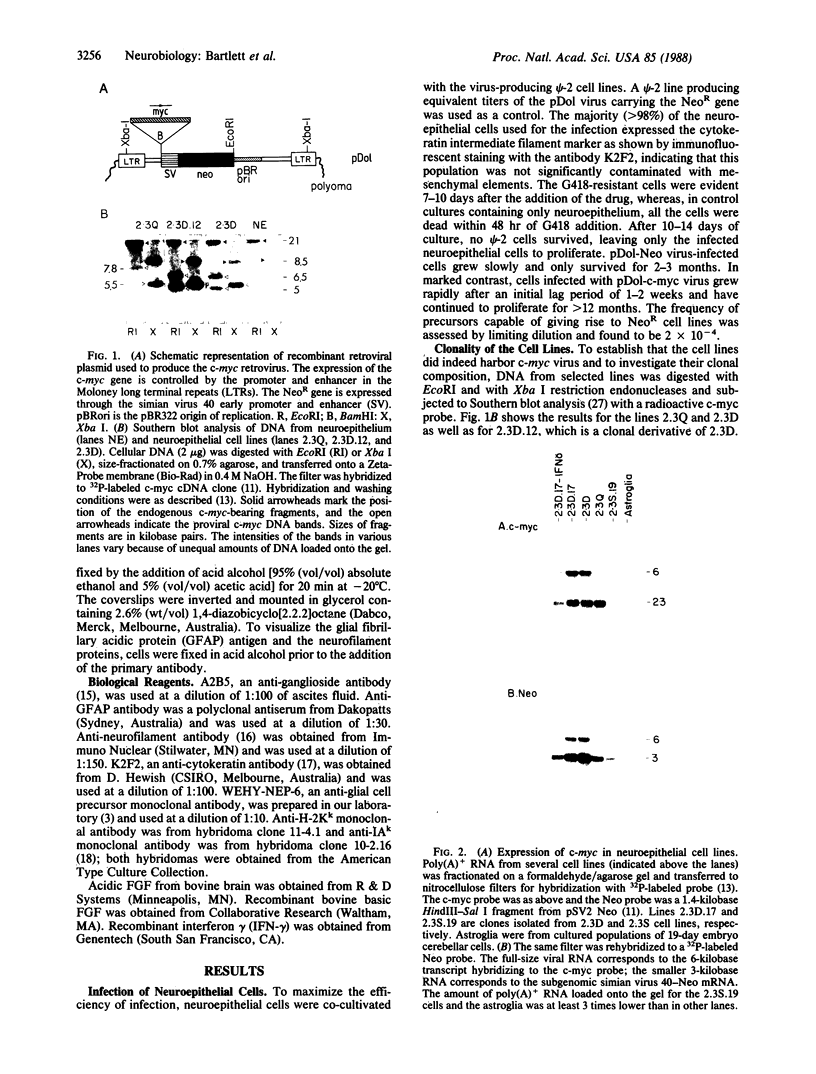
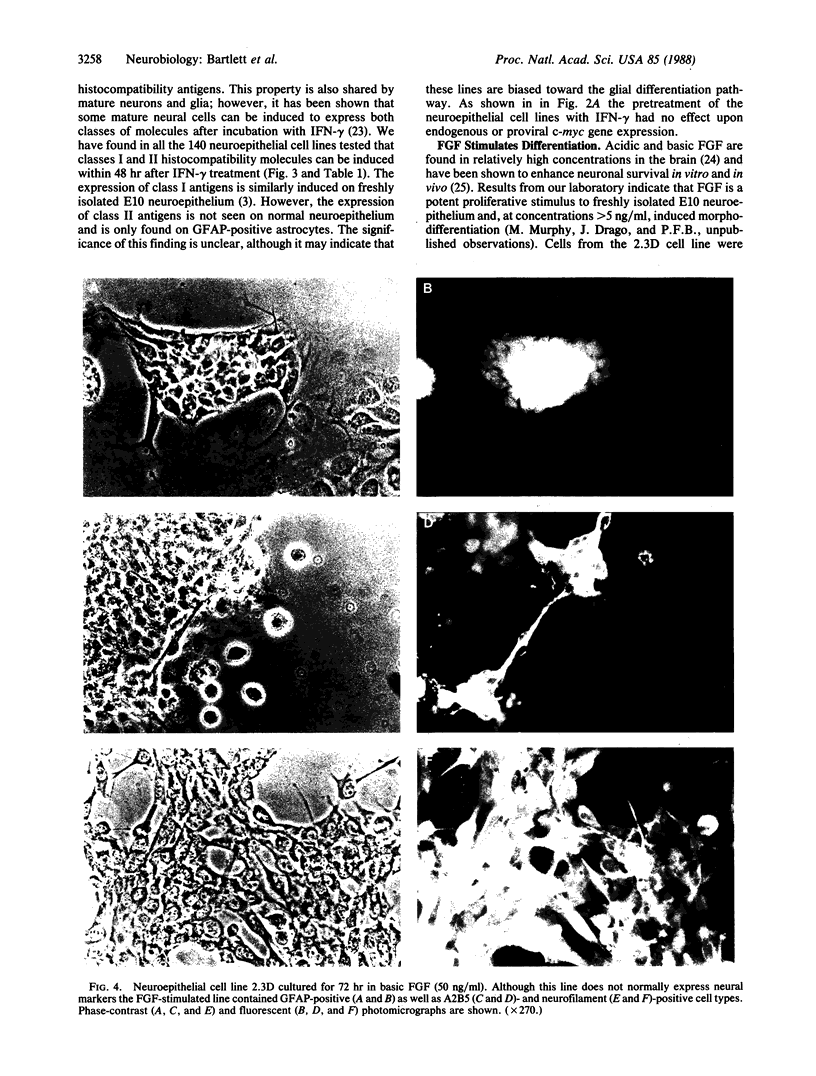
Abstract
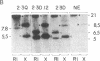
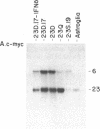
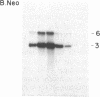
Immortalized cell lines have been generated from embryonic mouse neuroepithelium by infection with a retrovirus containing the c-myc oncogene. The morphology and the antigenic phenotype of the cloned cell lines are characteristic of normal neuroepithelium. Although the cell lines are stable and do not spontaneously differentiate, morphological changes can be induced with both acidic and basic fibroblast growth factor. Fibroblast growth factor at 5 ng/ml stimulates differentiation of the neuroepithelial cells, and it has been shown that the cloned cell line 2.3D can differentiate into astrocytes, containing glial fibrillary acidic protein, and neurons, expressing the A2B5 marker and neurofilaments. This indicates that some cells in the neuroepithelium at embryonic day 10 are multipotent and are not restricted to either the glial or neuronal cell lineage. The cell lines also can be induced with interferon gamma to express class I and class II histocompatibility antigens. The response of the c-myc-immortalized cell lines to these two factors is similar to that observed with freshly isolated neuroepithelium and suggests that such immortalized precursor populations are representative of the cells found in the developing neuroepithelium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Bartlett P. P., Raff M. C. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev Biol. 1981 Apr 30;83(2):301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Alexander W. S., Schrader J. W., Adams J. M. Expression of the c-myc oncogene under control of an immunoglobulin enhancer in E mu-myc transgenic mice. Mol Cell Biol. 1987 Apr;7(4):1436–1444. doi: 10.1128/mcb.7.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Casalbore P., Agostini E., Alemà S., Falcone G., Tatò F. The v-myc oncogene is sufficient to induce growth transformation of chick neuroretina cells. Nature. 1987 Mar 12;326(6109):188–190. doi: 10.1038/326188a0. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Bernard O., Bowtell D., Schrader S., Schrader J. W. Murine c-myc retroviruses alter the growth requirements of myeloid cell lines. Oncogene Res. 1987 Jun;1(1):61–76. [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Edwards D. L., Kleinschmidt J. Neurofilaments contain alpha-melanocyte-stimulating hormone (alpha-MSH)-like immunoreactivity. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6408–6412. doi: 10.1073/pnas.80.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French P. W., Hewish D. R. Localization of low-sulfur keratin proteins in the wool follicle using monoclonal antibodies. J Cell Biol. 1986 Apr;102(4):1412–1418. doi: 10.1083/jcb.102.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keath E. J., Caimi P. G., Cole M. D. Fibroblast lines expressing activated c-myc oncogenes are tumorigenic in nude mice and syngeneic animals. Cell. 1984 Dec;39(2 Pt 1):339–348. doi: 10.1016/0092-8674(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Sharma A., de Vellis J., Bradshaw R. A. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougneau E., Lemieux L., Rassoulzadegan M., Cuzin F. Biological activities of v-myc and rearranged c-myc oncogenes in rat fibroblast cells in culture. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5758–5762. doi: 10.1073/pnas.81.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]