Abstract
Summary
Osteoporotic post-menopausal women patients in two randomised trials comparing the anti-fracture efficacy of strontium ranelate with placebo were separated into tertiles according to their baseline levels of biochemical markers of bone formation and resorption. The vertebral anti-fracture efficacy of strontium ranelate was shown to be independent of baseline bone turnover levels.
Introduction
Bone turnover (BTO) levels vary among women at risk of osteoporotic fracture. Strontium ranelate is an anti-osteoporotic treatment increasing bone formation and reducing bone resorption. It was hypothesised that its anti-fracture efficacy would be independent of baseline BTO levels.
Methods
Post-menopausal women with osteoporosis from two pooled studies were stratified in tertiles according to baseline levels of two BTO markers: bone-specific alkaline phosphatase (b-ALP, n = 4995) and serum C-telopeptide cross-links (sCTX, n = 4891). Vertebral fracture risk was assessed over 3 years with strontium ranelate 2 g/day or placebo.
Results
In the placebo group, relative risk of vertebral fractures increased with BTO tertiles by 32% and 24% for patients in the highest tertile for b-ALP and CTX, respectively, compared to those in the lowest tertile. In the strontium ranelate group, incidences of vertebral fracture did not differ significantly across BTO tertiles. Significant reductions in vertebral fractures with strontium ranelate were seen in all tertiles of both markers, with relative risk reductions of 31% to 47% relative to placebo. Risk reduction did not differ among tertiles (b-ALP: p = 0.513; sCTX: p = 0.290).
Conclusion
The vertebral anti-fracture efficacy of strontium ranelate was independent of baseline BTO levels. Strontium ranelate offers clinical benefits to women across a wide range of metabolic states.
Keywords: Anti-fracture efficacy, Biochemical marker, Bone turnover, Osteoporosis, Strontium ranelate, Vertebral fracture
Introduction
Osteoporosis is a complex disease, and many factors may contribute to the skeletal fragility that underlies osteoporotic fractures [1]. Two processes are thought to be particularly important in post-menopausal osteoporosis. First, during adult life, in both men and women, resorption of bone tends to exceed bone formation at each of the basic multicellular units that are responsible for bone remodelling. Secondly, relative oestrogen deficiency in women after the menopause increases the rate of bone remodelling, accelerating the net loss of bone [2, 3]. During long-term treatment, anti-resorptive anti-osteoporotic agents act primarily by decreasing the rate of bone remodelling [4]. For example, during treatment with the bisphosphonate alendronate, some biochemical markers of bone resorption show a rapid decrease of 50% to 65% within 1 month of treatment. However, this is accompanied by a delayed decrease in markers of bone formation of approximately 50%, which reaches a nadir between 6 and 12 months [5]. It might be predicted that baseline bone turnover rates could influence the effects of treatment with anti-resorptive and other anti-osteoporotic agents. For example, anti-resorptive agents might be expected to be of greatest benefit to women with high levels of bone turnover, while bone formation agents might be most effective in women with low rates of bone formation.
Studies of the effects of anti-resorptive agents on bone mineral density (BMD) have generally shown larger treatment effects in women with high pre-treatment levels of bone turnover [6–10]. However, results for osteoporotic fracture risk have been less consistent [11, 12]. The effects of teriparatide, an agent that increases bone formation, on BMD were also greater in women with high bone turnover [13], but the reduction in the relative risk of osteoporotic fracture was independent of the pre-treatment bone turnover level [14].
Strontium ranelate is an oral anti-osteoporotic agent that reduces the risk of vertebral [15], non-vertebral and hip [16] fractures in post-menopausal osteoporotic women. Experiments in vitro and in animals [17, 18], as well as measurements of biochemical markers of bone turnover in osteoporotic women in a clinical trial [15], have shown that strontium ranelate simultaneously stimulates bone formation and reduces bone resorption, although individual effects are less pronounced than those induced by PTH or bisphosphonates. Two previous analyses have demonstrated that strontium ranelate reduces the risk to have a new vertebral fracture in patients with a wide range of osteoporosis severity: in osteopenic patients with and without previous fractures, in osteoporotic patients without prevalent vertebral fractures and in severe osteoporotic patients (at least two prevalent vertebral fractures) [19, 20]. The purpose of the present study was to determine whether the efficacy of strontium ranelate in increasing lumbar BMD and reducing vertebral fracture risk in post-menopausal women is influenced by the pre-treatment level of biochemical markers of bone turnover, using data obtained over 3 years in two large placebo-controlled clinical trials, the Spinal Osteoporosis Therapeutic Intervention (SOTI) study [15] and the Treatment of Peripheral Osteoporosis (TROPOS) study [16]. Given the specific effects on bone turnover and its wide efficacy profile to date, we hypothesise that its efficacy would be independent of pre-treatment bone turnover levels.
Methods
The present analysis is based on pooled data on vertebral fractures and markers of pre-treatment bone turnover taken from two randomised, double-blind, placebo-controlled, international studies in post-menopausal women with osteoporosis, that demonstrated the anti-fracture efficacy of strontium ranelate 2 g/day. The SOTI study [15] was aimed at vertebral anti-fracture efficacy, and the TROPOS study [16] was aimed at peripheral (non-vertebral) fractures. However, vertebral fractures were evaluated in TROPOS as a pre-specified secondary endpoint in those women who had a spinal radiograph at baseline and at least one post-baseline.
Patients
Patients for both the SOTI and TROPOS studies were included initially in a common, open-label run-in study, the FIRST study [21]. Detailed inclusion criteria have been published previously [15, 16, 21]. In brief, for inclusion in FIRST, women had to be Caucasian, post-menopausal for at least 5 years, ambulatory and considered to be osteoporotic and at high risk of fracture. During FIRST, the calcium and vitamin D status of all women was assessed, and they were given daily supplements of up to 1,000 mg of elemental calcium and up to 800 IU of vitamin D for a period of 2 weeks to 6 months. Supplementation doses and duration were adjusted for each patient according to their baseline calcium and 25-OH vitamin D status. After the run-in period, eligible women were proposed for enrolment in either the SOTI or TROPOS studies, and supplementation was continued at the same doses throughout the randomised treatment periods of both these studies. The SOTI study included women ≥50 years of age with low lumbar BMD (<0.840 g/cm2 measured with Hologic instruments, T-score ≤−2.4) and at least one prevalent vertebral fracture confirmed by spinal radiography. The TROPOS study included women with femoral neck BMD <0.600 g/cm2 and aged ≥74 years or 70–74 years with one additional risk factor (history of osteoporotic fracture after menopause, residence in a retirement home, frequent falls or maternal history of osteoporotic fracture of the hip, spine or wrist).
Study design and efficacy measurements
Patients were randomised to receive strontium ranelate 2 g/day or placebo for 5 years (TROPOS) or 4 years followed by a 1-year treatment-switch period (SOTI). In both studies, main efficacy analyses were performed at 3 years, and the vertebral fracture data over 3 years were used for the present analysis. Baseline refers to the commencement of the SOTI and TROPOS studies, not the time of inclusion in FIRST.
Vertebral fractures were determined from radiographs taken at baseline and annually thereafter and were analysed in the same way in both studies. Radiographs were analysed by the semi-quantitative method of Genant et al. [22, 23], using a four-point grading scale: grade 0—normal; grade 1—mild deformity (20–25% decrease in at least one vertebral height); grade 2—moderate deformity (25–40% decrease); and grade 3—severe deformity (>40% decrease). A new vertebral fracture was defined as a change from a non-fractured vertebra (grade 0) to a vertebra rated grade 1 or higher. All radiographs were analysed at a central facility (CEMO, France) blinded to treatment assignment but not to temporal sequence.
Lumbar L2–4 and femoral neck BMD were measured at baseline, and lumbar BMD was measured every 6 months post-baseline by dual-energy X-ray absorptiometry using Hologic devices. All scans were analysed centrally, and a programme of cross-calibration across centres was performed throughout both studies [24].
Blood samples were collected at baseline, 3 months, 6 months, and then every 6 months. Serum samples were stored at −80°C and analysed centrally after a maximum 6 months period of storage (University of Liège, Belgium). Serum concentration of bone-specific alkaline phosphatase (b-ALP), a marker of bone formation, was measured by immunoradiometric assay (Tandem® Ostase® Beckman Coulter, San Diego, CA, USA). Serum concentration of C-telopeptide cross-links (sCTX), a marker of bone resorption, was measured using an enzyme- linked immunosorbent assay (Serum CrossLaps®ELISA–Nordic Bioscience Diagnostic, formerly Osteometer BioTech, Herlev, Denmark).
All the assays were performed in duplicate per batch of maximum 140 and 86 unknown serum samples for b-ALP and sCTX, respectively. If the CV on the duplicate measurement was higher than 15%, the sample was re-assayed in a run control. In each assay run, two quality control samples (QCs) were assayed before and after the unknown samples. The assay run was validated if the CV on the duplicate measurement of a QC was lower or equal to 15%, if the QCs results were in their respective 2SD ranges determined previously and if the difference between the results obtained before and after the unknown samples did not exceed 15%.
Both clinical studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki, 1964, as revised in Hong Kong, 1989. The study protocol was approved by independent ethics committees in each country and/or centre. All patients gave written informed consent.
Statistical analysis
All analyses were performed in accordance with the intention-to-treat principle: The population included all patients having a baseline and post-baseline lumbar X-ray and having a baseline value for b-ALP or sCTX.
Groups were compared at baseline on the lumbar and femoral BMD and corresponding T-scores using an ANOVA analysis, adjusted or not on age.
Vertebral fracture risk was assessed as the number of patients with at least one new osteoporotic vertebral fracture, analysed by the Kaplan–Meier method. Patients were stratified into tertiles of baseline (pre-treatment) levels of b-ALP and sCTX. The boundaries of the tertiles and the normal ranges for b-ALP and sCTX are given in Table 1. Between-treatment differences in vertebral fracture risk over 3 years for each tertile were assessed using an unadjusted Cox model. Sensitivity analysis was performed using a Cox model adjusted for baseline lumbar BMD.
Table 1.
Tertile boundaries and normal ranges for markers of bone turnover (b-ALP and sCTX)
| Tertile 1 | Tertile 2 | Tertile 3 | |
|---|---|---|---|
| b-ALP (µg/L)a | ≤10.0 | >10.0–≤13.3 | >13.3 |
| sCTX (ng/mL)b | ≤0.423 | >0.423–≤0.626 | >0.626 |
ab-ALP, bone-specific alkaline phosphatase: normal range, 2.9–14.5 µg/L (premenopausal women); 3.8–22.6 µg/L (post-menopausal women)
bsCTX, serum C-telopeptide cross-links: normal range, 0.112–0.323 ng/mL (pre-menopausal women); 0.153–0.625 ng/mL (post-menopausal women)
Further between-treatment comparisons, using the same model, were performed for those patients who were in the lowest tertile for both b-ALP and sCTX (representing patients with the lowest bone turnover) and for patients in the highest tertile for both b-ALP and sCTX (representing those with the highest bone turnover).
Results
Patients
Of the 6,740 patients randomised in the two studies (1,649 in SOTI, 5,091 in TROPOS), 5,082 had a lumbar spinal radiograph at baseline and one or more post-baseline radiograph. Of these patients, 4,955 had a baseline measurement of b-ALP, and 4,891 patients had a baseline measurement of sCTX. These patients were then stratified into tertiles of b-ALP (n = 1,683 in tertile 1, n = 1,642 in tertile 2 and 1,630 in tertile 3) or sCTX (n = 1,631 in tertile 1, n = 1,630 in tertile 2 and n = 1,630 in tertile 3). Baseline characteristics of patients, stratified into tertiles of baseline b-ALP and sCTX, are shown in Tables 2 and 3. The mean age of patients was approximately 74 years. Most variables were similar across tertiles. However, there were significant progressive reductions in lumbar and femoral neck BMD (ANOVA, p < 0.001 for both sites), most obvious in the T-scores, with increasing tertiles of b-ALP and sCTX. There were no other relevant differences in baseline characteristics between tertiles, including in the levels of 25OH vitamin D, creatinine or PTH. Regarding treatment group differences, baseline characteristics were similar in the strontium ranelate and placebo groups regardless of the tertile considered (as an example, lumbar BMD values are described in Table 3).
Table 2.
Patients’ characteristics at baseline by tertiles of b-ALP and sCTX
| Tertile 1 | Tertile 2 | Tertile 3 | |
|---|---|---|---|
| According to b-ALP level | n = 1,683 | n = 1,642 | n = 1,630 |
| Age (years) | 74.5 ± 6.2 | 73.7 ± 6.3 | 73.8 ± 6.0 |
| Lumbar BMD (g/cm2) | 0.792 ± 0.146 | 0.781 ± 0.148 | 0.760 ± 0.149 |
| Lumbar BMD T-score | −2.9 ± 1.5 | −3.0 ± 1.5 | −3.2 ± 1.6 |
| Mean number of prevalent vertebral fractures | 2.5 ± 2.2 | 2.5 ± 2.2 | 2.6 ± 2.3 |
| Femoral neck BMD (g/cm2) | 0.573 ± 0.072 | 0.569 ± 0.073 | 0.560 ± 0.073 |
| Femoral neck T-score | −2.9 ± 0.7 | −3.0 ± 0.7 | −3.1 ± 0.7 |
| Mean number of previous peripheral fractures | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 |
| According to sCTX level | n = 1,631 | n = 1,630 | n = 1,630 |
| Age (years) | 73.6 ± 6.2 | 73.9 ± 6.3 | 74.4 ± 6.0 |
| Lumbar BMD (g/cm2) | 0.798 ± 0.149 | 0.778 ± 0.150 | 0.755 ± 0.145 |
| Lumbar BMD T-score | −2.8 ± 1.5 | −3.0 ± 1.6 | −3.3 ± 1.5 |
| Mean number of prevalent vertebral fractures | 2.6 ± 2.3 | 2.5 ± 2.2 | 2.5 ± 2.2 |
| Femoral neck BMD (g/cm2) | 0.579 ± 0.075 | 0.567 ± 0.070 | 0.556 ± 0.072 |
| Femoral neck T-score | −2.9 ± 0.7 | −3.0 ± 0.6 | −3.1 ± 0.6 |
| Mean number of previous peripheral fractures | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 1.0 |
Expressed as mean ± standard deviation
b-ALP bone-specific alkaline phosphatase, BMD bone mineral density, sCTX serum C-telopeptide cross-links
Table 3.
Lumbar BMD values at baseline by tertiles of b-ALP and sCTX and treatment
| Strontium ranelate | Placebo | |||||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |
| b-ALP | ||||||
| Lumbar BMD (g/cm²) | 0.793 ± 0.140 | 0.781 ± 0.153 | 0.759 ± 0.152 | 0.790 ± 0.153 | 0.781 ± 0.143 | 0.760 ± 0.146 |
| T-score | −2.8 ± 1.5 | −2.9 ± 1.6 | −3.2 ± 1.6 | −2.9 ± 1.6 | −3.0 ± 1.5 | −3.2 ± 1.5 |
| sCTX | ||||||
| Lumbar BMD (g/cm²) | 0.797 ± 0.145 | 0.780 ± 0.153 | 0.755 ± 0.148 | 0.800 ± 0.153 | 0.776 ± 0.146 | 0.755 ± 0.142 |
| T-score | −2.8 ± 0.5 | −3.0 ± 1.6 | −3.3 ± 1.5 | −2.8 ± 1.6 | −3.0 ± 1.5 | −3.3 ± 1.5 |
Expressed as mean ± standard deviation
b-ALP bone-specific alkaline phosphatase, BMD bone mineral density, sCTX serum C-telopeptide cross-links
Vertebral anti-fracture efficacy
The incidence of new vertebral fractures among patients treated with placebo was higher in patients in the highest tertile than in patients in the lowest tertile (26.5% vs 21.1% in b-ALP tertiles 3 and 1, p = 0.010, and 26.3% vs 21.2% in sCTX tertiles 3 and 1, p = 0.043, respectively). Compared with the low turnover group (tertile 1), the relative risk to have a new vertebral fracture in patients with a high bone turnover level was increased over 3 years by 32% when considering b-ALP (RR = 1.32, 95% CI [1.06; 1.62]) and 24% when considering sCTX (RR = 1.24, 95% CI [1.00; 1.54]). This result was confirmed when comparing the incidence of new vertebral fracture in placebo patients in the subset with the lowest tertile for both b-ALP and sCTX with placebo patients in the highest tertile for both b-ALP and sCTX (RR = 1.47, 95% CI [1.08; 1.97], p = 0.012).
Strontium ranelate was associated with a reduction in the relative risk of vertebral fracture, relative to placebo, of 40% (RR = 0.60, 95% CI [0.53–0.70], p < 0.001). When patients were stratified by tertiles of baseline levels of bone turnover markers, significant RR reductions with strontium ranelate were seen in each tertile of b-ALP (31%, 42% and 42% for tertiles 1, 2 and 3, respectively). The same results were observed for tertiles of sCTX, with RR reductions of 37%, 32% and 47% for tertiles 1 to 3, respectively (Table 4, Fig. 1). The magnitudes of the treatment effects were not significantly different between tertiles (interaction test p = 0.513 for b-ALP tertiles, p = 0.290 for sCTX tertiles). Results were similar after adjustment on lumbar BMD.
Table 4.
Incidence of vertebral fracture over 3 years of treatment with strontium ranelate (SR) compared with placebo, according to tertiles of pre-treatment b-ALP and sCTX level
| Tertile 1 | Tertile 2 | Tertile 3 | ||||
|---|---|---|---|---|---|---|
| SR | Placebo | SR | Placebo | SR | Placebo | |
| By b-ALP level | ||||||
| Eventsa | 114 | 155 | 107 | 175 | 115 | 203 |
| Incidence (%) | 14.9 | 21.1 | 14.3 | 23.7 | 16.4 | 26.5 |
| Relative risk [95% CI] | 0.69 [0.54; 0.88] | 0.58 [0.46; 0.74] | 0.58 [0.46; 0.73] | |||
| p value | 0.003 | <0.001 | <0.001 | |||
| Relative risk reduction (%) | 31 | 42 | 42 | |||
| Absolute risk reduction (%) | 6.2 | 9.4 | 10.2 | |||
| NNT | 17 | 11 | 10 | |||
| By sCTX level | ||||||
| Eventsa | 105 | 153 | 122 | 181 | 103 | 195 |
| Incidence (%) | 13.8 | 21.2 | 16.9 | 24.1 | 14.7 | 26.3 |
| Relative risk [95% CI] | 0.63 [0.49; 0.81] | 0.68 [0.54; 0.85] | 0.53 [0.42; 0.67] | |||
| p value | <0.001 | <0.001 | <0.001 | |||
| Relative risk reduction (%) | 37 | 32 | 47 | |||
| Absolute risk reduction (%) | 7.4 | 7.2 | 11.6 | |||
| NNT | 14 | 14 | 9 | |||
CI confidence interval, NNT number needed to treat
aTotal number of patients having at least one new vertebral fracture during the 3-year period
Fig. 1.
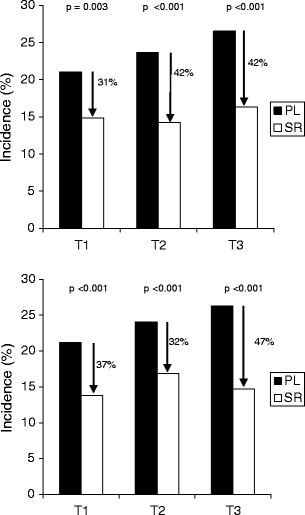
Incidence of vertebral fractures over 3 years according to tertiles of b-ALP (upper panel) and sCTX (lower panel). SR strontium ranelate, PL placebo
Among patients who were in the lowest tertile for both b-ALP and sCTX (n = 881), strontium ranelate treatment produced a RR reduction of 33% compared to placebo (RR = 0.67, 95% CI [0.47; 0.95], p = 0.023; Fig. 2). Among patients in the highest tertile for both b-ALP and sCTX (n = 867), the relative risk reduction with strontium ranelate was 49% (RR = 0.51, 95% CI [0.37; 0.70], p <0.001). The fracture incidences in the strontium ranelate group were comparable, and the magnitude of the treatment effect was not significantly different between patients in the lowest and highest tertiles for both markers (interaction test p = 0.254).
Fig. 2.
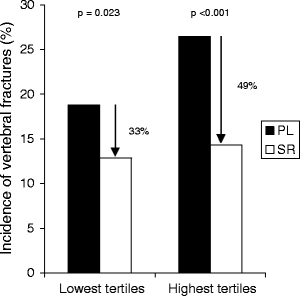
Incidence of vertebral fractures over 3 years in patients in the lowest (n = 881) and highest (n = 867) tertiles for both b-ALP and sCTX. SR strontium ranelate, PL placebo
Given the increasing incidence of fractures with increasing bone turnover in patients treated with placebo, the absolute reduction in fracture risk with strontium ranelate was larger for higher tertiles of bone turnover markers. The number needed to treat (NNT) for 3 years to prevent one first new vertebral fracture ranged from 17 and 14 for the lowest tertiles of b-ALP and sCTX, respectively, to 10 and 9 for the highest tertiles (Table 4).
Bone mineral density
Lumbar BMD increased progressively during the 3-year analysis period in patients treated with strontium ranelate, but remained virtually unchanged in placebo-treated patients (Fig. 3). The increase in lumbar BMD with strontium ranelate, relative to baseline, at 3 years was 12.5%, 14.6% and 16.5% in b-ALP tertile 1, 2 and 3, respectively, and 12.6%, 13.9% and 16.9% in sCTX tertile 1, 2, and 3, respectively (p < 0.001 in all tertiles; Fig. 3). At each yearly time point, significant between-group differences in favour of strontium ranelate were observed in all tertiles (p < 0.001 vs placebo at all time points for all tertiles of both b-ALP and sCTX).
Fig. 3.

Changes in lumbar bone mineral density (BMD) at 12, 24 and 36 months by tertiles of b-ALP (upper panel) and sCTX (lower panel) and treatment group. SR strontium ranelate, PL placebo
Discussion
The main result from this analysis is that 3 years of treatment with strontium ranelate produced similar reductions in the risk of vertebral fracture, relative to placebo, in women with post-menopausal osteoporosis, irrespective of their baseline bone turnover level, consistent with our stated hypothesis. Substantial and significant reductions in fracture risk were seen across all tertiles of pre-treatment b-ALP (a marker of bone formation) and all tertiles of sCTX (a marker of bone resorption), and the size of the treatment effect did not differ significantly between tertiles of either biochemical marker. When women who were in the lowest tertile for both b-ALP and sCTX were compared with those in the highest tertile for both markers, significant relative risk reductions were seen in both groups, with a similar magnitude between the two groups. We further reported that in the placebo group, a high level of bone turnover at baseline is associated with a higher risk to have a new vertebral fracture in accordance to previous studies [25].
Bone turnover markers increase in women after the menopause. In one study, b-ALP, assayed using the same method as in the present study, was significantly higher in post-menopausal (13.7 μg/L) than pre-menopausal women (10.8 μg/L, p < 0.0001) [26]. Other studies have found even lower values in healthy pre-menopausal women, of 8.2 μg/L [27] and 8.8 μg/L [28]. Reported mean values for post-menopausal women with osteoporosis range from approximately 12.5 μg/L [13] to 16.7 μg/L [27] and 18.1 μg/L [29]. The boundaries of the middle tertile for b-ALP in our sample were >10.0 and ≤13.3 μg/L and were slightly lower than the corresponding boundaries for osteoporotic subjects in the fracture intervention trial (FIT, 11.7 and 14.9 μg/L) [12]. Regarding sCTX, levels in healthy pre-menopausal women have been measured at 1,748 pmol/L (corresponding to 0.225 ng/mL) compared with 2,952 pmol/L (corresponding to 0.380 ng/mL) in post-menopausal women [30]. Similarly, Garnero et al. [5] obtained levels of 0.299 and 0.556 ng/mL in pre- and post-menopausal women. The boundaries of the middle tertile for sCTX in our sample of post-menopausal osteoporotic women was >0.423 to ≤0.626 ng/mL (or 3,283 to ≤4,861 pmol/L), slightly higher than in the FIT study (2,337 to 3,665 pmol/L) [12]. Thus, the baseline levels of bone turnover markers in the present analysis are consistent with those in previous studies in post-menopausal women.
At baseline, higher tertiles of b-ALP and sCTX were associated with lower BMD, both at the lumbar spine and the femoral neck. Previous studies have reported that high bone turnover is correlated with low BMD [25, 31] and predicts higher rates of future bone loss in post-menopausal women [32, 33]. High bone turnover has also been associated with increased fracture risk, even after adjustment for BMD [31, 34, 35]. In our analysis, rates of prevalent vertebral and peripheral osteoporotic fractures at baseline did not differ between tertiles of bone turnover markers. However, the incidence of vertebral fractures during the study in the placebo group increased across ascending tertiles of both bone markers by 24% or more depending on the marker considered, with significant differences when comparing the lowest and highest tertiles (b-ALP or CTX independently or both b-ALP and CTX), suggesting that high bone turnover is a risk factor for fracture.
Strontium ranelate produced substantial increases in lumbar BMD independently of the baseline level of b-ALP or sCTX. Larger effects of treatment on BMD in women with higher baseline bone turnover level have been reported for many anti-osteoporotic drugs, including anti-resorptive agents such as calcitonin [6], hormone replacement therapy [7] and bisphosphonates [8–10] and the bone formation agent, teriparatide [13]. Strontium ranelate treatment increased lumbar BMD in post-menopausal women across the range of baseline bone turnover and produced significant increases in BMD, relative to placebo, at each yearly time point in the present analysis.
The influence of baseline bone turnover level on the efficacy of anti-osteoporotic drugs on fracture risk has been less widely studied than BMD, and the results have been less consistent. In an analysis of a subgroup of 1,593 patients from three randomised trials of risedronate [11], vertebral anti-fracture efficacy was compared in women with baseline bone turnover levels, assessed by urinary excretion of deoxypyridinoline, above and below the normative median. At 3 years, the relative risk of vertebral fracture in patients with high bone turnover was 0.52, similar to that in patients with low bone turnover (0.54). A recent analysis in 6,459 osteoporotic and non-osteoporotic women in the FIT study [12] concluded that the efficacy of alendronate in reducing non-vertebral fractures was greater in those with higher baseline bone turnover levels, although there was some inconsistency between different biochemical markers. The vertebral anti-fracture efficacy of alendronate was also influenced by baseline bone turnover in non-osteoporotic women, but no significant influence was found among osteoporotic women [12].
In the case of the bone formation agent, teriparatide, the relative risk reduction for osteoporotic fractures (vertebral and non-vertebral combined) was found to be similar for women in all tertiles of baseline bone turnover markers [14]. However, in that analysis, the risk of fracture increased markedly across tertiles of bone turnover markers, in both the placebo and teriparatide-treated groups. For example, the risks of fracture in the teriparatide group were 0.03, 0.04 and 0.08 in the low, middle and high tertiles of b-ALP, respectively. Thus, the absolute risk reduction with teriparatide was influenced by baseline bone turnover, and the number needed to treat to prevent one fracture decreased with higher tertiles of bone turnover markers. In the present study, the risk of fracture in the strontium ranelate group was similar across tertiles of baseline b-ALP and sCTX, whereas the fracture risk in women treated with placebo increased. The absolute reduction in fracture risk achieved with strontium ranelate treatment was therefore greater in women with higher pre-treatment bone turnover.
In a range of in vitro and in vivo experimental models, strontium ranelate has been shown to simultaneously reduce bone resorption and increase bone formation [18, 36, 37], without any change in bone mineralization [38–40]. Thus, strontium ranelate rebalances bone turnover in favour of bone formation. This effect of strontium ranelate on bone turnover may contribute to its anti-fracture efficacy in women with widely differing bone turnover status.
It is increasingly recognised that osteoporosis is a multifactorial disease. BMD is widely used both in diagnosis and fracture risk prediction. However, BMD accounts for only a modest proportion of fracture risk, and the majority of osteoporotic fractures occur in women who do not meet the World Health Organization definition of osteoporosis based on BMD (T-score −2.5 or less). Other clinical risk factors also contribute substantially to fracture risk [41, 42]. The recently introduced FRAX fracture risk assessment tool provides a framework for estimating fracture risk in individuals from clinical risk factors, including age, body mass index, previous fracture, parental history of fracture and current smoking, with or without the use of BMD [43]. A previous study demonstrated that the efficacy of a 3-year treatment with strontium ranelate on the risk of vertebral fractures is independent of baseline BMD and all of the above clinical risk factors [19]. The present analysis indicates that elevated levels of bone turnover markers is another risk factor for vertebral fracture and shows that the 3-year efficacy of strontium ranelate is also independent of the baseline bone turnover level. Three-year treatment with strontium ranelate therefore reduces vertebral fracture risk in post-menopausal women with a wide spectrum of risk factors for these fractures.
The main limitation of this study is that the results were based on post hoc analyses using pooled data from two studies with different entry criteria. However, both studies included women from a common run-in study (the FIRST study), and vertebral fracture, BMD and bone turnover data were collected using the same methodology. There were no significant differences in patients’ characteristics at baseline between the strontium ranelate and placebo groups, and the only differences among patients in the tertiles of bone turnover markers are related to lumbar and femoral neck BMD. Pooling of data was therefore unlikely to have affected the conclusions of the study.
On the other hand, pooling of data allowed an adequate sample size and number of fractures to compare treatments after stratification of patients into tertiles and ensured that women with a wide range of disease severity and bone turnover were included in the analysis.
In conclusion, strontium ranelate showed significant vertebral anti-fracture efficacy in post-menopausal osteoporotic women in each tertile of markers of pre-treatment bone formation and resorption. The relative reductions in vertebral fracture risk achieved by strontium ranelate were independent of baseline bone turnover level. These results indicate that strontium ranelate offers clinical benefits to women across a wide range of metabolic states and disease severity.
Acknowledgments
Conflicts of interest
Dr. Collette has no conflict of interest; Dr. Bruyère and Dr. Boonen received some consulting fees; Dr. Kaufman, Dr. Lorenc, Pr Felsenberg and Dr. Spector are investigators in SOTI and TROPOS studies; Pr Reginster received consulting fees, lecture fees and research grants from Servier.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bouxsein ML, Karasik D. Bone geometry and skeletal fragility. Curr Osteoporos Rep. 2006;4:49–56. doi: 10.1007/s11914-006-0002-9. [DOI] [PubMed] [Google Scholar]
- 2.Seeman E. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am. 2003;32:25–38. doi: 10.1016/S0889-8529(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E. Invited review: pathogenesis of osteoporosis. J Appl Physiol. 2003;95:2142–2151. doi: 10.1152/japplphysiol.00564.2003. [DOI] [PubMed] [Google Scholar]
- 4.Stepan JJ, Alenfeld F, Boivin G, et al. Mechanisms of action of antiresorptive therapies of postmenopausal osteoporosis. Endocr Regul. 2003;37:227–240. [PubMed] [Google Scholar]
- 5.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47:694–702. [PubMed] [Google Scholar]
- 6.Civitelli R, Gonnelli S, Zachei F, et al. Bone turnover in postmenopausal osteoporosis. Effect of calcitonin treatment. J Clin Invest. 1988;82:1268–1274. doi: 10.1172/JCI113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonnelli S, Cepollaro C, Pondrelli C, et al. The usefulness of bone turnover in predicting the response to transdermal estrogen therapy in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:624–631. doi: 10.1359/jbmr.1997.12.4.624. [DOI] [PubMed] [Google Scholar]
- 8.Gonnelli S, Cepollaro C, Pondrelli C, et al. Boner turnover and the response to alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int. 1999;65:359–364. doi: 10.1007/s002239900713. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto J, Takeda T, Sato Y, et al. Determinants of one-year response of lumbar bone mineral density to alendronate treatment in elderly Japanese women with osteoporosis. Yonsei Med J. 2004;45:676–682. doi: 10.3349/ymj.2004.45.4.676. [DOI] [PubMed] [Google Scholar]
- 10.Kim SW, Park DJ, Park KS, et al. Early changes in biochemical markers of bone turnover predict bone mineral density response to antiresorptive therapy in Korean postmenopausal women with osteoporosis. Endocr J. 2005;52:667–674. doi: 10.1507/endocrj.52.667. [DOI] [PubMed] [Google Scholar]
- 11.Seibel MJ, Naganathan V, Barton I, et al. Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res. 2004;19:323–329. doi: 10.1359/JBMR.0301231. [DOI] [PubMed] [Google Scholar]
- 12.Bauer DC, Garnero P, Hochberg MC, et al. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the Fracture Intervention Trial. J Bone Miner Res. 2006;21:292–299. doi: 10.1359/JBMR.051018. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–970. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 14.Delmas PD, Licata AA, Reginster JY, et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone. 2006;39:237–243. doi: 10.1016/j.bone.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. New Engl J Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 16.Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 17.Marie PJ, Ammann P, Boivin G, et al. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 18.Marie PJ. Strontium ranelate: a novel mode of action of optimizing bone formation and resorption. Osteoporos Int. 2005;16(Suppl 1):S7–S10. doi: 10.1007/s00198-004-1753-8. [DOI] [PubMed] [Google Scholar]
- 19.Roux C, Reginster J-Y, Fechtenbaum J, et al. Vertebral fracture risk reduction with strontium ranelate in women with postmenopausal osteoporosis is independent of baseline risk factors. J Bone Miner Res. 2006;21:536–542. doi: 10.1359/jbmr.060101. [DOI] [PubMed] [Google Scholar]
- 20.Seeman E, Devogelaer J-P, Lorenc R, et al. Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res. 2008;23:433–438. doi: 10.1359/jbmr.071105. [DOI] [PubMed] [Google Scholar]
- 21.Meunier PJ, Reginster JY. Design and methodology of the phase 3 trials for the clinical development of strontium ranelate in the treatment of women with postmenopausal osteoporosis. Osteoporos Int. 2003;14(Suppl 3):S66–S76. doi: 10.1007/s00198-002-1341-8. [DOI] [PubMed] [Google Scholar]
- 22.Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 23.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 24.Slosman DO, Provvedini DM, Meunier PJ, et al. The use of different dual x-ray absorptiometry brands in a multicenter clinical trial. J Clin Densitom. 1999;2:37–44. doi: 10.1385/JCD:2:1:37. [DOI] [PubMed] [Google Scholar]
- 25.Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 26.Broyles DL, Nielsen RG, Bussett EM, et al. Analytical and clinical performance characteristics of Tandem-MP Ostase, a new immunoassay for serum bone alkaline phosphatase. Clin Chem. 1998;44:2139–2147. [PubMed] [Google Scholar]
- 27.Garnero P, Shih WJ, Gineyts E, et al. Comparison of new biochemical markers of bone turnover in late postmenopausal women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700. doi: 10.1210/jc.79.6.1693. [DOI] [PubMed] [Google Scholar]
- 28.de Papp AE, Bone HG, Caulfield MP, et al. A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone. 2007;40:1222–1230. doi: 10.1016/j.bone.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Bauer DC, Garnero P, Bilezikian JP, et al. Short-term changes in bone turnover markers and bone mineral density response to parthyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2006;91:1370–1375. doi: 10.1210/jc.2005-1712. [DOI] [PubMed] [Google Scholar]
- 30.Rosenquist C, Fledelius C, Christgau S, et al. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44:2281–2289. [PubMed] [Google Scholar]
- 31.Melton LJ, 3rd, Khosla S, Atkinson EJ, et al. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–1091. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 32.Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15:1398–1404. doi: 10.1359/jbmr.2000.15.7.1398. [DOI] [PubMed] [Google Scholar]
- 33.Löfman O, Magnusson P, Toss G, et al. Common biochemical markers of bone turnover predict future bone loss: a 5-year follow-up study. Clin Chim Acta. 2005;356:67–75. doi: 10.1016/j.cccn.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Ravn P, Rix M, Andreassen H, et al. High bone turnover is associated with low bone mass and spinal fracture in postmenopausal women. Calcif Tissue Int. 1997;60:255–260. doi: 10.1007/s002239900225. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Sornay-Rendu E, Claustrat B, et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 36.Buehler J, Chappuis P, Saffar JL, et al. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis) Bone. 2001;29:176–179. doi: 10.1016/S8756-3282(01)00484-7. [DOI] [PubMed] [Google Scholar]
- 37.Bonnelye E, Chabadel A, Saltel F, et al. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2007;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Ammann P, Shen V, Robin B, et al. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res. 2004;19:12–20. doi: 10.1359/JBMR.040906. [DOI] [PubMed] [Google Scholar]
- 39.Barbara A, Delannoy P, Denis BG, et al. Normal matrix mineralization induced by strontium ranelate in MC3T3–E1 osteogenic cells. Metabolism. 2004;53:532–537. doi: 10.1016/j.metabol.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Farlay D, Boivin G, Panczer G, et al. Long-term strontium ranelate administration in monkeys preserves characteristics of bone mineral crystals and degree of mineralization of bone. J Bone Miner Res. 2005;20:1569–1578. doi: 10.1359/JBMR.050405. [DOI] [PubMed] [Google Scholar]
- 41.Leslie WD, Metge C, Ward L. Contribution of clinical risk factors to bone density-based absolute fracture risk assessment in postmenopausal women. Osteoporos Int. 2003;14:334–338. doi: 10.1007/s00198-003-1375-6. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 43.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


