Abstract
Background
Klotho knockout mice (klotho-/-) have increased renal expression of sodium/phosphate co-transporters (NaPi2a), associated with severe hyperphosphatemia. Such serum biochemical changes in klotho-/-mice lead to extensive soft tissue anomalies and vascular calcification. To determine the significance of increased renal expression of the NaPi2a protein and concomitant hyperphosphatemia and vascular calcification in klotho-/-mice, we generated klotho and NaPi2a double knockout (klotho-/-/NaPi2a-/-) mice.
Methods and Results
Genetic inactivation of NaPi2a activity from klotho-/-mice reversed the severe hyperphosphatemia to mild hypophosphatemia or normophosphatemia. Importantly, despite significantly higher serum calcium and 1,25-dihydroxyvitamin D levels in klotho-/-/NaPi2a-/- mice, the vascular and soft tissue calcifications were reduced,. Extensive soft tissue anomalies and cardiovascular calcification were consistently noted in klotho-/-mice by 6 weeks of age; however, these vascular and soft tissue abnormalities were absent even in 12-week-old double knockout mice. Klotho-/-/NaPi2a-/- mice also regained body weight and did not develop the generalized tissue atrophy often noted in klotho-/-single knockout mice.
Conclusion
Our in vivo genetic manipulation studies have provided compelling evidence for a pathologic role of increased NaPi2a activities in regulating abnormal mineral ion metabolism and soft tissue anomalies in klotho-/- mice. Notably, our results suggest that serum phosphate levels are the important in vivo determinant of calcification, and that lowering serum phosphate levels can reduce or eliminate soft tissue and vascular calcification, even in presence of extremely high serum calcium and 1,25-dihydroxyvitamin D levels. These in vivo observations have significant clinical importance and therapeutic implications for chronic kidney disease patients with cardiovascular calcification.
Keywords: Klotho, Vitamin-D, NaPi2a, Calcification
Introduction
Understanding the molecular regulation of phosphate homeostasis has enormous clinical and biological importance, as it is involved in numerous essential biochemical reactions, including cell signaling process and energy metabolism. Adequate bone mineralization is closely dependent on the status of phosphate metabolism. Abnormal regulation of phosphate homeostasis can cause myopathy, cardiac dysfunctions, hematological abnormalities, and vascular/soft tissue calcifications 1-3. Recent studies have found that klotho, a transmembrane protein, is actively involved in regulation of mineral ion metabolism by affecting the functionality of ion channels and co-transporter proteins in the kidney 4,5. The in vivo importance of klotho in regulation of mineral ion metabolism is further evident in klotho knockout mice, as these mice have severely impaired mineral ion homeostasis 5,6.
The klotho-/-mice develop severe hyperphosphatemia by three weeks of age, and remain hyperphosphatemic throughout their life 7,8. The hyperphosphatemia in klotho-/- mice is associated with increased renal expression of the NaPi2a co-transporter protein in the proximal tubular epithelial cells 3,7,9. To assess the significance of increased renal expression of NaPi2a in soft tissue anomalies and vascular calcification in klotho-/- mice, we have generated a new mouse model by genetically ablating both the klotho and NaPi2a genes.
Hyperphosphatemia and reduced serum levels of 1,25-dihydroxyvitamin D are the major biochemical changes detected in patients with chronic kidney disease (CKD). The current treatment approach of reducing serum phosphate levels and providing vitamin D analogs in patients with CKD often poses a dilemma, as studies linked vitamin D treatment to subsequent vascular calcification. Importantly, about 50% of mortalities in CKD patients undergoing dialysis treatment are due to the cardiovascular complication of vascular calcification. Our current study provides the in vivo beneficial effects of reducing serum phosphate levels on preventing vascular calcification, even in the presence of extremely high serum 1,25-dihydroxyvitamin D.
Materials and methods
Generation of double mutant mice
We have crossbred heterozygous klotho mutants [Lexicon Genetics; Mutant Mouse Regional Resource Centers, University of California at Davis, CA] with heterozygous NaPi2a mutants to obtain compound heterozygous animals, which were then interbred to generate the desired double homozygous mutants [klotho-/-/NaPi2a-/-] 7,9-11. Routine PCR was used to identify the genotypes of various mice (Supplementary Fig. 1). All studies performed were approved by the institutional animal care and use committee at the Harvard Medical School, Boston, MA.
Gross phenotype and body weight
The total body weight of each of wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice was taken every week starting at 3 weeks of age until 20 weeks of age. The maximum survival of klotho−/− mice was around 15 weeks.
Biochemical measurements
Blood was obtained by cheek-pouch bleeding of wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice. Serum was isolated by centrifugation at 3000 g for 10 minutes and stored at −80°C. Serum and urinary phosphorus and calcium were determined by colorimetric measurements using the Stanbio Phosphorus Liqui-UV Test and Calcium (Arsenazo) LiquiColor Test, respectively, as used in our earlier publications 12. The level of 1,25-hydroxyvitamin-D [1,25(OH)2D3] was measured in serum obtained from wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice using a commercial kit (Immunodiagnostic Systems Ltd. Fountain Hills, AZ). The serum level of parathyroid hormone (PTH) was measured using a commercial kit (Immutopics, Inc. San Clemente, CA). The serum level of Fgf23 was measured by ELISA using a commercial kit (Kainos Laboratories, Japan), described in a previous publication 7.
Histological analyses
Soft tissues, obtained from wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice at 9-12 weeks were fixed with 4% paraformaldehyde, 10% buffered formalin, or Carnoy's solution and were subsequently embedded in paraffin. Four to six-micrometer paraffin sections of various tissues were mounted on SuperFrost Plus slides. Sections were then routinely stained with hematoxylin and eosin, and von Kossa 13,14. Histological changes were observed by light microscopy.
Calcification analyses
To determine the effects of hyperphosphatemia on soft tissue and vascular calcification in klotho−/− mice, sections were prepared from heart, lung, kidney, liver, spleen, aorta and the gastrointestinal tract, and were stained with von Kossa to visualize mineralized tissues by light microscopy. The von Kossa stained sections of klotho-/-/NaPi2a-/- mice were compared with similarly stained sections from wild-type, klotho−/−, and NaPi2a-/- mice. The von Kossa staining procedure is detailed in an earlier publication 15.
Immunofluorescence staining
Immunostaining was performed as described previously 16-18. Briefly, kidneys obtained from wild-type and klotho−/− mice were embedded in OCT and stored at −80°C. Frozen sections were incubated in a blocking solution for 30 minutes and then overnight with polyclonal anti-NaPi2a antibody (dilution 1:100; Alpha Diagnostic, San Antonio, TX) at 4°C. The slides were washed with PBS and incubated with fluorescein isothiocyanate-labeled anti-rabbit secondary antibody (dilution, 1:100) for 30 minutes. After a PBS wash, cover-slips were placed on slides using 4,6-diamidino-2-phenylindole (DAPI)-containing mounting media. The expression of NaPi2a was visualized using an immunofluorescence microscopy. Rabbit serum, in place of primary antibody, was used as a negative control. Kidney sections prepared from NaPi2a-/- mice were simultaneously stained for NaPi2a, and used as additional negative control.
Quantitative real-time PCR
Total RNA isolated from the at least three or more kidneys and aortas of wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice was used to detect the relative expression of Ennp-1, ANK, Pit-1, and RUNX2 mRNA as described previously 12,19. Real-time PCR was performed in duplicate. The quantity of mRNA was calculated by normalizing the CT (threshold cycle value) of Ennp-1, ANK, Pit-1 or RUNX2 to the CT of the housekeeping gene GAPDH. The sequences of the primers used to detect expression patterns of various genes are reported in our earlier publications 7.
Statistical analysis
Statistically significant differences between groups were evaluated either by the Student's t-test or by Mann-Whitney U-test for a comparison between two groups. All values were expressed as mean ± SE. A p value of less than 0.05 was considered to be statistically significant. All analyses were performed using Microsoft Excel.
Results and discussion
The identification of specific phosphate transporters has enhanced our understanding of the regulation of renal and intestinal phosphate handling. The type II family of NaPi co-transporters consists of three highly homologous isoforms: type IIa (NaPi2a) and type IIc (NaPi2c), which are mostly expressed in the brush-border membrane of the renal proximal tubules 20, and type IIb (NaPi2b), which is expressed in the epithelial cells of the small intestine and is thought to be involved in intestinal phosphate absorption. Renal phosphate transport through NaPi2a is an important mechanism for maintaining systemic phosphate balance.
To determine the expression pattern of NaPi2a in klotho−/− mice, we stained kidney sections from wild-type and klotho−/− mice with NaPi2a antibody. Compared with wild-type mice, increased expression of NaPi2a protein, in the luminal side of the proximal tubules, was noted in sections from klotho−/− mice (Supplementary Fig. 2). It is presumed that increased expression of NaPi2a is associated with increased renal reuptake of phosphate 21, resulting in hyperphosphatemia and extensive vascular calcification in klotho−/− mice (Supplementary Fig. 3). To test this hypothesis, we have generated klotho-/-/NaPi2a-/- mice and compared their physical, morphological, and biochemical phenotype with klotho-/-mice.
As mentioned, genetic deletion of klotho results in hyperphosphatemia 8,9. To determine whether hyperphosphatemia in klotho−/− mice is a NaPi2a-dependent process, we generated a new mouse model that is deficient in both klotho and NaPi2a genes (klotho-/-/NaPi2a-/-), by interbreeding heterozygous klotho mice with heterozygous NaPi2a mice. The klotho-/-/NaPi2a-/- mice were viable and larger in size to klotho−/− mice. At birth, klotho-/-/NaPi2a-/- mice were indistinguishable from their littermates of other genotypes. At 3 weeks of age, klotho-/-/NaPi2a-/- mice were slightly larger in size than klotho-/- mice (12.8±0.5 g vs. 10.4±0.5 g), but smaller than wild-type mice (16.0±0.5 g). Double knockout mice were similar in size to NaPi2a-/-animals (13.4±0.27 g). At 9 weeks of age, klotho-/-/NaPi2a-/- mice were still smaller than their wild-type littermates (20.2±0.85 g vs. 28.8±1.1 g), but their body weight was significantly higher than that of klotho-/- mice (11.3±0.9 g). At 9 weeks of age, the average body weight of NaPi2a-/- mice was 25.7±0.76 g (Fig. 1). Furthermore, klotho-/- mice had a maximum survival of around 15 weeks, while all the klotho-/-/NaPi2a-/- mice were capable of survival beyond 15 weeks and were alive until 20 weeks of observation period.
Figure-1. Macroscopic phenotype of klotho-/-/NaPi2a-/- mice.
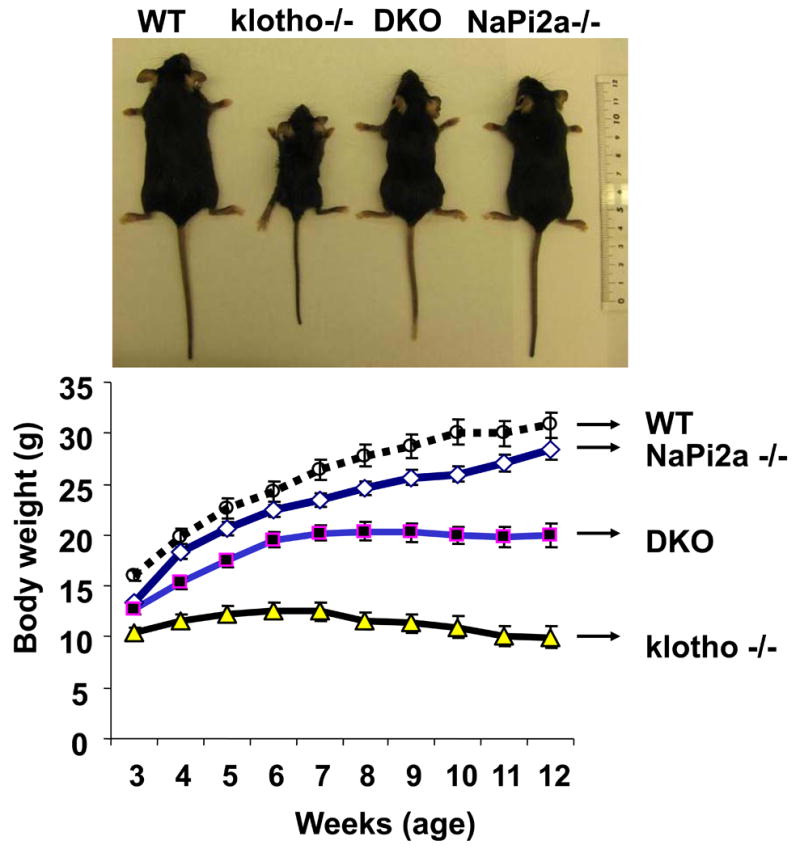
Gross phenotype of wild-type (WT), klotho-/-, klotho-/-/NaPi2a-/- (DKO), and NaPi2a-/- mice at around 12 weeks of age (upper panel). Body weight curves (lower panel) for all four genotypes, showing DKO mice (n=34) are smaller than WT mice (n=22), but larger than klotho-/- mice (n=23), suggesting that inactivation of NaPi2a function from klotho-/- mice helped in regaining the body weight in DKO mice. The average body weight of the NaPi2a-/- mice (n=42) is more than DKO mice. The statistical analyses among the groups were compared through Student's unpaired two-tail t-test (p<0.0001 at all time points for WT vs. klotho-/- mice; p<0.001 at all time points for WT vs. DKO mice; p<0.0001 at all time points for klotho-/- vs. NaPi2a-/- mice; p<0.0001 at all time points for klotho-/- vs. DKO mice).
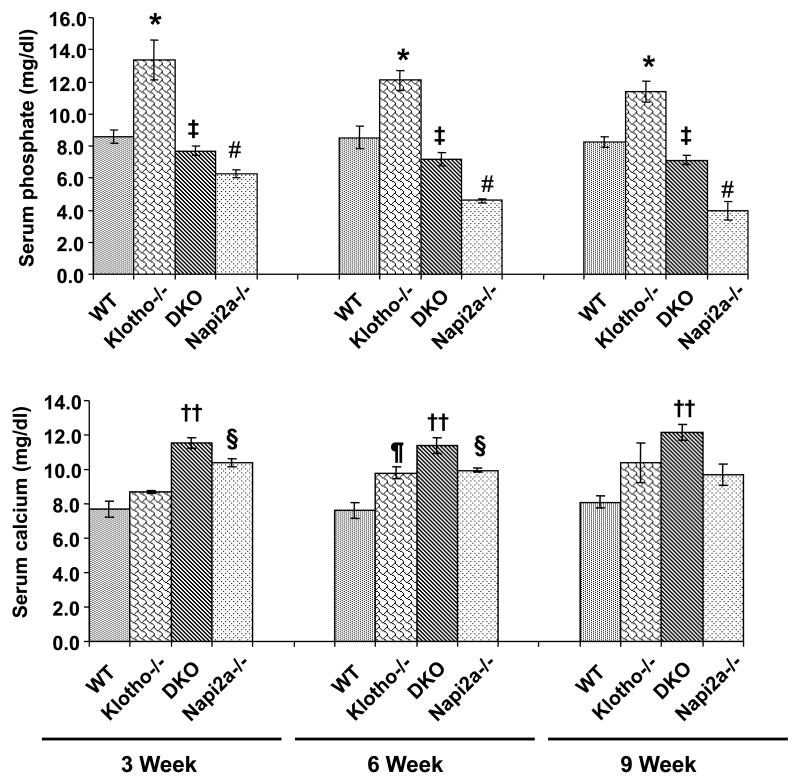
Next, we measured serum phosphate and calcium levels in 3-, 6-, and 9-week-old wild-type, klotho−/−, NaPi2a-/-, and klotho-/-/NaPi2a-/- mice. The double knockout mice were slightly hypophosphatemic by 6 weeks of age (7.2±0.41 mg/dl, n=10), compared to wild-type mice (8.5±0.69 mg/dl, n=5). The low serum phosphate levels were also noted in age-matched NaPi2a-/- mice (4.6±0.14 mg/dl, n=10). Serum phosphate levels were slightly high in klotho−/− mice (12.1±0.64 mg/dl, n=11) (Fig. 2). Significant reduction of serum phosphate levels were also noted in 9-week-old double knockout mice, compared to klotho−/− mice. Collectively, these findings suggest that inactivation of NaPi2a function can reverse hyperphosphatemia to hypophosphatemia or normophosphatemia at 3-, 6-, and 9-weeks of age.
Figure 2. Biochemical analysis of serum phosphate and calcium.
The serum phosphate (upper panel) and calcium (lower panel) levels are higher in klotho−/− mice, compared to the wild-type (WT) mice at 3-, 6-, and 9- weeks of age. In contrast to the klotho−/− mice, serum phosphate levels are markedly reduced in klotho-/-/NaPi2a-/- (DKO). Serum phosphate levels are also low in NaPi2a-/- mice (*: p < 0.05, vs. WT; #: p < 0.001, vs. WT; ‡: p < 0.001, vs. klotho-/-). As for serum calcium levels, compared to the WT controls, increased serum levels of calcium are noted in all three mutant mice at different time points. At around 6 weeks of age, serum calcium in klotho-/-/NaPi2a-/- mice (11.4±0.44 mg/dl, n=12) is significantly higher than in wild-type mice (7.6±0.46 mg/dl, n=6). The higher serum levels of calcium are also noted in klotho−/− mice (9.8±0.34 mg/dl, n=10) and NaPi2a-/- mice (10.0±0.12 mg/dl, n=10) of similar age. The statistical analyses among the groups were compared through Student's unpaired two-tail t-test (¶: p < 0.01, vs. WT; ††: p < 0.001, vs. WT; §: p < 0.001, vs. WT).
Serum calcium levels were higher in the klotho−/− mice at around 6 weeks of age, as also noted in both NaPi2a-/- and klotho-/-/NaPi2a-/- mice (Fig. 2). At around 6 weeks of age, serum calcium in klotho-/-/NaPi2a-/- mice (11.4±0.44 mg/dl, n=12) was significantly higher than in wild-type mice (7.6±0.46 mg/dl, n=6). The higher serum levels of calcium were also noted in klotho-/- mice (9.8±0.34 mg/dl, n=10) and NaPi2a-/- mice (10.0±0.12 mg/dl, n=10) of similar age (Fig. 2).
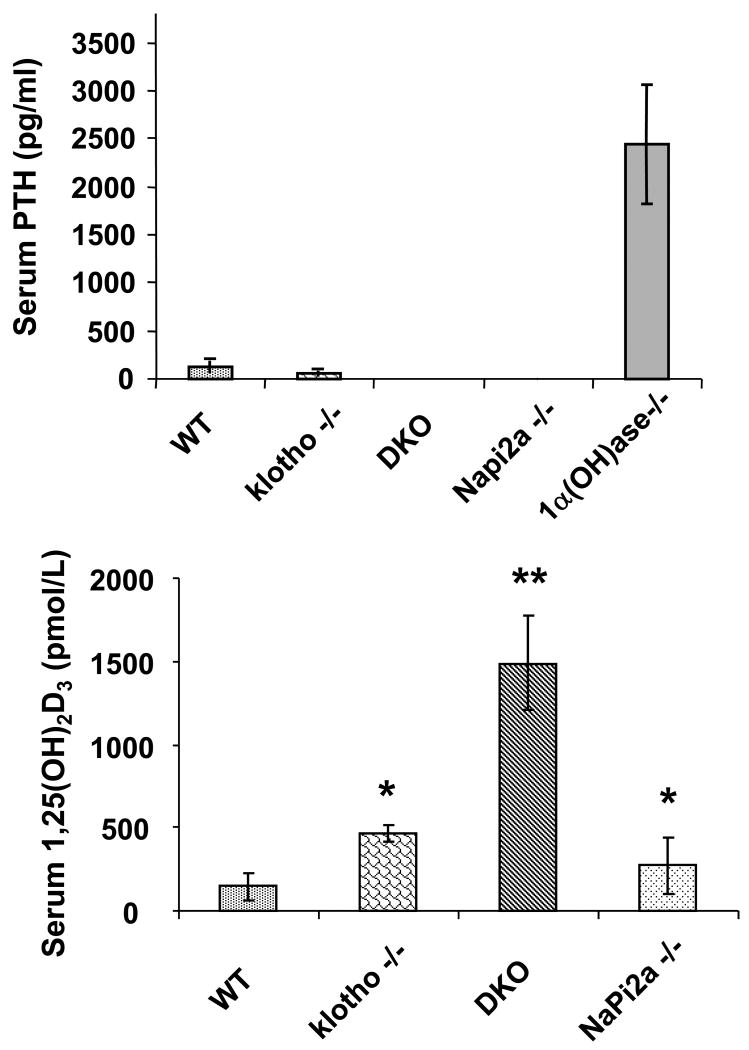
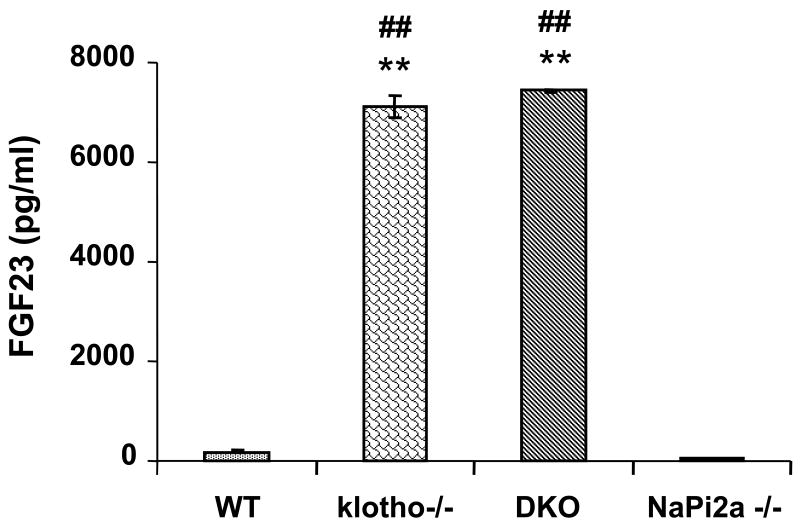
Despite reversal of serum phosphate level from severe hyperphosphatemia to mild hypophosphatemia or normophosphatemia in klotho-/-/NaPi2a-/- mice, the serum 1,25(OH)2D3 levels in klotho-/-/NaPi2a-/- mice were extremely high. Statistically significant increased serum levels of 1,25(OH)2D3 were also detected in klotho−/− and NaPi2a-/- mice (Fig. 3). In contrast to 1,25(OH)2D3, serum PTH levels were markedly reduced in klotho−/− and klotho-/-/NaPi2a-/- mice (Fig. 3); serum PTH was also extremely low in NaPi2a-/- mice. Of relevance, compared to the control (175 pg/ml), serum FGF23 levels were extremely high in the klotho−/− mice (7107 pg/ml) at 6 weeks of age 22. Markedly increased serum levels of FGF23 were also noted in the in klotho-/-/NaPi2a-/- mice (7440 pg/ml); the serum FGF23 levels were low in NaPi2a-/- mice (53 pg/ml) (Fig. 4).
Figure 3. Biochemical measurements of serum PTH and 1,25(OH)2D3 in various genotypes.
Compared to the wild-type (WT) mice (n=3; 121.8±60 pg/ml), serum PTH levels are markedly reduced in klotho−/− mice (n=5; 58.3±28 pg/ml). Serum PTH levels are low and undetectable in both klotho-/-/NaPi2a-/- (DKO) and NaPi2a-/- mice, respectively. We also measured serum PTH levels in 1-alpha hydroxylase knockout mice as a positive control and found significantly increased levels (n=4; 2443.4±610 pg/ml), compared to the controls. As for serum 1,25(OH)2D3 levels, compared to the WT mice (n=5; 144.8±77 pmol/L), markedly increased serum levels are noted in all three mutant mice; in klotho−/− mice (n=5; 465.4±47 pmol/L), in DKO (n=7; 1487.9±279 pmol/L), and in NaPi2a-/- mice (n=5; 272.6±169 pmol/L) (*: p < 0.01, vs. WT; **: p < 0.005, vs. WT).
Figure 4. Biochemical measurements of serum FGF23 in various genotypes.
The average serum levels of FGF23 are higher in klotho−/− mice (n=6; 7107 pg/ml) compared to wild-type mice (n=9; 176 pg/ml). Similarly increased FGF23 serum levels are also noted in klotho-/-/NaPi2a-/- (DKO) mice (n=7; 7440 pg/ml). The serum FGF23 levels is extremely low (n=6; 53 pg/ml) in NaPi2a-/- mice. Data presented after adding dilution factors (**: p < 0.001, vs. wild-type; ##: p < 0.001, vs. NaPi2a).
The major finding of our in vivo genetic manipulation studies is that NaPi2a regulates systemic phosphate homeostasis in klotho−/− mouse. Inactivation of the NaPi2a gene from klotho−/− mice reversed severe hyperphosphatemia to hypophosphatemia/normophosphatemia, despite significantly higher serum levels of calcium and 1,25(OH)2D3.
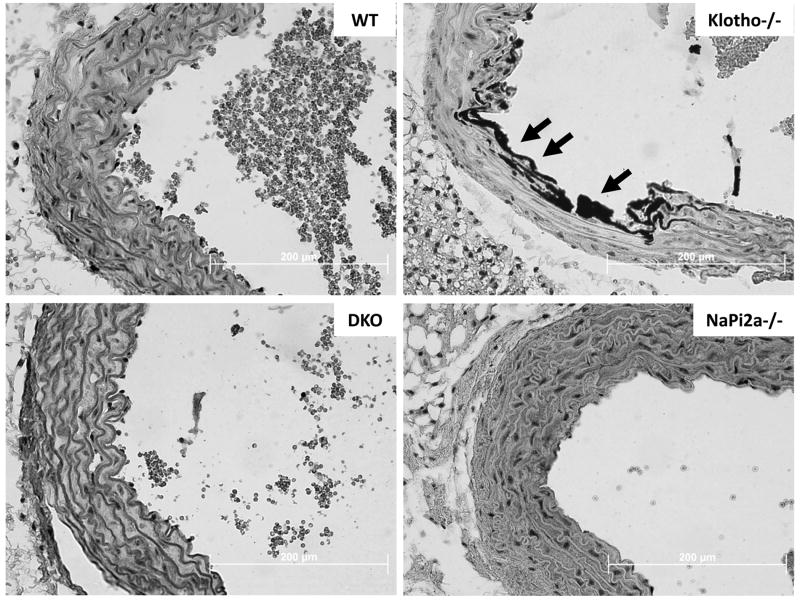
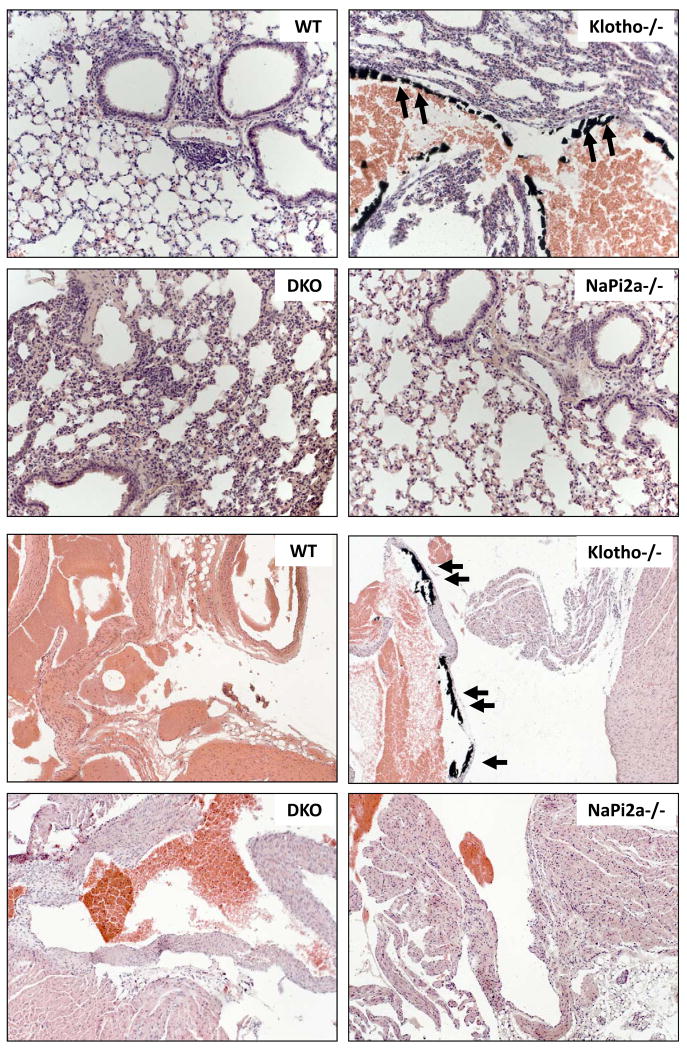
We then examined the effects of reducing serum phosphate from klotho−/− mice on vascular and soft tissue calcification. Extensive vascular and soft tissue calcifications were present in the heart, lung, kidney, aorta and other organs in klotho−/− mice, as determined by von Kossa staining (Supplementary Fig. 3). The extensive calcification observed in klotho−/− mice by 6 weeks was significantly reduced or eliminated in klotho-/-/NaPi2a-/- mice, even at 12 weeks of age (Figs. 5 and 6). These results indicate that high serum phosphate is an important determinant of calcification, and the lowering of serum phosphate can reduce/eliminate calcification, even in presence of higher serum calcium and 1,25(OH)2D3 levels (Fig. 3).
Figure 5. Aortic calcification.
Sections prepared from aorta of wild-type (WT), klotho-/-, klotho-/-/NaPi2a-/- (DKO) and NaPi2a-/- mice showing extensive calcifications in the aortic wall of only in klotho−/− mice. No such calcification is detected in aorta obtained from DKO mice or NaPi2a-/- mice (von Kossa staining; ×60).
Figure 6. Vascular calcification in lung and heart.
Lung sections (upper four panels) prepared from wild-type (WT), klotho-/-, klotho-/-/NaPi2a-/- (DKO) and NaPi2a-/- mice showing extensive calcifications in the lung parenchyma and vessel wall of klotho−/− mice. Inactivation of NaPi2a from klotho-/- mice reduced such calcification from DKO mice. Similarly, vascular calcification is also noted in heart (lower four panels) of klotho-/- mice but is absent from DKO mice (von Kossa staining; lung ×20, heart ×10).
Lowering serum phosphate levels in klotho−/− mice significantly rescued soft tissue anomalies, helped restore fertility, and markedly reduced extensive soft tissue and vascular calcifications in klotho-/-/NaPi2a-/- mice (Table 1). These results suggest that the phenotypes of klotho−/− mice are mostly derived from high serum phosphate levels. Despite significantly high serum levels of calcium and 1,25(OH)2D3, the opposing phenotypes of klotho−/− and klotho-/-/NaPi2a-/- mice suggest that such dissimilarities are due to phosphate toxicity. Our results provide compelling genetic evidence of the in vivo importance of NaPi2a in the regulation of systemic phosphate homeostasis in klotho−/− mice, and that abnormal regulation of NaPi2a co-transporters can lead to phosphate toxicity to induce severe soft tissue anomalies, including general tissue atrophy, and reduction of skeletal muscle mass. Of relevance, hyperphosphatemia and severe muscle wasting are common clinical problems encountered in CKD patients.
Table-1.
Phenotypes of various mutant mice compared to wild-type littermates at 6 to 9 weeks of age.
E: extremely; M: moderately; S: slightly; D: diffuse; F: focal
| Wild-type | klotho-/- | klotho-/-/NaPi2a-/- | NaPi2a-/- | |
|---|---|---|---|---|
| Gross appearance | ||||
| Body weight | Normal | Reduced (E) | Reduced (M) | Reduced (S) |
| Growth retardation | Absent | Present (E) | Present (S) | Present (S) |
| Generalized atrophy | ||||
| Spleen atrophy | Absent | Present (D) | Absent | Absent |
| Muscle wasting | Absent | Present (D) | Absent | Absent |
| Skin atrophy | Absent | Present (D) | Absent | Absent |
| Intestinal atrophy | Absent | Present (D) | Absent | Absent |
| Morphological changes | ||||
| Atherosclerosis/arteriosclerosis | Absent | Present | Absent | Absent |
| Vascular calcifications | Absent | Present | Absent | Absent |
| Emphysema | Absent | Present (D) | Present (F) | Present (F) |
| Biochemical changes | ||||
| Serum 1,25(OH)2D3 | Normal | High | High | High |
| Serum phosphate | Normal | High | Low/normal | Low |
| Serum calcium | Normal | High | High | High |
| Serum PTH | Normal | Low | Low | Low |
| Serum FGF23 | Normal | High | High | Low |
| Overall affect | ||||
| Physical activity | Normal | Sluggish | Normal | Normal |
| Infertility | Absent | Present | Absent | Absent |
| Lifespan (until 20 weeks) | Normal | Short | Normal | Normal |
Extensive vascular and soft tissue calcifications were widely present in the lung, kidney, aorta, and other organs in klotho−/− mice, as detected by von Kossa staining (Fig. 5, Supplementary Fig. 3). The extensive calcification noted in klotho−/− mice by 6 weeks of age was completely eliminated in klotho-/-/NaPi2a-/- mice and was not detected even in 12-week-old double mutant mice. These results indicated that high serum phosphate is an important determinant of calcification, and lowering serum phosphate can reduce/eliminate calcification, even in presence of higher serum 1,25(OH)2D3 levels (Fig. 3).
Phosphate retention and subsequent hyperphosphatemia, together with reduced circulating levels of 1,25-dihydroxyvitamin D, are the major biochemical changes detected in patients with CKD 23. Coronary calcification is the single most important pathologic condition that influences the mortality of CKD patients undergoing dialysis treatment 24. Hyperphosphatemia is believed to be an important risk factor for such cardiovascular calcification 25. The current approach of reducing serum phosphate levels and correcting vitamin D insufficiency/deficiency in CKD patients often poses a dilemma, as high doses of vitamin D/analog treatment are believed to affect the subsequent vascular calcification process. Our in vivo genetic manipulation study suggests that minimizing phosphate toxicity can reduce vascular calcification, even in presence of extremely high serum 1,25-dihydroxyvitamin D and calcium levels. One limitation of animal study is that the mechanisms of human vascular diseases in renal insufficiency may be different from vascular lesions in klotho−/− mice, and established medial and atherosclerotic lesions may persist even after molecular manipulations, as advanced stages of calcification may not be reversible. We believe that lowering serum phosphate levels can delay the progression of vascular lesions, but may not always reverse the established lesions.
Hyperphosphatemia in klotho−/− mice is associated with extensive soft tissue calcification (Fig. 5, Supplementary Fig. 3) 7,9,22. Imbalance between phosphate and pyrophosphate usually determines the ectopic calcification process 26,27. We have found that the expression of Ennp-1 (pyrophosphate generator) and ANK (pyrophosphate transporter) is slightly elevated in the kidneys and aortas of klotho−/− mice, and such elevation might be a compensatory response in these mutant mice (Supplementary Figs. 4, 5). Since elevation of pyrophosphate regulating molecules can not suppress calcification process, it appears likely that extensive calcification in klotho−/− mice is primarily associated with high serum phosphate levels.
In summary, the phenotypes of klotho-/-/NaPi2a-/- mice suggest that: 1) increased NaPi2a activity is the main cause for the severe hyperphosphatemia and ectopic calcification observed in klotho-/- mice, and 2) NaPi2a-mediated renal phosphate homeostasis is independent of serum 1,25(OH)2D3 levels in mice deficient for klotho. Notably, lowering phosphate burden, by reducing serum phosphate levels can modulate vascular and soft tissue calcification, despite the presence of extremely high serum 1,25(OH)2D3 levels. These results provide compelling genetic evidence of the importance of NaPi2a in regulating renal phosphate homeostasis in klotho-/- mice, and more importantly, suggest that reducing “phosphate toxicity” should be the single most important therapeutic priority in minimizing the risk of vascular calcification and eventual disease progression 23,28-30.
Supplementary Material
Acknowledgments
We thank Dr. A. K. M. Wara of Brigham and Women's Hospital at Boston, MA for helping us dissecting out aorta from the mice.
Sources of Funding: This research was partly funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK077276) to Dr. Razzaque.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–5. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- 3.Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque MS. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008;74:566–70. doi: 10.1038/ki.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 5.Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–64. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- 6.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. Faseb J. 2009 doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 9.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. Faseb J. 2009;23:433–41. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schuler C, Erben RG, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–7. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. Faseb J. 2006;20:720–2. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Nazneen A, Taguchi T, Razzaque MS. Low-dose local kidney irradiation inhibits progression of experimental crescentic nephritis by promoting apoptosis. Am J Nephrol. 2008;28:555–68. doi: 10.1159/000115290. [DOI] [PubMed] [Google Scholar]
- 14.Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, Razzaque MS. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am J Nephrol. 2008;28:755–764. doi: 10.1159/000128607. [DOI] [PubMed] [Google Scholar]
- 15.Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–61. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- 16.Razzaque MS, Taguchi T. Collagen-binding heat shock protein (HSP) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol. 1997;183:24–9. doi: 10.1002/(SICI)1096-9896(199709)183:1<24::AID-PATH1106>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Zha Y, Le VT, Higami Y, Shimokawa I, Taguchi T, Razzaque MS. Life-long suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–8. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]
- 18.Razzaque MS, Nazneen A, Taguchi T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod Pathol. 1998;11:1183–8. [PubMed] [Google Scholar]
- 19.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007;27:503–15. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- 21.Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009 doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 28.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009 doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–6. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razzaque MS. Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat Clin Pract Endocrinol Metab. 2007;3:788–9. doi: 10.1038/ncpendmet0667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.