Abstract
The identification of endothelial progenitor cells (EPCs) has led to a significant paradigm in the field of vascular biology and opened a door to the development of new therapeutic approaches. Based on the current evidence, it appears that EPCs may make both direct contribution to neovascularization and indirectly promote the angiogenic function of local endothelial cells via secretion of angiogenic factors. This concept of arterial wall repair mediated by bone marrow (BM)-derived EPCs provided an alternative to the local “response to injury hypothesis” for development of atherosclerotic inflammation. Increased oxidant stress has been proposed as a molecular mechanism for endothelial dysfunction, in part by reducing nitric oxide (NO) bioavailability. EPCs function may also be highly dependent on a well-controlled oxidant stress because EPCs NO bioavailability (which is highly sensitive to oxidant stress) is critical for their in vivo function. The critical question is whether oxidant damage directly leads to an impairment in EPCs function. It was revealed that activation of angiotensin II (Ang II) type 1 receptor stimulates nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase in the vascular endothelium and leads to production of reactive oxygen species. We observed that Ang II accelerates both BM- and peripheral blood (PB)-derived EPCs senescence by a gp91phox-mediated increase of oxidative stress, resulting in EPCs dysfunction. Consistently, both Ang II receptor 1 blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors have been reported to increase the number of EPCs in patients with cardiovascular disease. In this review, we describe current understanding of the contributions of oxidative stress in cardiovascular disease, focusing on the potential mechanisms of EPCs senescence.
Key Words: Endothelial progenitor cell, oxidative stress, senescence, angiotensin II, telomerase, nitric oxide.
INTRODUCTION
The identification of endothelial progenitor cells (EPCs) has led to a significant paradigm shift in the field of vascular biology. It has become evident that bone marrow (BM)-derived circulating EPCs can contribute to and amplify neovascularization. EPCs significantly contribute to adult vessel formation by physically incorporating and promoting vessel growth by paracrine mechanisms [1, 2]. It is believed that the majority of EPCs originate in the BM. The odyssey from BM to vascular endothelium can be divided into three stages. First, EPCs are mobilized or released from BM into systemic circulation in response to specific stimuli (mobilization). A number of cytokines and growth factors appear to promote this step. Subsequently, the cells appear to home to preferentially to sites of tissue injury (homing). Finally, some EPCs are incorporated into new blood vessels formed by the extension of existing vessels (angiogenesis) or possibly formed in situ (vasculogenesis). The concept of arterial wall repair mediated by BM-derived EPCs provided an alternative to the local “response to injury hypothesis” for the development of atherosclerotic inflammation. Based on the current evidence, it appears that EPCs may directly contribute to neovascularization and indirectly promote the angiogenic function of local endothelial cells via secretion of angiogenic factors. According to this new theory, the arterial wall can deal fairly well with multiple circulating and local noxious stimuli, as long as the BM-derived repair capacity, which induces competent EPCs and probably progenitors of other lineages, remains intact. Circulating EPCs are also indicators of overall cardiovascular health. Vasa et al. [3] initially showed that levels of circulating EPCs (CD34/kinase insert domain receptor (KDR)-positive) are higher in healthy volunteers than in patients with coronary artery disease. Hill et al. [4] analyzed “colony-forming units” of EPCs and found that this measurement negatively correlated with Framingham risk factor score. They also found that a reduction in EPCs colonies was a good predictor of impairment in flow-mediated brachial-artery reactivity. In a related study, it was reported that EPCs isolated from patients with type 2 diabetes mellitus display impaired proliferation and reduced incorporation into tube-like structures on Matrigel [5]. Conversely, statin (HMG-CoA reductase inhibitor) therapy increases circulating EPCs levels in patients with coronary artery disease [6]. Futhermore, an age-dependent decrease in EPCs mobilization has been reported [7]. Several of coronary risk factors are clustered in patients with cardiovascular disease. All of these risk factors are also associated with elevated markers of reactive oxygen species (ROS) [8].
The tissue microenvironment after ischemia is characterized by excessive production of ROS and oxidized metabolites. Prior studies have demonstrated that oxidative stress directly contributes to endothelial dysfunction and vascular disease [9-12]. These observations suggest that EPCs, in contrast to mature endothelial cells, are uniquely equipped with antioxidant defence systems to resist ROS-driven cytotoxicity if they are active participants in various repairs in ischemic tissues. Recently, two in vitro studies demonstrated that EPCs express higher levels of manganese superoxide dismutase (MnSOD) and glutathione peroxidase-1 (GPx-1) [13, 14]. It has also been shown that the collective inhibition of catalase, MnSOD, and GPx-1 increases ROS levels in EPCs and that this inhibition impairs EPCs survival and migration [15]. In fact, some studies have suggested that EPCs may be resistant to oxidative stress [15, 16]. Dernbach et al. [16] reported that EPCs are resistant to oxidative stress and uniquely equipped to repair damaged vessels in ischemic tissues. Some controversy does exist about the effects of oxidative stress on EPCs. Ingram et al. [17] reported that EPCs exhibited increased apoptosis and diminished tube-forming ability in vitro and in vivo in response to oxidative stress, which was directly linked to activation of a redox-dependent stress-induced kinase pathway. The current review describes the characterstics and properties of EPCs, focusing on the effects of oxidative stress on EPCs senescence.
EPCs DEFINITION AND CHARACTERIZATION
The ability of the BM to give rise to endothelial cells was first reported by Asahara et al [1]. This study was based on the finding that EPCs circulating in peripheral blood (PB) express the hematopoietic marker CD34. The EPCs were defined as cells positive for both hematopoietic stem cells and endothelial cell markers, such as CD34 and vascular endothelial growth factor (VEGF) receptor-2, respectively. The latter VEGF receptor-2 is often referred to as KDR.
The putative CD34+ EPCs are able to proliferate and differentiate to mature endothelial cells with expression of different endothelial markers such as KDR [2, 18], platelet-endothelial cell adhesion molecule (CD31) [2, 15], von Willebrand factor [2, 18, 20], VE-cadherin [2, 18], caveolin-1 [19, 21], and endothelial nitric oxide (NO) synthase (eNOS) [19, 21]. While in vitro, EPCs can form vascular-like structures [18, 21], and in vivo, incorporate into neovessels at sites of tissue ischemia [18, 20, 22]. Of note, CD34 antigen density is the highest on early progenitors and decreases progressively as the cells mature [23]; however, CD34 is expressed not only on EPCs but on mature endothelial cells, albeit at a lower density [24]. As noted, it remains to be determined whether or not CD34+/KDR+ cells fully reflect changes in EPCs capable of arterial repair and angiogenic activity [25]. Therefore, an early hematopoietic stem cells marker, CD133, was adopted as an alternative additional marker to indicate a “true” EPCs [26, 27]. In the present time, EPCs are thought to be a heterogenous population consisting of a more primitive CD133+/CD34+/VEGFR-2+ subpopulation and a more mature CD133-/CD34+/VEGFR-2+ subpopulation. The marker CD133 (also known as AC133) is a 120-kDa transmembrane polypeptide with as yet unknown biological function. It is expressed on hematopoietic stem cells and progenitor cells from human BM, fetal liver, and peripheral blood (PB) [28]. As progenitors develop to more mature endothelium-like cells, CD133 is rapidly down-regulated [27]. The CD133+ cells are able to form both early and late outgrowing colonies [27]. Thus, CD133 might provide a more reliable means of defining and tracking human angioblast-like EPCs and distinguishing these from mature endothelial or monocytic cells. Recently, Friedrich et al. [29] have demonstrated that CD34- /CD133+/ VEGFR-2+ EPCs are precursors of CD34+/CD133+/VEGFR-2+ EPCs with a higher potential for vascular repair. These data extend current knowledge about the heterogenous EPCs population and may have implications for the treatment of vascular disease and arterial injury. Several recently published data suggested that other populations of BM-derived, circulating, or tissue resident cells might also possess properties of EPCs. In particular, these sub-populations were characterized by expression of monocyte marker CD14, together with CD34 or VEGFR-2 [30, 31]. Furthermore, “early” isolated EPCs also displayed expression of monocyte marker (CD14, CD11b, CD11c), whereas the “late” endothelial outgrowth was CD14-negative and strongly expressed markers of mature endothelial cells [32, 33]. Hence, the definition of EPCs become more universal and indeed encompass rather heterogenous cell sub-populations of multiple origins and localization, which finally differentiate into functionally active mature endothelial cells. Despite the multiple EPCs origin, common characteristics of EPCs remain the expression of stem/ progenitor marker, their clonogenic potential (i.e. the formation of colony-forming units) and proliferative capacity (i.e. the development of late high-proliferative endothelial out-growth).
Considering the growing importance of the EPCs colonies in cardiovascular research, it is crucial to investigate the characteristics of the EPCs colony in detail. There are at least 2 morphological and functionally distinct endothelial cell populations in circulating MNCs [2]. The early spindle-like outgrowth cells possess a relatively low proliferative capacity and low ability to express mature endothelial proteins [18]. These cells presumably represent cells of different lineage, which include a subset of CD14+/CD34- monocytic cells, which have the potential to differentiate (transdifferentiate) into endothelial-like cells under certain environmental condition in the presence of special growth factors (e.g., VEGF, fibroblast growth factor, and so on) [34]. Late “out-growth cells” show a high proliferative potential and originate predominantly from BM donors and are considered as circulating angioblasts [2]. It is important to appreciate that although monocyte-derived EPCs have a lower in vitro proliferation potential than hematopoietic stem cells or cord blood-derived EPCs [35], the different progenitor types seem to have a similar ability to enhance neovascularization in experimental models [18, 36, 37]. One may speculate that proliferation capacity is not the decisive factor and that the reduced proliferation of the monocyte-derived EPCs is likely to be attributable to increased release of growth factors, which may act in a paracrine manner to support angiogenesis and arteriogenesis [38]. Hur et al. [39] have found that a specific subset of T cells (CD3+CD31+CXCR4+) make up the central cluster of EPC colonies. They also found that this subset of T cells enhances EPCs differentiation and angiogenesis, resulting in neovascularization in vivo.
In summary, a universal single or complex EPCs marker still remains to be identified, showing the heterogeneous of endothelial precursors. As a result, both different surface markers and culture properties have been used to define EPCs.
KINETICS OF EPCs FOR POSTNATAL NEOVASCULARIZATION
Mobilization
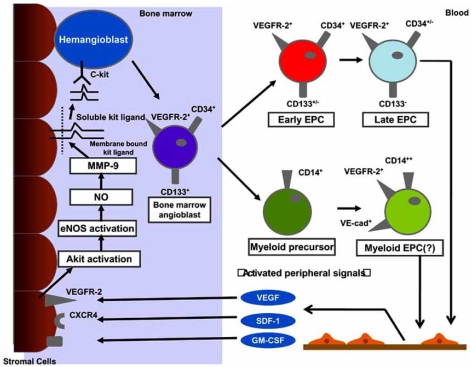
Recruitment of EPCs from the BM quiescent niche has been found to be associated with the activation of proteinases such as elastase, cathepsin G, and matrix metalloproteinases (MMPs) [40]. These enzymes proteolytically cleave the extracellular matrix- or cell membrane-bound molecules responsible for EPCs’ adhesive bonds on BM stromal cells (Fig. (1)). These cells express membrane-bound Kit ligand (mKitL), which binds to the EPCs membrane receptor c-kit when the ligand is in its soluble form (sKitL). MMP-9 proteolytically cleaves mKitL to sKitL, which then interacts with the EPCs c-kit receptor to conduct the signal essential for BM-EPCs differentiation and migration to the peripheral blood (PB) [41] (Fig. (1)). One of the models used in studying the recruitment of BM hematopoietic EPCs use BM cell suppression by cytotoxic agents. This suppression does not affect hematopoietic stem cells in the Go phase of the cell cycle. Therefore, these cells may serve as a cell population to reconstitute hematopoiesis and EPCs release. The introduction of cytotoxic suppression in the BM of MMP-9+/+ and MMP-9-/- mice resulted in poor recruitment and differentiation of hematopoietic cells only in the latter group of animals [40]. Treatment of MMP-9+/+ mice with VEGF, stromal-derived factor-1 (SDF-1), and granulocyte colony-stimulating factor (G-CSF) caused a marked increase in the concentration of plasma sKitL compared with untreated animals [40]. The results of this experiment proved that VEGF, SDF-1, and G-CSF play significant roles in the induction of MMP-9 precursor biosynthesis, secretion, and further mobilization of BM-EPCs to the PB [41-44] (Fig. (1)).
Fig. (1). Mobilization, recruitment, and differentiation of human, bone marrow-derived angiogenic progenitor cells.
Hemangioblast, originated from hematopoietic cell, is resident in bone marrow niches, in a quiescent state. The stimulation by circulating cytokines induces the activation of matrix metalloproteinase-9 (MMP-9) through an Akt, nitric oxide dependent pathway. MMP-9 promotes the transformation of membrane bound Kit-ligand to a soluble Kit-ligand. This activation is followed by detachment of early c-Kit+ progenitor cells from the bone marrow stromal niche and their subsequent movement to the vascular zone of the bone marrow. An important regulation is VEGF and SDF-1, which binds to its receptor VEGFR-2 and CXCR4, respectively, thus mediating further maturation of the cascade hemangioblast-angioblast-early endothelial progenitor cells (EPC)-late EPCs. Bone marrow-derived EPCs are of hematopoietic origin and possibly derive from the hemangioblast. These early progenitors (CD133+/CD34+/VEGFR-2+/CD14-) represent a small population with proliferative potential, capable to give rise to late endothelial outgrowth. Cells of myeloid origin (CD14+) may also trans-differentiate into endothelial cells and secret angiogenic factors, but their proliferative potential is limited and they did not generate a stable late outgrowth.
Growth Factors that Effect EPCs Function
SDF-1 and VEGF have been considered crucial in the differentiation and migration of EPCs [41-44]. Increased plasma levels of SDF-1 and VEGF are eventually accompanied by the mobilization of hematopoietic cells, among them EPCs, into the circulation (Fig. (1)). SDF-1 is a chemokine that binds specificially to the receptor designated as CXCR4 [43]. This receptor is expressed on the surface of hematopoietic stem cells, including EPCs [43, 45]. The SDF-1 acts as a key chemokine for the mobilization of EPCs and other progenitor cells from the BM compartment, e.g. by MMP-9-mediated cleavage of mKitL [46-48] (Fig. (1)). SDF-1 further mediates the recruitment of progenitor cells along hypoxic gradients and towards surface-adherent platelets after artrial injury [46-48]. Hristov et al. [49] revealed that blocking CXCR4 significantly reduced the adhesion of EPCs after arterial wire-injury in vivo. Additionally, SDF-1 mediated migration of isolated EPCs, enhanced their matrix arrest when acting as a soluble chemokine, and was further secreted by activated platelets and SMCs after arterial wire-injury [49, 50]. Within the clinical context, a dysregulation of the CXCR4 signaling in EPCs from patients with stable chronic coronary artery disease has been described [51]. Thus, the role of CXCR4 in EPCs biology appears to be more universal. Recent data has provided evidence for VEGF autocrine action in hematopoietic cells, including apoptosis protection and survival effect [42]. Granulocyte macrophage colony-stimulating factor (GM-CSF) is also proposed as a possible candidate for EPCs function regulation [52]. In a study performed by Cho et al., recombinant GM-CSF was able tomobilize EPCs and accelerated the re-endotheliazation process in hypercholesterolemic rabbits [52].
MECHANISMS OF CELLULAR SENESCENCE
Phenotype and Pathways
Cellular senescence is originally described as the finite replicative lifespan of human somatic cells in culture. Senescent cells remain viable, but they do not respond to mitogenic stimuli and their morphological characteristics and function change dramatically [53-58]. They lose their original shape, their volume increases, and they acquire a flattened cytoplasma (“fried egg” appearance) [59, 60]. These changes are accompanied by alterations in nuclear structure, gene expression, protein processing, and metabolism [58, 59]. These phenotypic changes of senescent cells are not observed in quiescent cells and have been implicated in aging and age-associated disease [61]. More recent data have shown that cells can enter senescence rapidly, independently of the number of cell divisions, in response to various physiological stresses (radiation, oxidative stress, lack of nutrients, DNA damage, and so on) [62-65]. This type of senescence has been termed stress-induced premature senescence [66].
Although initially believed to be a cell-culture phenomenon, cellular senescence recently was observed in vivo as well [67, 68]. The most common means of detecting cellular senescence is by colorimetric detection of β-galactosidase in cells under mildly acidic (pH 6.0) conditions, in contrast to the more strongly acidic conditions (pH 4.0) normally required to detect endogenous lysosomal β-galactosidase activity [69]. Other biomarkers include increased expression of p53, p21, and p16 [70-73].
Senescence is a fundamental cellular program that parallels that of programmed cellular death (apoptosis). Both molecular mechanisms restrict cellular proliferation. The reason a cell is driven to apoptosis versus senescence is not yet known [74-78]. The degree of stress [75] and cell-cycle phase [74] seem to be determining factors (eg, higher doses of oxidative stress induce apoptosis, whereas lower and long-acting doses induce senescence). Moreover, apoptosis appears to occur more easily in senescent endothelial cells, yet seems to be blocked in other senescent cell types [78]. At the same time, factors involved in senescence signaling, such as p53, are also involved in apoptosis regulation through interaction with the BCL2 family of proteins [79]. In any case, cellular senescence as a biological mechanism, as well as the role that senescence has in the living organism, is, in contrast to apoptosis, not well understood.
Telomeres and Telomerase
Significant progress has been made in our understanding of the mechanisms underlying cellular senescence. One widely discussed hypothesis of senescence is the telomere hypotheis [80]. Telomeres are non-nucleosomal DNA/protein complexes located at the ends of chromosomes that serve as protective caps and act as the substance for specialized replication mechanisms [81-83]. As a consequence of semiconservative DNA replication, the extreme terminals in successive shortening DNA replication, the extreame terminals of the chromosomes are not duplicated completely, resulting in successive shortening of the telomeres with each cell division. Critical telomere shortening is thought to trigger the onset of cellular senescence. Thus, telomere shortening has been proposed to act as a mitotic clock that ptevents unlimited proliferation of human somatic cells. However, telomeres are also involved in stress-induced premature senescence. It seems that this second pathway initiates not because of shortening, but because of changes in telomere structure (ie, alterations in the T loop and single-stranded overhang) [84, 85]. Thus both telomere length and structural integrity are necessary for proper chromosome function and avoidance of DNA damage response and its consequent triggering of senescence.
Telomerase is a cellular reverse transcriptase which catalyzes the synthesis and extension of telomeric DNA. Telomerase activity is consistently expressed in germline cells and in the majority of malignant tissue cells and is repressed in most human normal somatic cells. Strikingly, however, telomerase activation is expressed in a highly regulated manner in certain somatic cell populations, such as lymphocytes and hematopoietic stem cells. Studies on telomerase regulation in normal somatic cells have focused on expression of the two essential components of telomerase, human telomerase RNA template (hTER) and human telomerase reverse transcriptase (hTERT). There is a good correlation between the expression of hTERT mRNA and the presence of telomerase activity in extracts from tissue culture and normal and cancer tissues, whereas hTER is expressed constitutively in both cancer and normal cells, irrespective of the status of telomerase expression. The hTERT enzyme not only produces telomeric repeats that elongates telomeres, but also prevents alterations in telomere structure, protecting the telomere cap [86-88]. Early studies reported that telomerase activity was detected in cancer cells and stem cells but not in normal somatic cells [89, 90]. However, increasing evidence has suggested that telomerase activity regulates cell proliferation in normal somatic cells by telomere lengthening or telomere length-independent mechanisms [91, 92]. Human endothelial cells and VSMCs express telomerase activity, but the activity declines with in vitro aging due to a decrease of hTERT, leading to telomere shortening and cellular senescence [93, 94]. In fact, induction of telomerase extends the lifespan of both endothelial cells and VSMCs [95, 96], suggesting a critical role of telomere and telomerase in vascular senescence.
FUNCTIONAL AND ANTIOXIDATIVE CAPACITY OF EPCS
To contribute to tissue repair, EPCs and stem cells in general, have to be equipped with antioxidative defence system to survive, in necrotic and ischemic tissues. Interestingly, a high resistance to oxidative stress has been considered a characteristics feature of stem cells [97, 98]. Protection against oxidative stress by ROS is accomplished by a complex defense system composed of several antioxidative enzymes that reduce the damaging effects of ROS [99]. The most vulnerable organelles to oxidative stress are the mitochondria, because of the permanent potential for the production of superoxide anions. Superoxide anions are converted to hydrogen provided by superoxide dismutases, whereas hydrogen peroxide is detoxified by the enzymes by catalase and glutathione peroxidase. Because of the localization of MnSOD and GPx-1 in the matrix of the mitochondria, in close proximity to the production of ROS by the electron transport chain, these two enzymes are believed to be the primary antioxidant defense systems in the mitochondria. What is the specific mechanism by which ROS affect mobilzation and progenitor cell differentiation toward the endothelial lineage? Recently, two in vivo studies demonstrated that EPCs express high levels of MnSOD and GPx-1 [15, 16]. Galasso et al. [100] have addressed the role of GPx-1 for the functional capacity of EPCs in vivo. They investigated ischemia-induced neovascularization in GPx-1deficient mice and assessed the number and functional activity of EPCs. GPx-1-deficient mice showed a reduced blood flow recovery after hindlimb ischemia compared with their wild type. This was accompanied with reduced EPCs levels in response to the functional capacity of EPCs to migarate and promote angiogenesis in vivo. These findings support the knowledge that EPCs require antioxidative enzymes, especially GPx-1, for their functional capacity. However, is the reduction of EPCs in GPx-1-deficient mice caused by increased cell death or decreased differentiation or mobilization? Galasso et al. [100] demonstrated that EPCs derived from GPx-1-deficient mice show increased apoptosis sensitivity and decreased expression of Flk-1 are involved in the reduction of EPC levels from GPx-1-deficient mice. However, a reduced Flk-1 expression was demonstrated before and after ischemia, supporting the hypothesis that interfering with the antioxidant defense system of cells may also influence differentiation. Although the precise molecular mechanisms are unknown so far, one may speculate that the reduced bioavailability of NO reported for the GPx-1-deficient mice may contribute to the phenotype of the mice [101]. It is also important to note that eNOS-deficient mice show a similar impaired capacity to mobilize EPCs combined with a systemic dysfunction of isolated EPCs [102, 103]. The finding that GPx-1 expression is essential for EPC functions may also have clinical implications, given that patients with chronic heart failure [104] and with type 2 diabetes [105] showed a downregulation of GPx-1. This in turn may contribute to the reduced EPC numbers and function in patients with CAD and severe heart failure [106]. However, other antioxidative enzymes such as superoxide dismutase and catalases are also downregulated in these patients [105, 106]. In addition, some controversy does exist about the effects of oxidant stress on EPCs. Ingrum et al. [17] reported that clonogenic cord and adult blood-derived EPCs are sensitive to oxidant stress. Furthermore, EPCs treated with oxidants undergo increased apoptosis and decreased tube formation via apoptosis signal-regulating kinase 1 (ASK1) activation. Thus, it seems mandatory to emphasize the importance of designing therapeutic strategies to protect EPCs against oxidant stress to enhance new vessel formation.
EFFECTS OF ATHEROGENIC FACTORS ON EPCS SENESCENCE
Clinical studies clearly demonstrate that high EPCs levels are associated with reduced cardiovascular event rates underlying the vasculoprotective action of EPCs [107, 108]. The rejuvenation of the endothelium by circulating EPCs may represent a novel approach in the prevention of atherosclerotic disease. However, limitations in therapy may come from the negative influence of cardiovascular risk factors, which are apparently overwhelming the organism’s repair mechanisms, bringing the equilibrium between vascular repair and injury out of balance (Fig. (2)). Cardiovascular risk factors negatively influence EPCs number and function, whereas vasculoprotection is at least in part mediated by functional active EPCs. Therefore, EPCs may present a cellular risk marker, integrating the positive and negative mediators affecting the endothelial monolayer. Multiple factors seem to be involved in the aging-associated deterioration of EPCs quantity and function. The chronic exposure to cardiovascular risk factors continuously damages endothelial cells and requires their intensive replacement. Conversely, cardiovascular risk factors possibly affect EPCs mobilization, integration in injured vascular sites, and angiogenic capacity (Fig. (2)). Recent studies have underlined the detrimental effects of type 1 and 2 diabetes on EPCs function [5, 109]. Loomans et al. [109] have demonstrated that the media from EPCs culture of type 1 diabetic patients not only possess evidence of reduced angiogenic capacity, but also contain an inhibitor for in vitro tube formation. Tepper et al. [5] reported that the proliferation and tube formation of EPCs were impaired in patients with type 2 diabetes compared with normal subjects. In both studies, decreased number and dysfunction of EPCs was inversely related to the levels of hemoglobin A1c, implying that the degree of glycemic dysregulation was associated with EPCs pathophysiology. Although these studies have clarified an adverse effect of DM on the functional activity of EPCs, the underlying mechanisms remain unsolved. The EPCs dysfunction may also be result of their accelerated senescence. We showed that hyperglycemia (HG) increases the rate of EPCs senescence, which effect is inhibited by an inhibitor p38 MAPK, SB203580 [110]. We also have demonstrated that high glucose (HG) levels can accelerate the p38 MAPK pathway in EPCs [110]. Seeger et al [111] have demonstrated that the redunction of EPCs induced by high glucose (HG) in vitro is associated with a profound upregulation of p38 mitogen activated protein kinase (MAPK) phosphorylation and is completely blocked by p38 inhibitors. Furthermore, EPCs cultivated from patients with CAD show an increased p38 phosphorylation compared with EPCs from healthy control subjects. Interestingly, they have shown that HG further augment the phosphorylation of the p38 downstream kinase stress-activated kinase (MSK)1 and the transcription factor camp-responsive element-binding protein (CREB). Several studies demonstrate that p38 MAPK blockade inhibition is associated with increased angiogenesis [112-114]. Because NAD(P)H oxidase activation promotes p38 MAPK phosphorylation [111], it is tempting to speculate that increased p38 MAPK phosphorylation may represent an additional potential pathway whereby NAD(P)H oxidase may alter EPCs function in DM. Therefore, p38 MAPK inhibitors and/or NAD(P)H inhbition might be a promising tool to augment the yield of ex-vivo-expanded EPCs for cell therapy, specially for the patients with DM. In this context, Sorrentino et al. [115] have demonstrated that short-term in vivo rosiglitazone treatment in diabetic subjects reduced EPCs NAD(P)H oxidase activity and restored NO availability, suggesting that PPAR-γ agonist exerts a direct on NAD(P)H oxidase in diabetic EPCs. Of note, in vitro treatment with the PPAR-γ agonist pioglitazone prevented oxidative stress-induced apoptosis in human EPCs, further suggesting a role of PPAR-γ for EPCs function [116]. We have also shown that pioglitazone reduces Ang II-induced acceleration of senescence in EPCs [117]. Collectively, therapeutic interventions that improve vascular activity in EPCs by PPAR-γ agonists may have tremendous potential for the treatment of cardiovascular diseases.
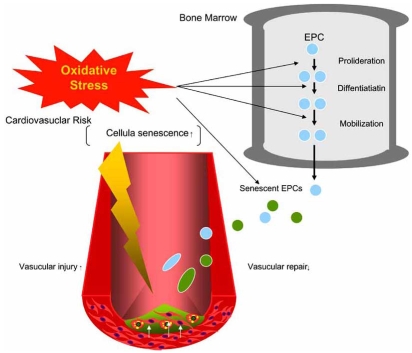
Fig. (2). Oxidative stresses on endothelial progenitor cells (EPCs) in cardiovascular diseases.
EPCs repair cardiovascular damage. Oxidative stress caused by dyslipidemia, diabetes mellitus, or hypertension interferes with the ability of EPCs proliferation, differentiation, and mobilization in bone marrow. Oxidative stress also induces EPCs senescence. These negative effects of oxidative stress on EPCs number and function bring the equilibrium between cellular repair and injury out of balance, resulting in the progression of cardiovascular damages.
The number of circulating EPCs in patients with CAD has been shown to decline with increasing age [3]. Furthermore, following coronary artery bypass grafting, EPCs mobilization is significantly impaired in older individuals compared with younger patients [118]. Besides changes in EPCs levels, the function of EPCs from older individuals also appear to be disrupted, based on in vitro examination of EPCs survival, proliferation, and migration [3, 119]. These results strongly suggest that age is an important determinant of EPCs function and further support the hypothesis that changes in EPCs function with age contribute to the impairment of cardiovascular repair mechanisms in the aging host. The age-associated impairment of cardiac angiogenic capacity in older mice, estimated as neovascularization of cardiac allografts, can be restored by implantation of BM-derived EPCs from young adult animals [120]. Progression of atheroscleorsis in apolipoprotein E-/- mice with persistent hypercholesterolemia seems delayed by chronic administration of BM-derived progenitor cells from young mice [120]. This treatment was much less effective when donors were older animals with atherosclerosis, indicating that progressive age-dependent reduction in EPCs may accelerate the development of atherosclerosis, particularly in the presence of hypercholesterolemia [120].
Reduced levels of angiogenic and mobilizing cytokines have been related to age-dependent impairment of EPCs mobilization in vivo. Indeed, vascular endothelial growth factor (VEGF) and NO production have been reported to decrease with age [118-120]. Specifically, eNOS expression and subsequent NO production are crucial in EPCs mobilization (Fig. (1)) [121, 122]. In this context, eNOS is a central downstream mediator in VEGF-signaling pathways [123-125]. Moreover, we and others have shown that ox-LDL, which accumulates with age, also suppresses eNOS expression and impairs EPCs survival and function [126, 127]. We showed that ox-LDL accelerates the onset of EPCs senescence, which leads to impairment of proliferative capacity and network formation [127]. However, the mechanisms by which ox-LDL accelerates the onset of EPCs senescence remain unclear. We also demonstrated that, in the presence of VEGF, ox-LDL reduces the number of adherent EPCs through dephosphorylation of the Akt kinase on Ser473 in EPCs [128]. Dimmeler et al. [129] showed that VEGF, as well as statins, induces EPCs differentiation via the PI3-K/Akt pathway, as showed by the inhibitory effect of pharmacological PI3-K blockers or the overexpression of a dominant negative Akt construct. Interestingly, Breitschopf et al. [130] showed that a dominant-negative Akt significantly reduced telomerase activity in HUVECs. Therefore, it is possible that ox-LDL accelerates the onset of EPCs senescence through telomerase inactivation, which may be related to inactivation.
POTENTIAL ROLE OF OXIDATIVE STRESS IN EPCS SENESCENCE IN ACTIVATION OF RENIN-ANGIOTENSIN SYSTEM
Ang II and Oxidative Stress
Ang II increases oxidative stress, inflammation, and alters endothelial function [131, 132]. The principle sources of ROS in the human vasculature is NAD(P)H oxidase, activated by a number of proatherogenic stimuli including Ang II. Overall NAD(P)H oxidase activity in cells is achieved by several components. These included cell-membrane-associated p22phox and gp91phox (or gp91phox [nox2] homologues, nox1 and nox4), as well as cytosolic p47phox and p67phox [132]. Vascular NAD(P)H oxidase is upregulated by vasoactive factors, stretch, shear stress and pulsatile strain [132]. At low concentrations ROS serve a physiological role as signalling molecules involved in endothelial function and vascular contractility [131]. However, pathological increases in ROS results in a plethora of effects that damage the vessel wall [133].
Ang II and EPCs Senescence
We have shown that in animal models of hypertension, as well as in subjects with essential hypertension, EPCs become precociously senescent and dysfunctional [134]. Higher blood pressure is associated with lower EPCs levels in the general population [135], and in diabetic subjects [136]. Hyperreactivity of the renin-angiotensin system (RAS) has been recognized as one link between hypertension and altered EPCs biology. Bahlmann et al. [136] documents that angiotensin receptor antagonists increase the number of EPCs in patients with type II diabetes mellitus. This effect seems to be a class effect, because they have demonstrated it with standard doses of 2 long acting ARBs (olmesartan or irbesartan). In contrast, in patients treated with standard antihypertensives, they did not observe any effects of EPCs. Ramipril is an angiotensin-converting enzyme (ACE) inhibitor used to reduce RAAS activation in patients with stable CAD. Min et al. [137] showed that increased numbers of EPCs could be cultured from ramipril-treated patients with stable CAD and that ACE inhibition resulted in improved functional properties like adhesion, proliferation, migration, and in vitro vasculogenesis assay, independent of any impact on blood pressure. These results show that EPCs are sensitive to Ang II signalling and that this should indeed impact on number and function. Our group has shown that Ang II increases the rate of senescence of EPCs and that this appears to be a consequence of its ability to stimulate expression of gp91phox and thus O2- formation [138]. In addition, we have demonstrated that the ability of Ang II to induce senescence also involves the suppression of telomerase [138]. Our group also noticed an expected increase in formation of a marker of oxidative stress-peroxynitrite, which is formed of O2- with NO in the Ang II-treated EPCs [138]. Increased O2- production is a feature of Ang II-dependent hypertension [139]. Thus, under conditions of Ang II excess, Ang II most likely contributes to the decline in formation of the vascular endothelium at least in part by the mechanisms via Ang II-induced EPCs senescence (Fig. (3)). On the other hand, it has been demonstrated that Ang II and angiotensin peptides promote hematopoietic progenitor cell proliferation and hematopoietic recovery after radiation therapy and chemotherapy [140]. Murohara et al reported that the Ang II-AT1 receptor pathway plays an important role in angiogenesis associated with ischemia and tumor growth [141, 142]. These results appear to be contradictory to protective effects of RAS suppression on EPCs function. Recent studies suggested that the intracellular redox state is a critical modulator of the balance between self-renewal and differentiation in dividing precursor cells and that anti-oxidant may preserve their stemness [143]. It is plausible that a reduction in oxidative stress resulted in restoration of the impaired faction of EPCs in spontaneous hypertensive rats as well as patients with metabolic disorders, although it remains to be determined whether RAS inhibition stimulates EPCs function under physiological conditions in healthy subjects.
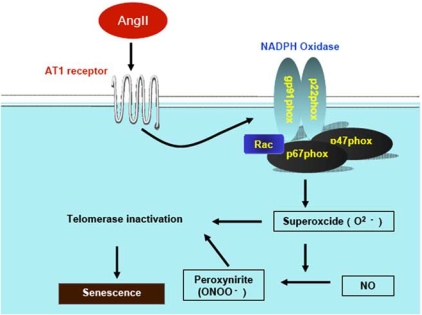
Fig. (3). Potential mechanisms of Ang II-induced EPCs senescence.
Ang II stimulates gp91phox expression, a subunit of NADPH oxidase, via the angiotensin type 1 (AT1) receptor, which leads to the increase in superoxide (O2-). Furthermore, peroxynitrite is formed form the inteaction of O2- with nitric oxide (NO). Both superoxide and peroxynitrite inactivate telomerase activity, which induces the impairment of telomere structure integrity, resulting in senescence.
PHARMACOLIGIC MODULATIONS ON EPCS SENESCENCE
HMG-CoA Reductase Inhibitors (Statins)
Pharmacologically, statins have been shown to enhance EPCs-mediated angiogenesis in models of ischemic tissue injury. In vivo, statin treatment increases the numbers of circulating EPCs and enhances both neovascularization in corneal assays and reendotheliazation of injured vessels, promoting incorporation of labeled BM-derived cells into these vessels [144-146]. Mechanistically, statin treatment in vitro appears to inhibit EPCs senescence, via induction of telomere repeat binding factor-2, which inhibits induction of the DNA damage checkpoint-kinase 2 [147]. Simvastatin activates the serine-threonine kinase Akt in endothelial cells, promoting endothelial cell survival and migration [148]. Akt also acts downstream of VEGF and may therefore represent a key regulator of VEGF-mediated neovascularization [149]. Thus, these data suggest that statin therapy may constitute an important approach in the development of strategies to improve EPCs survival and function and to improve cardiac repair pathways in the aging population. Indeed, the TOPCARE-AMI clinical trial demonstrated that the treatment of ex vivo cultured blood-derived progenitor cells with atorvastatin was found to be safe and potentially effective for the enhancement of cardiac regeneration [150].
Ang II Receptor Blockers (ARBs)
ARBs have been shown to be antioxidant and vasoprotective through downregulation of vascular NAD(P)H oxidase expression in patients with CAD [151]. Treatment with either an ACE inhibitor or ARB lowered levels of vascular superoxide. Bahlmann et al. [136] reported that ARBs increase the number of EPCs in patients with type II diabetes mellitus. Our group reported that Ang II accelerates EPCs senescence via the AT1 receptor and through induction of oxidative stress [138], (Fig. (3)). Exposure of cultured EPCs to Ang II significantly accelerated the rate of senescence compared with a control and impaired proliferative activity. We also showed that Ang II-induced EPCs senescence was significantly inhibited by pretreatment with either valsartan or superoxide dismutase (SOD) [138]. Ang II also significantly diminished telomerase activity, and this effect was significantly reduced by pretreatment with either an AT1 receptor antagonist, valsartan, or SOD [138]. In addition, Yao et al. [152] reported that EPCs colony formation was markedly lower in SHR-SP than in WKY rats. Losartan improved the reduced colony formation with inhibition of oxidation from SHR-SP by reducing expression of gp91phox, p22phox, and p47phox. Trichlormethiazide did not affect the reduced colony formation in EPCs. Thus, EPCs function was altered in Ang II-dependent hypertension with oxidative stress. These data indicate that EPC has an Ang II-generating system that accelerates senescence of EPCs and may directly contribute to vascular injury in hypertension.
Estrogens
No direct studies of effect of estrogen therapy on EPCs in humans are available, but increased blood estrogen levels in women do correlate with numbers of circulating EPCs [130]. In an animal carotid injury model, estradiol treatment showed stimulatory effects on EPCs mobilization, proliferation, mitogenic and migratory activity, as well as inhibited EPCs apoptosis [123]. Mechanistically, we have demonstrated that estrogen augments differentiation and delays the onset of the senescence in BM-EPCs from SHR/Izm, accompanied with telomerase activation via up-regulation of hTERT mRNA in a PI3-K/Akt dependent manner [153]. Importantly, the inhibition of BM-EPCs senescence by estrogen in vitro resulted in improved functional activity of BM-EPCs [153]. We showed that 17β-estradiol dose-dependently inhibited the senescence of cultured human PB-MNCs by increasing the catalytic activity of telomerase [154]. We also showed that telomerase activity in EPCs is upregulated by treatment with 17β-estradiol. Furthermore, we have demonstrated that this activation accompanied upregulation of the hTERT mRNA [154]. We speculated that estrogen delays the onset of senescence through telomerase activation, which may be related to estrogen-induced upregulation of the expression of hTERT. However, with regard to senescence, the structure of the telomere appears at least as important as its absolute length, in relation to telomere function [155]. In addition, we can not rule out the possibility that a telomere-independent mechanism regulates replicative senescence. Further studies are required to elucidate the mechanisms underlying the inhibitory effects of 17β-estradiol on senescence in EPCs. Importantly, the inhibitory effects of 17β-estradiol on EPCs senescence may not be of only a beneficial nature. Telomere shortening is probably one of the body’s anticancer mechanisms, and estrogen is considered to be a potentially cancer-promoting substance. Indeed, estrogens increase the incidence of various malignancies [156]. It is tempting to speculate that the induction of neoplasia during estrogen treatment might be dependent on the effects of EPCs.
CONCLUSIONS AND IMPLICATIONS
Recent data show that the vascular regenerative potential of patients with cardiovascular risk factors may be impaired as a consequence of reduced number and function of circulating EPCs that can support endothelial maintenance and ischemia-induced neovascularization. Recent studies have demonstrated a critical role of oxidative stress in the EPCs dysfunction. Notably, oxidative stress-induced EPCs premature senescence is involved in this process. Autologous transplatation of progenitor cells that are affected by cardiovascular risk factors, may not only be hampered by a dysfunctional nature of these cells but in fact may stimulate proatherogenic mechanisms, such as monocyte recruitment or vascular smooth muscle cell proliferation. Therefore, attention should be paid to transplantation of autologous BM cells or circulating EPCs not to promote atherosclerosis. The therapeutic goal must be the rebalancing between endothelial injury and repair. In the future, the use of a vascular repair index may be important for choosing therapy strategies with a maximized benefit for the patient. With this knowledge in mind, we need to search for more effective proregenerative therapeutic strategies not only for neoangiogenesis but, more importantly, for regeneration of the dysfunctional vascular wall, which represents the common trunk for all cardiovascular diseases.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasa M, Fichtlscherer S, Aisher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 4.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 5.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 6.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 7.Scheubel RJ, Zorn H, Silber RE, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 9.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: Molecular and cellular mechanism. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 11.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 12.Ogita H, Liao J. Endothelial function and oxidative stress. Endothelium. 2004;44:248–252. doi: 10.1080/10623320490482664. [DOI] [PubMed] [Google Scholar]
- 13.Blokhina O, Virolainen E, Fagerstedt KY. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dernbach E, Urbich C, Brandes RP, et al. Antioxidative stress-associated genes in circulating progenitor cells: Evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 15.He T, Peterson TE, Holmuhamedov EL, et al. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 16.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 17.Ingram DA, Krier TR, Mead LE, et al. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 18.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 19.Gulati R, Jevremovic D, Peterson TE, et al. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Raffi S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 21.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 23.Civin CI, Banquerigo ML, Strass LC, Loken MR. Antigenic analysis of hematopoiesis. VI. Flow cytometric characterization of My-10 positive progenitor cells in normal human bone marrow. Exp Hematol. 1987;15:10–17. [PubMed] [Google Scholar]
- 24.Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 25.Dong C, Crawford L, Goldschmidt-Clermont PJ. Endothelial progenitor obsolescence and atherosclerotic inflammation. J Am Coll Cardiol. 2005;45:1458–1460. doi: 10.1016/j.jacc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 27.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 28.Miraglia S, Godfrey W, Yin AH, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 29.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 30.Romagnani P, Annunziato F, Liotta F, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 31.Elsheikh E, Uzunel M, He Zhong, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 32.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 33.Gulati R, Jevrempovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 34.Rockmaaker MB, Vergeer M, van Zonneveld Aj, Rabelink TJ, Verhaar MC. Endothelial progenitor cells: mainly derived from the monocyte/macrophage containing CD34- but also partly derived from the hematopoetic stem cell containing CD34+ mononuclear population. Circulation. 2003;108:150e. doi: 10.1161/01.CIR.0000100885.93909.FB. [DOI] [PubMed] [Google Scholar]
- 35.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells utilizing human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Heeschen C, Aisher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 37.Kalka C, Matsuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbich C, Dimmeler S. Endothelial progenitor cells: functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Hur J, Yang HM, Yoon CH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characteristics of endothelial progenitor cell colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 40.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne A, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 44.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 45.Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- 46.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 47.Schober A, Karshovska E, Zernecke A, Weber C. SDF-1α-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc Med. 2006;16:103–108. doi: 10.1016/j.tcm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Massberg S, Konrad I, Schurzinger K, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to artrial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100:590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 50.Zernecke A, Schober A, Bot I, et al. SDF-1α/CXCR4 axis is instumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 51.Walter DH, Haendeler J, Reinhold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 52.Cho HJ, Kim HS, Lee M, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendotheliazation and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108:2918–2925. doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Porath I, Weinberg RA. When cells get stressed: An integrative view of cellular senescence. J Clin Invest. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright WE, Shay JW. Histological claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 55.Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 56.Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:2495–2502. [PubMed] [Google Scholar]
- 57.Sitte N, Merker K, Von Zglinicki T, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts. I. Effects of proliferative senescence. FASEB J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 58.Narita M, Nunez S, Heard E, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh's dilemma. Am J Physiol Heart Circ Physiol. 2006;290:1729–1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 60.Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. doi: 10.1016/s0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- 61.Faragher RG, Kipling D. How might replicative senescence contribute to human agening? Bioassays. 1998;20:985–991. doi: 10.1002/(SICI)1521-1878(199812)20:12<985::AID-BIES4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 62.Gire Roux P, Wynford-Thomas D, Brondello JM, Dulic V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J. 2004;23:2554–2563. doi: 10.1038/sj.emboj.7600259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaneko T, Tahara S, Taguchi T, Kondo H. Accumulation of oxidative DNA damage, 8-oxo-2'-deoxyguanoside, and change of repair systems during in vitro cellular aging of cultured human skin fibroblasts. Mutat Res. 2001;487:19–30. doi: 10.1016/s0921-8777(01)00100-8. [DOI] [PubMed] [Google Scholar]
- 64.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 66.Llond AC. Limits to lifespan. Nat Cell Biol. 2002;4:E25–E27. doi: 10.1038/ncb0202-e25. [DOI] [PubMed] [Google Scholar]
- 67.Satyanarayana A, Wiemann SU, Buer J, et al. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J. 2003;22:4003–4013. doi: 10.1093/emboj/cdg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 69.Dimri GP, Lee X, Basile G, et al. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuju KS, Lehman JM. Increased p53 protein associated with aging in human diploid fibroblasts. Exp Cell Res. 1995;217:336–345. doi: 10.1006/excr.1995.1095. [DOI] [PubMed] [Google Scholar]
- 71.Dirac AM, Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J Biol Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- 72.Castro ME, del Valle Guijarro M, Moneo V, Carnero A. Cellular senescence induced by p-53-ras cooperation is independent of p21waf1 in murine embryo fibroblasts. J Cell Biochem. 2004;92:514–524. doi: 10.1002/jcb.20079. [DOI] [PubMed] [Google Scholar]
- 73.Favetta LA, Robert C, King WA, Betts DH. Expression profiles of p53 and p66shc during oxidative stress-induced senescence in fetal bovine fibroblasts. Exp Cell Res. 2004;299:36–48. doi: 10.1016/j.yexcr.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Chen QM, Liu J, Merret JB. Apoptosis or senescence-like growth arrest: Influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bladier C, Volvetang EJ, Hutchinson P, de Haan JB, Kola I. Response of a primary human fibroblast cell line to H2O2: Senescence-like growth arrest or apoptosis? Cell Growth Differ. 1997;8:589–598. [PubMed] [Google Scholar]
- 76.Rincheval V, Renaud F, Lemaire C, et al. Bcl-2 can promote p-53 dependent senescence versus apoptosis without affecting the G1/S transition. Biochem Biophy Res Commun. 2002;298:282–288. doi: 10.1016/s0006-291x(02)02454-3. [DOI] [PubMed] [Google Scholar]
- 77.Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Patel JM, Block ER. Enhanced apoptosis in prolonged cultures of senescent porcine pulmonary artery endothelail cells. Mech Ageing dev. 2002;123:613–625. doi: 10.1016/s0047-6374(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 79.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 80.Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cencer and aging: the telomere connection. Exp Gerontol. 2001;36:1619–1637. doi: 10.1016/s0531-5565(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 81.Blackburn EH. Telomeres and telomerase: Their mechanism of action of action and the effects of altering their function. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 82.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 83.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 84.Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- 85.Li C-Z, Eller MS, Firoozabadi R, Gilchrest BA. Evidence that exposure of the telomere 3’ overhang sequence induces senescence. Proc Natl Acad Sci USA. 2003;100:527–531. doi: 10.1073/pnas.0235444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bodnar AG, Quellete M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cell. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 87.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 88.Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-trasnformed human cells without net telomere lengthening. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of huma telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 90.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 91.Lee HW, Blasco MA, Gottlieb GJ, Horner JW 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 92.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 93.Minamino T, Kourembanas S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res. 2001;89:237–243. doi: 10.1161/hh1501.094267. [DOI] [PubMed] [Google Scholar]
- 94.Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001;21:3336–3342. doi: 10.1128/MCB.21.10.3336-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsiao R, Sharma HW, Ramakrishnan S, Keith E, Narayanan R. Telomerase activity in normal human endothelial cells. Anticancer Res. 1997;17:827–832. [PubMed] [Google Scholar]
- 96.Yang J, Chang E, Cherry AM, et al. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 97.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 98.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness” transcriptional profiling of embrypnic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 99.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galasso G, Schickofer K, Sato K, et al. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormialities in vascular and cellular function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 102.Aicher A, Heeschen C, Midner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 103.Laufs U, Wemer N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointimal formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 104.Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 105.Colak E, Majkic-Singh N, Stankovie S, et al. Parameters of antioxidative defense in type 2 diabetes patients with cardiovascular complications. Ann Med. 2005;37:613–620. doi: 10.1080/07853890500330193. [DOI] [PubMed] [Google Scholar]
- 106.Valgimigli M, Rigolin GM, Fucili A, et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 107.Scheubel RJ, Zorn H, Silber RE, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 108.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 109.Loomans CJM, deKoening EJP, Staal FJT, et al. Endothelial progenitor cell dysfunction. A novel concept in the pathogenesis of vascular complications of type I diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 110.Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via an activation of p38 mitogen-activated protein kinase. Circ J. 2006;70:1076–1081. doi: 10.1253/circj.70.1076. [DOI] [PubMed] [Google Scholar]
- 111.Seeger FH, Haendeler J, Walter DH, et al. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 112.Adams RH, Porras A, Alonso G, et al. Essential role of p38 MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6:106–116. [PubMed] [Google Scholar]
- 113.Matsumoto T, Turesson I, Bock M, Gerwins P, Claesson-Welsh L. p38 MAP kinase negatively regulates endothelial cell survaival, proliferation, and differentiation in FGF-2 stmulated angiogenesis. J Cell Biol. 2002;156:149–160. doi: 10.1083/jcb.200103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 115.Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress in vivo reendotheliazation capacity of endothelial progenitor cells from patients with type 2 diabeted mellitus. Resoration by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 116.Gensch C, Clever YP, Werner C, et al. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 117.Imanishi T, Kobayashi K, Kuroi A, et al. Pioglitazone inhibits angiotensin II-induced senescence of endothelial progenitor cell. Hypertens Res (in press) 2009 doi: 10.1291/hypres.31.757. [DOI] [PubMed] [Google Scholar]
- 118.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial progenitor cells resotre aging-improved cardiac angiogenic function. Circ Res. 2002;90:e89–e93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 119.Scheubel RJ, Zorn H, Rolf-Edgar S, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing croronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 120.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem cell and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 121.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 122.Bernardini D, Ballabio E, Mariotti M, Maiser JA. Differential expression of EDF-1 and endothelial nitric oxide synthase by proliferating, quiescent and senescent microvascular endothelial cells. Biochem Biophys Acta. 2005;1745:265–272. doi: 10.1016/j.bbamcr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 123.Iwakura A, Luedemann C, Shastry S, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendotheliazation after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 124.Papapetropoulos A, Gracia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endohtleial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 126.Ma FX, Zhou B, Chen Z, et al. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res. 2006;47:1227–1237. doi: 10.1194/jlr.M500507-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31:407–413. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 128.Imanishi T, Hano T, Matsuo Y, Nishio I. Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharmacol Physiol. 2003;30:665–670. doi: 10.1046/j.1440-1681.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- 129.Dimmeler S, Aisher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breitschopf K, Zeiher AM, Dimmeler S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 2001;493:21–25. doi: 10.1016/s0014-5793(01)02272-4. [DOI] [PubMed] [Google Scholar]
- 131.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 132.Sowers JR. Hypertension, Angiotensin II, and Oxidative Stress. N Engl J Med. 2002;346:1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 133.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 134.Imanishi T, Moriwaki C, Hano T, et al. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with hypertension. J Hypertens. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 135.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 136.Bahlmann FH, de Groot K, Mueller OH, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells-a new putative therapeutic effect of angiotensin II receptor antagonism. Hypertension. 2005;45:526–529. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 137.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–209. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 138.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23:97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 139.Heitzer T, Wenzel U, Hink U, et al. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 140.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18:287–294. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 141.Sasaki K, Murohara T, Ikeda H, et al. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest. 2002;109:603–611. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Egami K, Murohara T, Shimada T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function. Ann NY Acad Sci. 2003;991:251–271. doi: 10.1111/j.1749-6632.2003.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 144.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendotheliazation: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 145.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendotheliazation and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 146.Llevadot J, Murasawa S, Kureishi Y, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spyridopoulos I, Haendeler J, Urbich C, et al. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004;110:3136–3142. doi: 10.1161/01.CIR.0000142866.50300.EB. [DOI] [PubMed] [Google Scholar]
- 148.Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 150.Assmus B, Schachinger V, Teupe C, et al. Transplatation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 151.Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: Protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 152.Yao EH, Fukuda N, Matsumoto T, et al. Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect. Hypertens Res. 2007;30:1119–1128. doi: 10.1291/hypres.30.1119. [DOI] [PubMed] [Google Scholar]
- 153.Imanishi T, Kobayashi K, Hano T, Nishio I. Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens Res. 2005;28:763–772. doi: 10.1291/hypres.28.763. [DOI] [PubMed] [Google Scholar]
- 154.Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005;23:1699–1706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- 155.Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, Weinberg RA, Stewart SA, Hahn WC. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 156.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D. HERS Research Group. JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]