and Egr-1 [10,11]. At high concentrations our results are in agreement with Fukuda et al.’s [10] work where no significant changes were observed in the c-Fos and Egr-1 expression in PC12 cells stimulated with 100 µM propofol for 60 min. However, our work demonstrates that propofol has a significant effect at low concentrations. In fact, in a clinical setting, propofol concentration in the plasma is about 16.8 µM [17], and at this concentration the drug induced a 6 and a 2.5-fold increase in the expression of c-Fos and Egr-1. These results suggest that clinically relevant doses of propofol can potentially induce long-term changes of neuronal functions by inducing changes in the gene expression.
GABA-A receptor complex is a major mediator of rapid synaptic inhibition in the brain, as well as an important drug target. It is thought that propofol acts primarily through the potentiation of the GABA-A receptor [1,2]. However, our results show that both GABA-A receptor agonists (GABA) and GABA-A receptor antagonists (bicuculline) did not induce c-Fos and Egr-1 expression in N2A cells. In addition, several studies suggest that neuronal cell lines, such as N2A, do not possess a functional GABA-A receptor [18]. These results suggest that propofol could potentially activate a mechanism distinct from the GABA-A pathway, leading to c-Fos and Egr-1 expression.
MAPKs are known to be important mediators of signal transduction from the cell surface to the nucleus in all eukaryotes [19]. MAPK/ERK pathways are activated by a wide range of stimuli and have the ability to activate transcription factors, protein kinases as well as cytoskeleton-associated proteins [19]. Several studies have shown that general anesthetics may interfere with cellular targets, including MAPK/ERK pathways [20–22]. Previous reports suggest the involvement of the MAPK/ERK pathway in the induction of c-Fos and Egr-1 expression by growth hormone [23] or midazolam [10]. Our results show that PD98059, a MAPK/ERK inhibitor, blocked the propofol-induced c-Fos and Egr-1 expression, thus suggesting the possible involvement of MAPK/ERK pathway in propofol-induced IEG expression.
Conclusion
In summary, we demonstrated that propofol induces a time and dose-dependent transcription of IEGs, c-Fos, and Egr-1 in neuronal cells. We demonstrate for the first time that propofol induced IEG expression was mediated through a GABA-A independent, MAPK/ERK dependent pathway.
Acknowledgements
The authors thank Dr Martin Yarmush for valuable discussions regarding the manuscript. We also thank Dr Monica Casali for her valuable suggestions regarding PCR experiments. This study was supported by NIH/NIDDK Grant K01DK090241, by Park Slope Anesthesia Associates, and by the core facilities of Shriners Childrens Hospital.
References
- 1.Nagashima K, Zorumski CF, Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005;103:318–326. doi: 10.1097/00000542-200508000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA-A receptor beta 3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 3.Jones C, Griffiths RD, Humphris G. Disturbed memory and amnesia related to intensive care. Memory. 2000;8:79–94. doi: 10.1080/096582100387632. [DOI] [PubMed] [Google Scholar]
- 4.Perouansky M. General anesthetics and long-term neurotoxicity. Handb Exp Pharmacol. 2008;182:143–157. doi: 10.1007/978-3-540-74806-9_7. [DOI] [PubMed] [Google Scholar]
- 5.Pang R, Quartermain D, Rosman E, Turndorf H. Effect of propofol on memory in mice. Pharmacol Biochem Behav. 1993;44:145–151. doi: 10.1016/0091-3057(93)90292-2. [DOI] [PubMed] [Google Scholar]
- 6.Veselis RA, Feshchenko VA, Reinsel RA, Beattie B, Akhurst TJ. Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology. 2005;102:26–34. doi: 10.1097/00000542-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WC, Dragunow M, Tate WP. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol. 1991;5:297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- 9.Dragunow M. A role for immediate-early transcription factors in learning and memory. Behav Genet. 1996;26:293–299. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K, Shoda T, Mima H, Uga H. Midazolam induces expression of c-Fos and EGR-1 by a non-GABAergic mechanism. Anesth Analg. 2002;95:373–378. doi: 10.1097/00000539-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Jouvert P, Pain L, Aunis D, Zwiller J. The anesthetics propofol and ketamine inhibit cocaine-induced egr-1 gene expression in rat forebrain. Eur J Pharmacol. 2002;449:239–243. doi: 10.1016/s0014-2999(02)02035-6. [DOI] [PubMed] [Google Scholar]
- 12.Hamaya Y, Takeda T, Dohi S, Nakashima S, Nozawa Y. The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesth Analg. 2000;90:1177–1183. doi: 10.1097/00000539-200005000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Kubota I, Tsuboi Y, Shoda E, Kondo M, Masuda Y, Kitagawa J, et al. Modulation of neuronal activity in CNS pain pathways following propofol administration in rats: Fos and EEG analysis. Exp Brain Res. 2007;179:181–190. doi: 10.1007/s00221-006-0779-x. [DOI] [PubMed] [Google Scholar]
- 14.Dickey CA, De Mesquita DD, Morgan D, Pennypacker KR. Induction of memory-associated immediate early genes by nerve growth factor in rat primary cortical neurons and differentiated mouse Neuro2A cells. Neurosci Lett. 2004;366:10–14. doi: 10.1016/j.neulet.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 15.Stephanova E, Topouzova-Hristova T, Konakchieva R. Mitochondria are involved in stress response of A549 alveolar cells to halothane toxicity. Toxicol In Vitro. 2008;22:688–694. doi: 10.1016/j.tiv.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Engdahl O, Abrahams M, Bjornsson A, Vegfors M, Norlander B, Ahlner J, Eintrei C. Cerebrospinal fluid concentrations of propofol during anesthesia in humans. Br J Anaesth. 1998;81:957–959. doi: 10.1093/bja/81.6.957. [DOI] [PubMed] [Google Scholar]
- 18.Hales TG, Tyndale RF. Few cell lines with GABAA mRNAs have functional receptors. J Neurosci. 1994;14:5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- 21.Dahmani S, Tesniere A, Rouelle D, Toutant M, Desmonts JM, Mantz J. Effects of anesthetic agents on focal adhesion kinase (pp125FAK) tyrosine phosphorylation in rat hippocampal slices. Anesthesiology. 2004;101:344–353. doi: 10.1097/00000542-200408000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Kozinn J, Mao L, Arora A, Yang L, Fibuch EE, Wang JQ. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology. 2006;105:1182–1191. doi: 10.1097/00000542-200612000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 1998;273:31327–31336. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]