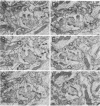
Abstract
The "kindling" phenomenon is associated with long-lasting facilitation of synaptic transmission. A possible mechanism of such facilitation could involve changes in the number of synaptic contacts. However, previous attempts to demonstrate a synaptic morphological alteration that could account for the long-term effects of kindling had failed, possibly due to the unavailability, at the time, of unbiased methods for synapse quantitation. Using the unbiased stereological disector technique, we estimated the number of synapses per neuron in the middle molecular layer of the hippocampal dentate gyrus in rats kindled by electrical stimulation of the medial perforant path with implanted electrodes. Unkindled but stimulated (coulombic control) and unstimulated but implanted rats served as controls. Animals were coded and killed 4 weeks after reaching the kindling criterion of five generalized seizures. The most important results were obtained when axospinous synapses with continuous or discontinuous postsynaptic densities ("nonperforated" or "perforated" synapses) were differentially analyzed. Kindling resulted in a selective loss of nonperforated synaptic contacts in contrast to preservation of perforated ones. Furthermore, the ratio of perforated to nonperforated synapses was increased by 45% or 40% in kindled rats relative to unstimulated or coulombic controls, respectively. These findings suggest that synaptic efficacy may depend on a balance of the two synaptic types; selective elimination of nonperforated synapses may augment the potency of remaining synaptic contacts, a process reminiscent of synaptic remodeling during development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Blackstad T. W., Lömo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1(3):236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Excitatory synapses on hippocampal apical dendrites activated by entorhinal stimulation. Acta Physiol Scand. 1966 Apr;66(4):461–472. doi: 10.1111/j.1748-1716.1966.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Anderson P., Lomo T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp Brain Res. 1966;2(3):247–260. [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendgaard H., Gundersen H. J. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986 Oct;18(1-2):39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. Changes in the numerical density of synaptic contacts with long-term potentiation in the hippocampal dentate gyrus. J Comp Neurol. 1986 Nov 22;253(4):466–475. doi: 10.1002/cne.902530404. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., de Toledo-Morrell L., Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986 Nov 29;398(2):266–275. doi: 10.1016/0006-8993(86)91486-1. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., de Toledo-Morrell L., Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986 May;83(9):3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard G. V. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967 Jun 3;214(5092):1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goddard G. V., Douglas R. M. Does the engram of kindling model the engram of normal long term memory? Can J Neurol Sci. 1975 Nov;2(4):385–394. doi: 10.1017/s0317167100020539. [DOI] [PubMed] [Google Scholar]
- Goddard G. V., McIntyre D. C., Leech C. K. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969 Nov;25(3):295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., West R. W., DeVoogd T. J. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978 Dec 8;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J Comp Neurol. 1972 Oct;146(2):219–232. doi: 10.1002/cne.901460206. [DOI] [PubMed] [Google Scholar]
- Jonec V., Walsterlain C. G. Effect of inhibitors of protein synthesis on the development of kindled seizures in rats. Exp Neurol. 1979 Dec;66(3):524–532. doi: 10.1016/0014-4886(79)90199-7. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- McNamara J. O., Byrne M. C., Dasheiff R. M., Fitz J. G. The kindling model of epilepsy: a review. Prog Neurobiol. 1980;15(2):139–159. doi: 10.1016/0301-0082(80)90006-4. [DOI] [PubMed] [Google Scholar]
- Morrell F., Tsuru N., Hoeppner T. J., Morgan D., Harrison W. H. Secondary epileptogenesis in frog forebrain: effect of inhibition of protein synthesis. Can J Neurol Sci. 1975 Nov;2(4):407–416. doi: 10.1017/s0317167100020552. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Hoff S. F., Cotman C. W. Perforated postsynaptic densities: probable intermediates in synapse turnover. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5718–5722. doi: 10.1073/pnas.79.18.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969 Sep 22;100(4):487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Racine R., Tuff L., Zaide J. Kindling, unit discharge patterns and neural plasticity. Can J Neurol Sci. 1975 Nov;2(4):395–405. doi: 10.1017/s0317167100020540. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Bradburne R. M., Harris A. B. A preferential loss of GABAergic, symmetric synapses in epileptic foci: a quantitative ultrastructural analysis of monkey neocortex. J Neurosci. 1982 Dec;2(12):1725–1735. doi: 10.1523/JNEUROSCI.02-12-01725.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P. The postsynaptic density: a possible role in long-lasting effects in the central nervous system. Proc Natl Acad Sci U S A. 1985 May;82(10):3494–3498. doi: 10.1073/pnas.82.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag A. M., Greenough W. T. Differential rearing effects on rat visual cortex synapses. II. Synaptic morphometry. Brain Res. 1985 Apr;351(2):215–226. doi: 10.1016/0165-3806(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987 Jan 2;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Sutula T., Steward O. Facilitation of kindling by prior induction of long-term potentiation in the perforant path. Brain Res. 1987 Sep 8;420(1):109–117. doi: 10.1016/0006-8993(87)90245-9. [DOI] [PubMed] [Google Scholar]
- Sutula T., Steward O. Quantitative analysis of synaptic potentiation during kindling of the perforant path. J Neurophysiol. 1986 Sep;56(3):732–746. doi: 10.1152/jn.1986.56.3.732. [DOI] [PubMed] [Google Scholar]
- Thompson W. J. Activity and synapse elimination at the neuromuscular junction. Cell Mol Neurobiol. 1985 Jun;5(1-2):167–182. doi: 10.1007/BF00711091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuff L. P., Racine R. J., Adamec R. The effects of kindling on GABA-mediated inhibition in the dentate gyrus of the rat. I. Paired-pulse depression. Brain Res. 1983 Oct 24;277(1):79–90. doi: 10.1016/0006-8993(83)90909-5. [DOI] [PubMed] [Google Scholar]
- Vrensen G., Cardozo J. N. Changes in size and shape of synaptic connections after visual training: an ultrastructural approach of synaptic plasticity. Brain Res. 1981 Aug 10;218(1-2):79–97. doi: 10.1016/0006-8993(81)90990-2. [DOI] [PubMed] [Google Scholar]