Abstract
Background
Hyperuricemia, a known correlate of oxidative stress, is a marker for adverse prognosis among individuals with heart failure. However, the relationship between hyperuricemia and the risk for incidence of heart failure in a community-based population has not been studied.
Methods and Results
We prospectively analyzed the relationship between serum uric acid concentration at baseline and subsequent heart failure among the participants of the Framingham Offspring cohort (mean baseline age 36 years, women 52%). Using Cox regressions we calculated the risk of heart failure with increasing serum uric acid after adjusting for sex, age, smoking, body mass index, renal dysfunction, diuretics, systolic blood pressure, valvular heart disease, diabetes, alcohol, and use of anti-hypertensive medications. The incidence rates of heart failure was ~6 fold higher among those at the highest quartile of serum uric acid (>6.3 mg/dl) compared to those at the lowest quartile (<3.4mg/dl). The adjusted hazard ratio for the highest quartile of serum uric acid compared to the lowest was 2.1 (1.04–4.22). The relationship between hyperuricemia and heart failure was found in participants without metabolic syndrome and other subgroups as well.
Conclusions
Hyperuricemia is a novel, independent, risk factor for heart failure in a group of young general community dwellers. This has implications for development of preventive strategies for heart failure.
Keywords: uric acid, heart failure, risk
Nearly 5 million Americans currently suffer from heart failure and approximately 550,000 new cases of heart failure are now diagnosed each year1. Heart failure is associated with high risk of morbidity, mortality and hospital utilization in the United States2. The established risk factors for heart failure include male sex, hypertension, valvular heart disease, coronary artery disease, and obesity3. In spite of the progress made in its management, the mortality from heart failure remains high, underlining the need for identification of novel risk factors that may be amenable to intervention.
Earlier studies have shown that heart failure is often associated with hyperuricemia4, 5. Hyperuricemia is associated with worse hemodynamic measures such as increased left atrial pressure and decreased cardiac index among patients with primary pulmonary hypertension, cor pulmonale and dilated cardiomyopathy in a small case series6. Among those with established heart failure, hyperuricemia is a risk factor for adverse outcomes including mortality5, 7–15.
Serum uric acid may be useful for prognostication among those with preexisting heart failure5, 10–15. Hyperuricemia can predict heart failure among those with preexisting hypertension16. There have not been any studies that examined hyperuricemia as independent risk factors for heart failure risk among the general population. The single available study from Austria, did not account for confounders such as valvular heart disease and diuretics, and renal disease suggested that highest quantiles of serum uric acid was associated with elevated risk for death from heart failure17.
Hyperuricemia can be easily detected in routine medical care. If indeed presence of hyperuricemia provides additional information on future heart failure risk (over and above other risk factors), it has the potential to be a screening tool. Accordingly, we hypothesized that hyperuricemia is a risk factor for heart failure independent of other known risk factors.
Methods
Study cohort and data source
We used data from the Framingham Offspring Study, a longitudinal observational study of children of the original Framingham Heart Study cohort and their spouses18. All participants of the Framingham Offspring Cohort that began in 1971 were eligible to be included in the present study. The data for our analyses were obtained from the National Heart Lung and Blood Institute (NHLBI) limited access dataset program. This analysis protocol was approved by the Stanford University institutional review board. We excluded all the subjects who did not have uric acid measurement.
Follow up and observation period
Participants were under surveillance for cardiovascular events and were followed up approximately every four years by study visits that included medical review, physical examination and laboratory testing. In the present analyses we used data collected from the first through seventh study visit. The exact number of days from baseline to each study visit/outcome event was utilized in our analyses. The median follow up of this cohort was 29 years and the cumulative observation time was 135,991 person-years.
For analyses of the effects of serum uric acid, each observation started in the first (baseline) visit and ended at the day of outcome event, death or last contact with the study. In using such a definition, we acknowledge that the duration of hyperuricemia for each individual in the observation period is an underestimation of the true duration of hyperuricemia. In all analyses, the observation ended at the time of death, last contact or the outcome event.
Measurement of covariates
Exercise, diet, drugs, and state of hydration, may result in transient fluctuations of uric acid levels and one measurement of uric acid may not be an accurate metric of the hyperuricemic `trait'. We examined this possibility in our data by calculating the probability of an individual participant changing the quartile of uric acid during the time interval between the first and second visit (i.e. transition probability) each of the uric aid stratum. Since this estimate was ~20%,, we deemed the variability to be too high and serum uric acid was measured at the first and the second visits and averaged to arrive at a mean value that replaced the single baseline measurement. Serum uric acid was assayed using the uricase method. Information on renal dysfunction, obesity measures, blood pressure, serum lipids, serum glucose, smoking, alcohol, aspirin, antihypertensive, and anti-diabetic medication use were available at all visits. Detailed information on individuals' diabetes/hypertesion medications such as name, dosage, duration of treatment were not available. For the purpose of this study, participants with a cardiac murmur at the time of the first study visit were assessed to have valvular heart disease, a risk factor for heart failure. Participants were evaluated for coronary artery disease at baseline and at subsequent visits by medical history, clinician assessment, and electrocardiogram.
The determination of renal dysfunction at baseline was made by the study physician. Serum creatinine or other laboratory measures of renal function was not available for this analysis. Gout was defined as a study physician diagnosis of definite gouty arthritis19.
Outcome Assessment
Heart failure
Heart failure events (both hospitalized and non-hospitalized) were adjudicated by a study physician panel according to predetermined Framingham criteria shown in Table 1. 20, 21. Heart failure was considered to be present if two major or one major and two minor criteria were present in the absence of alternative explanation for the clinical picture (please see Table 1 for further details). There were no participants with heart failure at baseline.
Table 1.
| A definite diagnosis of congestive heart failure requires that a minimum of two major or one major and two minor criteria be present concurrently. The presence of other conditions capable of producing the symptoms and signs were considered in evaluating the findings. |
| Major Criteria: |
|
| Minor criteria: |
|
Statistical analysis
Risk factors for heart failure
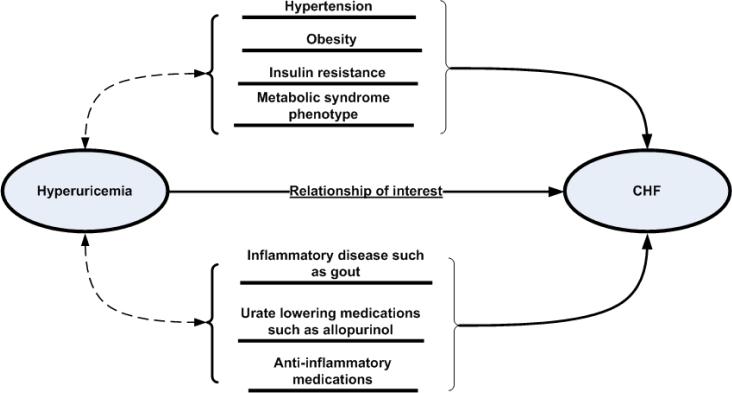
Our primary analyses addressed the question-Does elevated serum uric acid independently predict the risk for incident heart failure? We used Cox proportional hazards regression model to study the relationship between baseline serum uric acid level, and heart failure. In these regressions, the time variable was defined as the period (number of days) from the baseline date to the date of incidence of heart failure or the date of last study visit. Observations of patients who did not die or develop heart failure were censored at the time of last observation. In the primary analyses the baseline values of the covariates were used to adjust for confounding. However, the relationship between hyperuricemia and other cardiovascular risk factors are complex (Figure 1) since hyperuricemia is a risk factor for kidney disease, hypertension, and atherosclerotic cardiovascular diseases22–27. Changes in health conditions over time such as increased blood pressure and worse renal function can potentially be a cause and a consequence of hyperuricemia. Thus using time varying measures of these covariates may be problematic. Therefore, in addition to Cox regressions with time-varying values of covariates, we preformed extensive stratified analyses such as for those who did not meet the ATP criteria for metabolic syndrome baseline28, and who died of any cause during the follow-up, and those who survived until the cut-off date for observation (visit 7).
Figure 1.
Potential epidemiological pathways linking hyperuricemia and heart failure can be direct, mediated through risk factors such as hypertension, confounded by medication use, or a combination of these.
Results
Overall, of the 4989 participants in the Offspring study there were 4912 eligible participants with 196 incident cases of heart failure. Participants who developed heart failure were more likely to be older, male and with a worse traditional risk factor profile, have gout and currently used allopurinol, a uric acid reducing medication. These individuals had a greater prevalence of gout and higher serum uric acid concentration. Increasing serum concentrations of serum uric acid was associated with worse cardiovascular risk (Table 2).
Table 2.
Baseline characteristics of Framingham Offspring Study participants according to serum uric acid concentrations (n=4912)
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p value for trend | ||
|---|---|---|---|---|---|---|
| Metric | 1.2–4.34 mg/dl | 4.35–5.2 mg/dl | 5.3–6.2 mg/dl | 6.3–13.7 mg/dl | ||
| Number of participants | 1164 | 1194 | 1227 | 1329 | ||
| Age in years | (mean ± SD) | 34± 10 | 36± 10 | 36±11 | 38± 11 | <0.001 |
| Proportion of men | % | 5 | 30 | 65 | 88 | <0.001 |
| Body mass index in kg/m2 | (mean ± SD) | 23±3 | 25±4 | 26±4 | 28±4 | <0.001 |
| Alcohol use | % | 81 | 84 | 85 | 90 | <0.001 |
| Proportion of current smokers | % | 60 | 65 | 62 | 68 | <0.001 |
| Diuretic users | % | 2 | 3 | 4 | 6 | <0.001 |
| Systolic blood pressure in mm Hg | (mean ± SD) | 114±13 | 119±15 | 123±15 | 129±17 | <0.001 |
| Diastolic blood pressure in mm Hg | (mean ± SD) | 73±9 | 77±10 | 79±10 | 84±11 | <0.001 |
| Fasting glucose (mg/dl) | (mean ± SD) | 93±17 | 99±23 | 104±33 | 108±32 | <0.001 |
| Total Cholesterol (mg/dl) | (mean ± SD) | 187±37 | 191±38 | 199±41 | 205±40 | <0.001 |
| LDL cholesterol (mg//dl) | (mean ± SD) | 115±33 | 121±35 | 130±36 | 131±35 | <0.001 |
| HDL cholesterol (mg/dl) | (mean ± SD) | 57±14 | 53±15 | 48±13 | 45±13 | <0.001 |
| Triglycerides (mg/dl) | (mean ± SD) | 101±69 | 112±73 | 131±107 | 163±162 | <0.001 |
| Serum uric acid (mg/dl) | (mean ± SD) | 3.7±0.5 | 4.8±0.3 | 5.7±0.3 | 7.2±0.8 | |
| Valvular heart disease | % | 8.3 | 7.7 | 6.0 | 7.0 | 0.16 |
| History of gout at baseline | % | 0.1 | 0.6 | 0.3 | 6.9 | <0.001 |
| History of diabetes at baseline | % | 0.9 | 1.8 | 2.7 | 2.3 | 0.008 |
Valvular heart disease was defined for this study as presence of cardiac murmur at baseline. Gout was determined based on physician diagnosis. Diabetes was defined using the American Diabetes Association Criteria and/or use of anti-diabetes medications.
Over the follow-up period, the cumulative incidence of gout were 12.6% (n=171) and 4.5% (n=192) among heart failure and no heart failure groups respectively (p<0.001). Overall, 155 participants with gout reported using allopurinol during the follow up. Only 2 participants without gout reported using allopurinol.
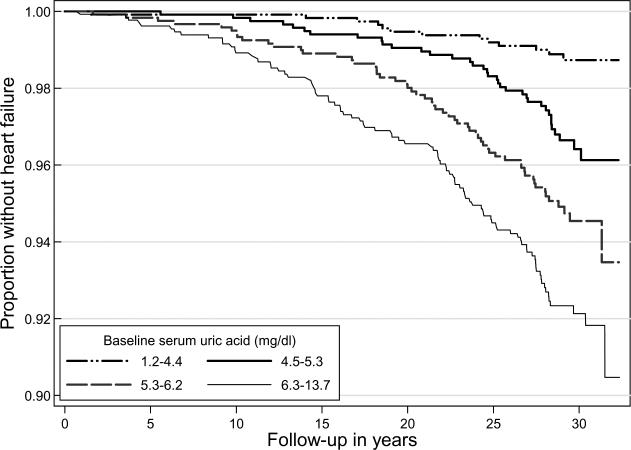
Figure 2 shows the heart failure-free survival curve. Those in the higher quartiles of serum uric acid had greater incidence of heart failure (Table 3). Proportional hazards assumptions were met in all the Cox regression models. In these models, increasing level of serum uric acid was associated with increased risk for heart failure, in unadjusted, and age-sex adjusted models (Table 3). In multivariable regressions, the increased risk relationship between uric acid level and heart failure was most evident in the highest quartile.
Figure 2.
Kaplan-Meier estimates for heart failure-free follow-up among the 4989 participants of the Framingham Offspring Study by quartiles of serum uric acid. For this survival model, the observation started at the first study visit and ended at the time of incident heart failure (n=201). Note that the Y axis scale is adjusted for the sake of clarity.
Table 3.
Incident heart failure according to baseline uric acid quartiles in Framingham Offspring Cohort (n=4912)
| Characteristic | 1.2–4.34 mg/dl (n=1164) | 4.35–5.2 mg/dl (n=1193) | 5.3–6.2 mg/dl (n=1227) | 6.3–13.7 mg/dl (n=1328) | P value for trend |
|---|---|---|---|---|---|
| Number of incident heart failure | 13 | 36 | 57 | 90 | |
| Rate per 10,000 person years (95% CI) | 3.9(2.3–6.8) | 10.8(1.8–15.0) | 17.3(13.4–22.4) | 25.8(21.0–31.7) | <0.001* |
| Unadjusted hazard ratio | 1.00 | 2.8(1.5–5.3) | 4.5(2.5–8.3) | 7.0(3.9–12.4) | <0.001 |
| Age-sex adjusted hazard ratio | 1.00 | 1.9(1.0–3.7) | 2.6(1.3–4.9) | 3.3(1.7–6.3) | <0.001 |
| Model 1: Multivariable-adjusted hazard ratio with baseline values of covariates | 1.00 | 1.6(0.8–3.2) | 1.7(0.9–3.3) | 2.1(1.0–4.2) | 0.007 |
| Model 2: Multivariable-adjusted hazard ratio with time-varying values of covariates | 1.00 | 1.6(0.7–3.5) | 2.1(0.9–4.0) | 2.3(1.0–5.1) † | <0.01 |
| Model 3: Multivariable-adjusted hazard ratio with time-varying values of covariates among the sub group without metabolic syndrome | 1.00 | 1.5(0.6–3.5) | 2.0(0.8–4.8) | 2.5(1.0–6.2) ‡ | <0.01 |
CI: Confidence interval ;Multivariable models were adjusted for : sex, baseline values of age , smoking systolic blood pressure serum total cholesterol: high density lipoprotein ratio, alcohol use renal dysfunction, coronary artery disease, valvular heart disease, diuretic use and non-diuretic blood pressure medications
Age-sex adjusted trend confidence interval
(1.01–5.10)
confidence interval (1.04–6.18)
In this cohort of relatively young adults (median baseline age 36 years, inter-quartile range 28–44), the prevalence of documented coronary artery disease at baseline was infrequent (n=6) and exclusion of these individuals did not change our overall risk estimate. There were no participants with renal dysfunction at the baseline. Multivariable Cox regressions were performed for each of the following sub groups: participants who did not use diuretics, any blood pressure medications, non-diabetics, participants who did not develop renal dysfunction anytime during follow up, and those who did not develop the metabolic syndrome (Table 4). The link between hyperuricemia and heart failure was consistent across all these analyses.
Table 4.
Multivariable adjusted risk of heart failure among various sub-groups of the Framingham Offspring Cohort
| Number of participants in the model | Hazard ratio for each mg/dl increase in serum uric acid | 95% confidence interval | |
|---|---|---|---|
| No renal dysfunction anytime during the observation | 3587 | 1.3 | 1.10–1.50 |
| Non-uses of any non-diuretic blood pressure medications | 3677 | 1.20 | 1.01–1.44 |
| Non-users of diuretics | 3639 | 1.20 | 1.00–1.41* |
| Non diabetics | 3517 | 1.28 | 1.01–1.52 |
| No metabolic syndrome at study end-date | 3765 | 1.26 | 1.07–1.47 |
p=0.048. Unless specified otherwise, multivariable models were adjusted for : sex, time varying measures of age , smoking systolic blood pressure serum total cholesterol: high density lipoprotein ratio, alcohol use renal dysfunction, coronary artery disease, valvular heart disease, diuretic use and non-diuretic blood pressure medications
Analyses for survivor effect
When the multivariable analyses were repeated separately among the 892 participants who died during follow-up from any cause and those who survived until the 7th visit, each unit increase in serum uric acid increased the risk for incident heart failure for the deceased (n=125, hazard ratio 1.2(0.9–1.5)) and survivors (n=76, hazard ratio 1.1(0.9–1.5)) although neither reached statistical significance.
Gender effects
In multivariable regressions, the impact of such statistical interaction was tested for but was found to be statistically insignificant (p=0.21). Further, when data were analyzed for men and women separately, the risk estimates were greater than unity but not statistically significant in both groups owing to small number of events in each.
Discussion
We report for the first time that hyperuricemia is a risk factor for heart failure in a large prospective study of a community dwelling population. Similar to the Vorarlberg study, this risk was most evident at serum uric acid levels greater than ~6 mg/dl- a cut-off point close to the solubility of urate in the normal human body17.
Our observation is not unexpected given the knowledge about the significance of hyperuricemia as a marker of abnormal oxidative metabolism29. Serum uric acid level is an index of oxidative stress in the human body 30. Serum uric acid is known to contribute to endothelial dysfunction by impairing nitric oxide production31. Serum uric acid has also been shown to be inversely correlated with the measures of functional capacity and maximal oxygen intake5. Among patients with chronic heart failure, serum uric acid concentrations are associated with greater activity of superoxide dismutase and endothelium dependent vasodliatation32.
Another potential pathophysiological link between hyperuricemia and heart failure might be through inflammation. Asymptomatic hyperuricemia is a pro-inflammatory state associated with higher levels of serum markers of inflammation, such as CRP, interleukin-6, and neutrophil count 31, 33 34. Among patients with heart failure, hyperuricemia is associated with higher levels of markers of endothelial activation such as the soluble intercellular adhesion molecule(ICAM)-1 and inflammatory markers such as interleukin-6, tumor necrosis factor- α and its receptors 12. Similar observations have been made in other population-based studies 35 and hospital-based studies 11, 12. The risk of heart failure was proportionate to the degree of elevation of serum uric acid among patients with gout 36. Locally, even when there is no active arthritis, the synovial fluid of patients with gout show low grade inflammatory activity37.
Elevated levels of serum uric acid among normal individuals predict hypertension38 38, 39, renal dysfunction 25 coronary artery disease22, and portends reduced life expectancy40. Lowering of serum uric acid with allopurinol can reduce blood pressure among hypertensives41, 42. This raises the possibility of the hyperuricemia-heart failure link being mediated by hypertension, a hypothesis that cannot be directly tested in observational studies such as ours. Nevertheless, other studies have shown that hyperuricemia is an independent risk factor for heart failure among those who already have hypertension 16. In our study, this link was consistently observed in a) time-varying Cox models where incident hypertension was adjusted for and b) in stratified analyses of participants who did not develop hypertension.
The significance of our observation lies in its use for developing a risk prediction rule for heart failure. While observations we have made raise the possibility of primary prevention of heart failure, the literature is conflicting on whether a reduction in serum uric acid will result in measurable clinical benefit among those with established heart failure43, 44. Some even argue that increased serum uric acid cause by diuretic use might have a beneficial role in itself 44. On the other hand, the uricosuric property of Losartan, an antihypertensive has been thought to have a beneficial effect among patients with hypertension and left ventricular hypertrophy in the LIFE study45. The putative mechanisms by which uric acid reduction treatments have shown benefit is also unclear. Specifically, it is unclear if the observed benefit from the use of Xanthine Oxidase inhibitors is mediated through reduction in serum uric acid levels or some other mechanism. Inhibition of Xanthine Oxidase enzyme by allopurinol has beneficial effects in terms of improved peripheral vasodilator capacity, systemic blood flow, and clinical outcomes 46 47. Randomized controlled studies have also been unclear about the putative benefit of allopurinol or its metabolite oxypurinol on established heart failure. While La Plata study showed improvement in left ventricular ejection fraction with use of allopurinol48, the OPT-CHF study did not show an overall benefit49. In our study, the majority of patients with gout were treated with allopurinol; the number of participants with gout but not on allopurinol was too few for meaningful comparison. If indeed allopurinol is protective from heart failure, the excess risk for serum uric acid we have found is likely to be an underestimate.
Limitations apply to our analysis. Our observational data on serum uric acid are essentially left-truncated. In other words, we know the severity of hyperuricemia but not the duration of hyperuricemia. Additionally, the long interval between follow-up visits (~4 years) may be too long to capture heart failure that results in death in shorter time. : The distribution of serum uric acid concentrations among men and women were different, the former having higher concentrations. Thus the lowest quartile of the pooled data was constituted mainly by women and the highest quartile by men. The gender-uric acid statistical interaction was insignificant, but limitations in statistical power precluded a more detailed analysis.
In summary, this large prospective study found that hyperuricemia is associated with greater incidence of heart failure. Future studies of various urate reduction strategies with adequate power to detect small improvement in clinical outcomes would be needed to determine whether, if at all, heart failure is preventable. Given the increasing prevalence and serious health impact of heart failure, even such small clinical benefit can translate into substantial public health benefit.
Hyperuricemia and incident heart failure.
Heart failure is an incurable condition that is responsible for at least 287,000 deaths annually. Heart failure is the most common reason for hospitalization among people on Medicare and the number of hospitalizations have been increasing over time. We propose that hyperuricemia is a useful biomarker for estimating risk for heart failure and tested this hypothesis using the data from Framingham Offspring Study. We observed that the incidence of heart failure among those with serum uric acid concentrations >6.3 mg/dl was 6 fold higher than that among participants with serum uric acid <3.4 mg/dl. The adjusted risk for heart failure was double among those with hyperuricemia. Our findings have implications for early identification of those at risk for heart failure and put forward a new target for intervention.
Acknowledgments
Funding Sources: The Framingham Offspring Study (FOS) is conducted and supported by the National Heart Lung Blood Institute (NHLBI) in collaboration with the FOS Study Investigators. This manuscript was prepared using a limited access dataset Dr. Krishnan obtained from the NHLBI and does not necessarily reflect the opinions or views of the FOS or the NHLBI. Dr Krishnan conceived the manuscript idea, designed the analysis plan, performed statistical analysis, interpreted the results, drafted the manuscript and will serve as the guarantor.
This publication was made possible by in part Grant Number KL2 RR024154-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NHLBI or the NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. No commercial products are discussed in this manuscript.
Disclosures Dr. Krishnan has received grant support from Takeda Pharmaceuticals of North America Inc. Deerfield, IL. (formerly TAP Pharmaceutical Products, Inc) and had held stock in Savient Pharmaceuticals. He has served as an advisor/consultant for both these companies. Proprietary products manufactured by these companies are not named/discussed in this manuscript.
References
- 1.Heart disease and stroke statistics-2006 update. American Heart Association; Dallas, TX: 2006. [DOI] [PubMed] [Google Scholar]
- 2.Gwadry-Sridhar FH, Flintoft V, Lee DS, Lee H, Guyatt GH. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Narula J, Vasan RS. Risk factors for heart failure. Med Clin North Am. 2004;88:1145–1172. doi: 10.1016/j.mcna.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RD, Newill A, Morgan DB. The cause of the raised plasma urea of acute heart failure. Postgrad Med J. 1979;55:10–14. doi: 10.1136/pgmj.55.639.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJ. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18:858–865. doi: 10.1093/oxfordjournals.eurheartj.a015352. [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Hohlfeld JM, Fabel H. Hyperuricaemia in patients with right or left heart failure. Eur Respir J. 1999;13:682–685. doi: 10.1183/09031936.99.13368299. [DOI] [PubMed] [Google Scholar]
- 7.Pascual-Figal DA, Hurtado-Martinez JA, Redondo B, Antolinos MJ, Ruiperez JA, Valdes M. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail. 2007;9:518–524. doi: 10.1016/j.ejheart.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 9.Niizeki T, Takeishi Y, Arimoto T, Okuyama H, Nozaki N, Hirono O, Tsunoda Y, Watanabe T, Nitobe J, Miyashita T, Takahashi H, Koyama Y, Kubota I. Hyperuricemia associated with high cardiac event rates in the elderly with chronic heart failure. J Cardiol. 2006;47:219–228. [PubMed] [Google Scholar]
- 10.Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Yamagishi M, Tei C, Hiraoka H, Sonoda M, Tsuchihashi K, Shimoyama N, Honda T, Ogata Y, Matsui K, Ogawa H. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study) Am J Cardiol. 2005;96:489–495. doi: 10.1016/j.amjcard.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Olexa P, Olexova M, Gonsorcik J, Tkac I, Kisel'ova J, Olejnikova M. Uric acid--a marker for systemic inflammatory response in patients with congestive heart failure? Wien Klin Wochenschr. 2002;114:211–215. [PubMed] [Google Scholar]
- 12.Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- 13.Doehner W, von Haehling S, Anker SD. Uric acid as a prognostic marker in acute heart failure--new expectations from an old molecule. Eur J Heart Fail. 2007;9:437–439. doi: 10.1016/j.ejheart.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Kittleson MM, John ME, St, Bead V, Champion HC, Kasper EK, Russell SD, Wittstein IS, Hare JM. Increased levels of uric acid predict haemodynamic compromise in patients with heart failure independently of B-type natriuretic peptide levels. Heart. 2007;93:365–367. doi: 10.1136/hrt.2006.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation. 2003;107:1951–1953. doi: 10.1161/01.CIR.0000066420.36123.35. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsson O, Wilhelmsen L, Pennert K, Berglund G. Angina pectoris, intermittent claudication and congestive heart failure in middle-aged male hypertensives. Development and predictive factors during long-term antihypertensive care. The Primary Preventive Trial, Goteborg, Sweden. Acta Med Scand. 1987;221:23–32. [PubMed] [Google Scholar]
- 17.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, Diem G, Pfeiffer K, Ulmer H. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 2008;54:273–284. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 19.Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol. 1988;41:237–242. doi: 10.1016/0895-4356(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 22.Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005;118:816–826. doi: 10.1016/j.amjmed.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54:2688–2696. doi: 10.1002/art.22014. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 25.Avram Z, Krishnan E. Hyperuricaemia--where nephrology meets rheumatology. Rheumatology (Oxford) 2008;47:960–964. doi: 10.1093/rheumatology/ken070. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan E. Gout and coronary artery disease: epidemiologic clues. Curr Rheumatol Rep. 2008;10:249–255. doi: 10.1007/s11926-008-0039-0. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–440. doi: 10.1016/j.amjhyper.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Alcaino H, Greig D, Chiong M, Verdejo H, Miranda R, Concepcion R, Vukasovic JL, Diaz-Araya G, Mellado R, Garcia L, Salas D, Gonzalez L, Godoy I, Castro P, Lavandero S. Serum uric acid correlates with extracellular superoxide dismutase activity in patients with chronic heart failure. Eur J Heart Fail. 2008;10:646–651. doi: 10.1016/j.ejheart.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF, 2nd, Kullo IJ. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Lauretani F, Bandinelli S, Senin U, Ferrucci L. Uric acid and inflammatory markers. Eur Heart J. 2006;27:1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 36.Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, Nuki G. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67:960–966. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual E. Persistence of monosodium urate crystals and low-grade inflammation in the synovial fluid of patients with untreated gout. Arthritis Rheum. 1991;34:141–145. doi: 10.1002/art.1780340203. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan E, Kwoh C, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrom. Hypertension. 2007:1–2. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 39.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 40.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, Odaka M, Yamaguchi M, Yosida H, Morisawa H, Murayama T. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–409. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 41.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 42.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doehner W, Anker SD. Uric acid in chronic heart failure. Semin Nephrol. 2005;25:61–66. doi: 10.1016/j.semnephrol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Reyes AJ. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur J Heart Fail. 2005;7:461–467. doi: 10.1016/j.ejheart.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlof B. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–1049. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 46.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 47.Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart. 2002;87:229–234. doi: 10.1136/heart.87.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail. 2006;12:491–498. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Cleland JG, Coletta AP, Clark AL. Clinical trials update from the Heart Failure Society of America meeting: FIX-CHF-4, selective cardiac myosin activator and OPT-CHF. Eur J Heart Fail. 2006;8:764–766. doi: 10.1016/j.ejheart.2006.10.001. [DOI] [PubMed] [Google Scholar]