Abstract
Drug addiction can be defined by a compulsion to seek and take drug and loss of control in limiting intake, and the excessive drug taking derives from multiple motivational mechanisms. One such mechanism is the emergence of a negative emotional state when access to the drug is prevented, reflecting hedonic homeostatic dysregulation. Excessive drug taking then results in part via the construct of negative reinforcement. The negative emotional state that drives such negative reinforcement is hypothesized to derive from dysregulation of key neurochemical elements involved in reward and stress within basal forebrain structures, including the ventral striatum and extended amygdala. Specific neurochemical elements in these structures include not only decreases in reward neurotransmission, such as decreases in dopamine and opioid peptide function in the ventral striatum, but also recruitment of brain stress systems, such as corticotropin-releasing factor (CRF), in the extended amygdala. Chronic exposure or extended access to self-administration of all major drugs of abuse produces during abstinence increases in reward thresholds, increases in aversive anxiety-like responses, increases in extracellular levels of CRF in the central nucleus of the amygdala, and increases in drug self-administration. CRF receptor antagonists block excessive drug intake produced by dependence. A combination of decreased reward system function and increased brain stress response system function is hypothesized to be responsible for hedonic homeostatic dysregulation that drives drug seeking behavior in dependence. Such hedonic dysregulation is hypothesized to extend into protracted abstinence to provide a residual negative emotional state that enhances the salience of cues eliciting drug seeking and relapse.
Keywords: addiction, hedonic dysregulation, stress, extended amygdala, corticotropin-releasing factor
Definitions and conceptual framework for hedonic dysregulation in addiction
Drug addiction is a chronically relapsing disorder characterized by (i) compulsion to seek and take the drug, (ii) loss of control in limiting intake, and (iii) emergence of a negative emotional state (e.g., dysphoria, anxiety, irritability). The third component reflects a motivational withdrawal syndrome (defined here as dependence) when access to the drug is prevented [1]. Although withdrawal per se is part of the diagnostic criteria for both the American Psychiatric Association and the World Health Organization, historically this has referred to physical or somatic signs. Critically important for the thesis of the present review is the hypothesis that the withdrawal state important for addiction is not physical or somatic signs but rather a motivational withdrawal syndrome that reflects dysregulation of hedonic homeostatic processes that form the bases of not only the motivational drive of acute withdrawal from chronic drugs of abuse but also a background state change that extends into protracted abstinence and contributes to reinstatement of drug seeking.
Drug addiction has been conceptualized as a disorder that involves elements of both impulsivity and compulsivity. Collapsing the cycles of impulsivity and compulsivity yields a composite addiction cycle comprised of three stages—preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect—in which impulsivity often dominates at the early stages and compulsivity dominates at terminal stages. As an individual moves from impulsivity to compulsivity, a shift occurs from positive reinforcement driving the motivated behavior to negative reinforcement driving the motivated behavior [2]. Negative reinforcement can be defined as the process by which removal of an aversive stimulus (e.g., negative emotional state of drug withdrawal) increases the probability of a response (e.g., dependence-induced drug intake). These three stages are conceptualized as interacting with each other, becoming more intense, and ultimately leading to the pathological state known as addiction [1] (Table 1). The present review will focus on the role of dysregulated reward and stress systems in the negative emotional states associated with the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle that drive drug seeking behavior. The focus will be on animal models of dependence, measures of compulsivity, and neurotransmitter systems that contribute to the negative emotional state of drug withdrawal.
Table 1.
| Stage | DSM-IV criteria | Neurocircuitry |
|---|---|---|
| Binge/intoxication | Persistent desire | reinforcing effects ...................... ventral striatum; extended amygdala reward system |
| Larger amounts taken than expected | ||
| Withdrawal/negative affect | Tolerance | negative affect ........................... extended amygdala reward system |
| Withdrawal | increased anxiety ....................... brain stress neurocircuitry | |
| Compromised social, occupational, or recreational activities | ||
| Preoccupation/anticipation | Proccupation with obtaining drug | drug-induced reinstatement ........ prefrontal cortex |
| Persistent physical/psychological problems | cue-induced reinstatement .......... basolateral amygdala | |
| stress-induced reinstatement ....... extended amygdala |
Three neurobiological circuits have been identified that have heuristic value for the study of the neurobiological changes associated with the development and persistence of drug dependence. The acute reinforcing effects of drugs of abuse that comprise the binge/intoxication stage most likely involve actions with an emphasis on the ventral striatum and extended amygdala reward system and inputs from the ventral tegmental area and arcuate nucleus of the hypothalamus. In contrast, the symptoms of acute withdrawal important for addiction, such as negative affect and increased anxiety associated with the withdrawal/negative affect stage, most likely involve decreases in function of the extended amygdala reward system but also recruitment of brain stress neurocircuitry. The preoccupation/anticipation (craving) stage involves intrinsic brain stress systems in the extended amygala (stress-induced reinstatement) and key afferent projections to the extended amygdala and nucleus accumbens, specifically the prefrontal cortex (for drug-induced reinstatement) and basolateral amygdala (for cue-induced reinstatement). Compulsive drug-seeking behavior is hypothesized to engage ventral striatalventral pallidal-thalamic-cortical loops that may subsequently engage dorsal striatal-pallidalthalamic-cortical loops [3, 4], both effects exaggerated by concomitant decreased reward function and activation of brain stress systems in the extended amygdala [5].
Animal models relevant to hedonic homeostatic dysregulation
Place aversion, animal models of anxiety, and reward thresholds
Animal models of the withdrawal/negative affect stage include measures of conditioned place aversion (rather than preference) to precipitated withdrawal or spontaneous withdrawal from chronic administration of a drug, increases in reward thresholds using brain stimulation reward, and increases in anxiety-like responses (for reviews, see [6, 7]). Increases in brain stimulation reward thresholds refers to the increase in current required to engender responding using a discrete trials procedure for stimulation of the medial forebrain bundle in rodents with permanent indwelling electrodes [8]. Here, all drugs of abuse when administered acutely decrease reward thresholds (decreased reward) [8] and during withdrawal from all drugs of abuse brain reward threshold increase (decreased reward) [9]. Animal models of anxiety in drug withdrawal studies involve anxiety-like responses such as decreased exploration as is observed in the elevated plus maze where there is a decreased exploration of open spaces or anxiety-like responses such as active burying of an electrified probe (defensive burying) [10].
Escalation in drug self-administration with prolonged access
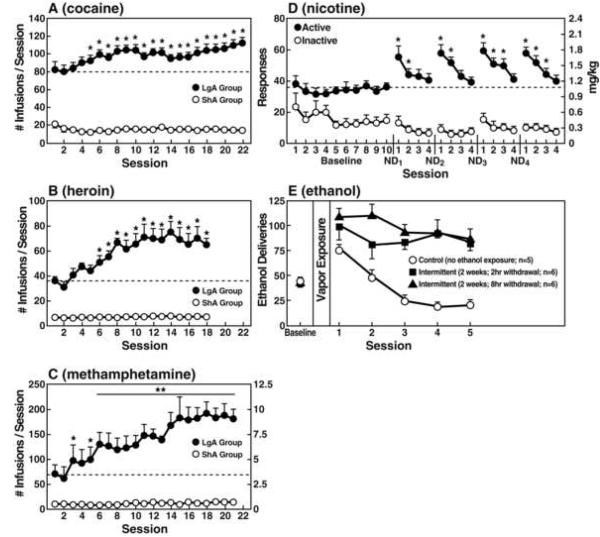
A progressive increase in the frequency and intensity of drug use is one of the major behavioral phenomena characterizing the development of addiction. A framework with which to model the transition from drug use to drug addiction can be found in recent animal models of prolonged access to intravenous cocaine self-administration. Historically, animal models of drug self-administration involved the establishment of stable behavior from day to day to allow the reliable interpretation of data provided by within-subject designs aimed at exploring the neuropharmacological and neurobiological bases of such reinforcing effects. However, in an effort to explore the possibility that differential access to intravenous cocaine self-administration in rats may produce different patterns of drug intake, rats were allowed to intravenously self-administer cocaine for 1 h or 6 h per day [11]. One hour access to intravenous cocaine per session produced low and stable intake as observed previously. In contrast, 6 h access to cocaine produced drug intake that gradually escalated over days (Figure 1). Escalation also is associated with an increase in breakpoint for cocaine in a progressive-ratio schedule of reinforcement, suggesting an enhanced motivation to seek cocaine or an enhanced efficacy of cocaine reward [12, 13]. Such increased self-administration in dependent animals has now been observed with cocaine, methamphetamine, nicotine, heroin, and alcohol [11, 14-17] (Figure 1).
Figure 1.
(A) Effect of drug availability on cocaine intake (mean ± SEM). In 6 h long-access (LgA) rats (n = 12) but not in 1 h short-access (ShA) rats (n = 12), mean total cocaine intake started to increase significantly from session 5 (p < 0.05, sessions 5 to 22 compared with session 1) and continued to increase thereafter (p < 0.05, session 5 compared with sessions 8-10, 12, 13, 17-22). (Taken with permission from [11].) (B) Effect of drug availability on total intravenous heroin self-infusions (mean ± SEM). During the escalation phase, rats had access to heroin (40 mg per infusion) for 1 h (ShA rats, n = 5-6) or 11 h per session (LgA rats, n = 5-6). Regular 1 h (ShA rats) or 11 h (LgA rats) sessions of heroin self-administration were performed 6 days per week. The dotted line indicates the mean (± SEM) number of heroin self-infusions of LgA rats during the first 11 h session. *p < 0.05, compared with the first session (paired t-test). (Taken with permission from [14].) (C) Effect of extended access to intravenous methamphetamine self-administration as a function of daily sessions in rats trained to self-administer 0.05 mg/kg/infusion of intravenous methamphetamine during a 6 h session. Short access group (ShA), 1 h session (n = 6). Long access group (LgA), 6 h session (n = 4). All data were analyzed using two-way analysis of variance (dose × escalation session within ShA or LgA group). *p < 0.05, **p < 0.01, ***p < 0.001, compared with Day 1. (Taken with permission from [15].) (D) Total 23 h active and inactive responses after repeated cycles of 72 h of nicotine deprivation (ND) followed by 4 days of self-administration (*p < 0.05, compared with baseline). (Taken with permission from [17].) (E) Ethanol deliveries (mean ± SEM) in rats trained to respond for 10% ethanol and then either not exposed to ethanol vapor (control, n = 5) or exposed to intermittent ethanol vapor (14 h on/10 h off) for 2 weeks and then tested either 2 h (n = 6) or 8 h (n = 6) after removal from ethanol vapor. *p < 0.05, significant increase in operant self-administration of ethanol in rats receiving intermittent vapor exposure compared with control. No difference was observed between rats exposed to intermittent vapor and tested either 2 or 8 h after ethanol withdrawal. (Taken with permission from [16].)
The hypothesis that compulsive drug use is accompanied by a chronic perturbation in brain reward homeostasis has been tested in animal models of extended access to intravenous drug self-administration combined with measures of brain stimulation reward thresholds. Elevation in baseline reward thresholds temporally preceded and was highly correlated with escalation in drug intake [18, 19]. Extended access also is associated with an increase in breakpoint on a progressive ratio schedule for cocaine, methamphetamine, and opioids as well as for alcohol in dependence-induced drinking, suggesting an enhanced motivation to seek cocaine or an enhanced efficacy of drug reward ([12, 13, 20, 21]; also see Oleson and Roberts in this issue).
Motivation, opponent process, and hedonic homeostasis
Motivation is a state that can be defined as a “tendency of the whole animal to produce organized activity” [22], and such motivational states are not constant but rather vary over time. The concept of motivation was linked inextricably with hedonic, affective, or emotional states in addiction in the context of temporal dynamics by Solomon's opponent process theory of motivation. Solomon and Corbit [23] postulated that hedonic, affective, or emotional states, once initiated, are automatically modulated by the central nervous system with mechanisms that reduce the intensity of hedonic feelings. The a-process includes affective or hedonic habituation (or tolerance), and the b-process includes affective or hedonic withdrawal (abstinence).
The hedonic changes associated with drug addiction became a dramatic example of opponent process theory in which hedonic responses were exaggerated by pharmacological probes. The a-process in drug use reflected positive hedonic responses, occurred shortly after presentation of the drug, correlated closely with the intensity, quality, and duration of the reinforcer, and showed tolerance. In contrast, the b-process in drug use appeared after the a-process terminated, consisted of negative hedonic responses, was sluggish in onset, slow to build up to an asymptote, and slow to decay, and became larger with repeated exposure. The thesis here is that opponent hedonic processes begin early in drug-taking, reflect changes in the brain reward and stress systems, acquire allostatic-like physiological properties, and later form one of the major motivations for compulsivity in drug taking.
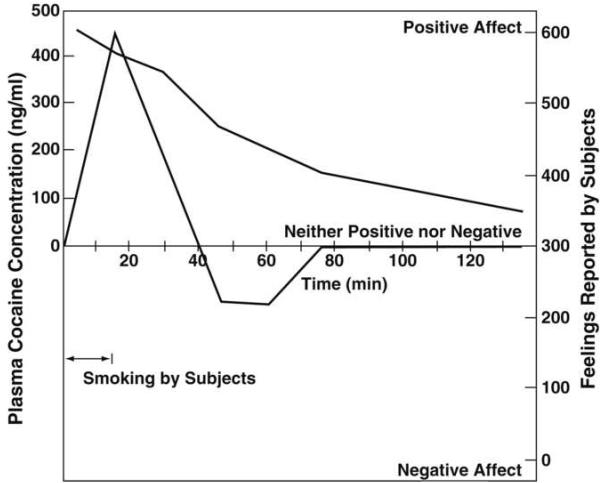
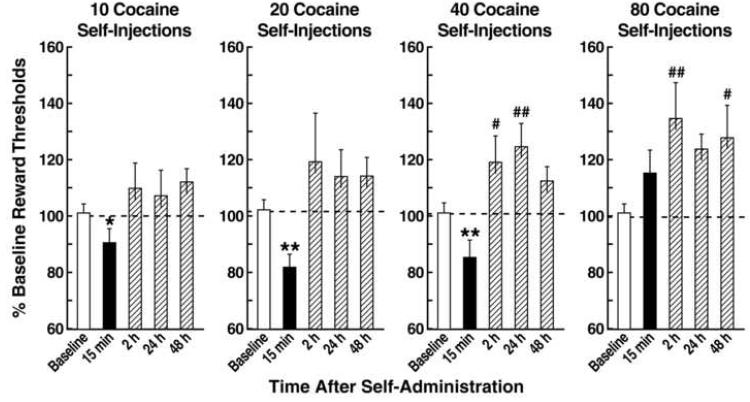
Rapid acute tolerance and opponent process-like effects in response to the hedonic effects of cocaine were reported in human studies of smoked coca paste [24] (Figure 2). After a single smoking session, the onset and intensity of the “high” were very rapid via the smoked route of administration, and rapid tolerance manifested. The “high” decreased rapidly despite significant blood levels of cocaine. With intravenous cocaine self-administration in animal models, such elevations in reward threshold begin rapidly and can be observed within a single session of self-administration [25] (Figure 3), bearing a striking resemblance to human subjective reports. These results demonstrate that the elevation in brain reward thresholds following prolonged access to cocaine failed to return to baseline levels between repeated, prolonged exposure to cocaine self-administration, thus creating a greater and greater elevation in “baseline” ICSS thresholds. Similar results have been observed showing dysphoria-like responses accompanying acute opioid and ethanol withdrawal [26, 27]. Here, naloxone administration following single injections of morphine or acute ethanol withdrawal increased reward thresholds, measured by ICSS, and increased thresholds with repeated morphine and naloxone-induced withdrawal experience [26, 27].
Figure 2.
Dysphoric feelings followed the initial euphoria in experimental subjects who smoked cocaine paste, although the concentration of cocaine in the plasma of the blood remained relatively high. The dysphoria is characterized by anxiety, depression, fatigue, and a desire for more cocaine. The peak feelings for the subjects were probably reached shortly before the peak plasma concentration, but the first psychological measurements were made later than the plasma assay. Therefore, the temporal sequence of the peaks shown cannot be regarded as definitive. (Taken with permission from [24].)
Figure 3.
Rats (n = 11) were allowed to self-administer 10, 20, 40, and 80 injections of cocaine (0.25 mg per injection), and intracranial self-stimulation reward thresholds were measured 15 min and 2, 24, and 48 h after the end of each intravenous cocaine self-administration session. The horizontal dotted line in each plot represents 100% of baseline levels. Data are expressed as mean + SEM percentage of baseline reward thresholds. *p < 0.05, **p < 0.01, compared with baseline; paired t-test. #p < 0.05, ##p < 0.01, compared with baseline; Fisher's LSD test after a statistically significant effect in the repeated-measures analysis of variance. (Taken with permission from [25].)
The dysregulation of brain reward function associated with withdrawal from chronic administration of drugs of abuse is a common element of all drugs of abuse. Withdrawal from chronic cocaine, amphetamine, opioids, cannabinoids, nicotine, and ethanol leads to increases in reward threshold during acute abstinence, and some of these elevations in threshold can last for up to 1 week [9]. These observations lend credence to the hypothesis that opponent processes can set the stage for compulsive drug intake in which negative reinforcement mechanisms are engaged.
More recently, the hedonic changes associated with opponent process theory have been extended into the domain of an allostatic model [2, 28]. In this formulation, addiction is conceptualized as a cycle of increasing dysregulation of brain reward/anti-reward mechanisms that results in a negative emotional state contributing to the compulsive use of drugs. Counteradaptive processes that are part of the normal homeostatic limitation of reward function fail to return within the normal homeostatic range. These counteradaptive processes are hypothesized to be mediated by two mechanisms: within-system neuroadaptations and between-system neuroadaptations.
In a within-system neuroadaptation, “the primary cellular response element to the drug would itself adapt to neutralize the drug's effects; persistence of the opposing effects after the drug disappears would produce the withdrawal response” [29]. Thus, a within-system neuroadaptation is a molecular or cellular change within a given reward circuit to accommodate overactivity of hedonic processing associated with addiction resulting in a decrease in reward function.
The emotional dysregulation associated with the withdrawal/negative affect stage also may involve between-system neuroadaptations in which neurochemical systems other than those involved in the positive rewarding effects of drugs of abuse are recruited or dysregulated by chronic activation of the reward system. Thus, a between-system neuroadaptation is a circuitry change in which another different circuit (anti-reward circuit) is activated by the reward circuit and has opposing actions, again limiting reward function. The purpose of this review is to explore the neuroadaptational changes that occur in the brain emotional systems to account for the neurocircuitry changes that produce opponent processes and are hypothesized to have a key role in the compulsivity of addiction.
Neural substrates for hedonic homeostatic dysregulation associated with addiction
Neural substrates for hedonic homeostatic dysregulation: Construct of the extended amygdala
The neuroanatomical entity termed the extended amygdala [30] may represent a common anatomical substrate integrating brain arousal-stress systems with hedonic processing systems to produce the between-system opponent process elaborated above. The extended amygdala is composed of the central nucleus of the amygdala, bed nucleus of the stria terminalis, and a transition zone in the medial (shell) subregion of the nucleus accumbens. Each of these regions has cytoarchitectural and circuitry similarities [30]. The extended amygdala receives numerous afferents from limbic structures such as the basolateral amygdala and hippocampus and sends efferents to the medial part of the ventral pallidum and a large projection to the lateral hypothalamus, thus further defining the specific brain areas that interface classical limbic (emotional) structures with the extrapyramidal motor system. The extended amygdala has long been hypothesized to have a key role not only in fear conditioning [31] but also in the emotional component of pain processing [32].
For the conceptual framework outlined here, the shell of the nucleus accumbens has high levels of dopamine and opioid peptides and forms an interface between the well established role of ventral striatal dopamine and opioid peptides in drug reward (“light” side of addiction) and of the central nucleus of the amygdala in anti-reward processes (“dark” side of addiction [2]). Both systems are hypothesized to contribute to the hedonic homeostatic dysregulation that drives drug-seeking behavior in addiction.
Within-system neuroadaptations that contribute to the negative emotional state of drug withdrawal
Within-system neuroadaptations to chronic drug exposure include decreases in function of the same neurotransmitter systems in the same neurocircuits implicated in the acute reinforcing effects of drugs of abuse. One prominent hypothesis is that dopamine systems are compromised in crucial phases of the addiction cycle, such as withdrawal, thus leading to decreased motivation for non-drug-related stimuli and increased sensitivity to the abused drug [33]. Activation of the mesolimbic dopamine system has long been known to be critical for the acute rewarding properties of psychostimulant drugs and to be associated with the acute reinforcing effects of other drugs of abuse [34], but decreases in activity of the mesolimbic dopamine system and decreases in serotonergic neurotransmission in the nucleus accumbens occur during drug withdrawal in animal studies [35]. Imaging studies in drug-addicted humans have consistently shown long-lasting decreases in the numbers of dopamine D2 receptors in drug abusers compared with controls [36]. Decreases in the number of D2 receptors, coupled with the decrease in dopaminergic activity, in cocaine, nicotine, and alcohol abusers results in decreased sensitivity of reward circuits to stimulation by natural reinforcers [36]. Under this conceptual framework, other within-system neuroadaptations triggered by chronic activation of reward systems, such as dopamine and opioid peptides, would include increased sensitivity of receptor transduction mechanisms in the nucleus accumbens, including adenylate cyclase, protein kinase A, cyclic adenosine monophosphate response-element binding protein (CREB), and ΔFosB (e.g., during opiate withdrawal; for review, see [37]). These findings suggest an overall reduction in the sensitivity of the dopamine component of reward circuitry to natural reinforcers and other drugs in drug-addicted individuals.
Between-system neuroadaptations that contribute to the negative emotional state of drug withdrawal
Brain neurochemical systems involved in arousal-stress modulation also may be engaged within the neurocircuitry of the brain stress systems in an attempt to overcome the chronic presence of the perturbing drug and to restore normal function despite the presence of drug. Both the hypothalamic-pituitary-adrenal axis and the brain stress system mediated by corticotropin-releasing factor (CRF) are dysregulated by chronic administration of all major drugs with dependence or abuse potential, with a common response of elevated adrenocorticotropic hormone, corticosterone, and amygdala CRF during acute withdrawal [38].
Acute withdrawal from all drugs of abuse also produces an aversive or anxiety-like state that can be reversed by CRF receptor antagonists [38]. The ability of CRF antagonists to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal would predict motivational effects of CRF antagonists in animal models of extended access to drugs. CRF antagonists selectively blocked the increased self-administration of drugs associated with extended access to intravenous self-administration of cocaine [39], nicotine [17], and heroin [40]. CRF antagonists also blocked the increased self-administration of ethanol in dependent rats but not in nondependent rats [41] (Table 2).
Table 2.
Role of corticotropin-releasing factor in dependence.
| Drug | CRF antagonist effects on withdrawal-induced anxiety-like responses |
Withdrawal-induced changes in extracellular CRF in CeA |
CRF antagonist effects on dependence-induced increases in self- administration |
|---|---|---|---|
| Cocaine | ↓[53] | ↑[57] | ↓[39] |
| Opioids | ↓*[54] | ↑[58] | ↓[40] |
| Ethanol | ↓[55] | ↑[59] | ↓[60] |
| Nicotine | ↓[17] | ↑[17] | ↓[17] |
| Δ9-tetrahydrocannabinol | ↓[56] | ↑[56] | nt |
, aversive effects with place conditioning.
nt, not tested.
CeA, central nucleus of the amygdala.
Norepinephrine functional antagonists (β1 antagonist and α2 agonist) injected into the lateral bed nucleus of the stria terminalis blocked precipitated opiate withdrawal-induced place aversions [42]. Functional norepinephrine antagonists block excessive drug intake associated with dependence on ethanol [43], cocaine [13], and opioids [44]. A focal point for many of these effects is the bed nucleus of the stria terminalis.
Dynorphin, an opioid peptide that binds to κ opioid receptors, has long been known to show activation with chronic administration of psychostimulants and opioids [37, 38], and κ opioid agonists produce aversive effects in animals and humans [45, 46]. A κ opioid antagonist blocks the excessive drinking associated with ethanol withdrawal and dependence [47]. The effects of dynorphin in producing negative emotional states have been hypothesized to be driven by activation of CRF systems [48], but other studies have shown that dynorphin may drive the CRF system [49, 50]. CRF1 knockout mice failed to show conditioned place aversion to opioid withdrawal and failed to show an opioid-induced increase in dynorphin mRNA in the nucleus accumbens [51].
Neuropeptide Y and nociceptin have cellular actions in the central nucleus of the amygdala and neuropharmacological actions in alcohol dependence that are the opposite to CRF, suggesting that the brain stress systems of the extended amygdala may be buffered by additional homeostatic mechanisms [38]. Other potential candidates contributing to such emotional dysregulation in the extended amygdala include vasopressin, substance P, orexin, neuropeptide Y, and nociceptin [38].
Hedonic dysregulation and allostasis
The overall conceptual theme argued here is that drug addiction represents a break with homeostatic brain regulatory mechanisms that regulate the emotional state of the animal. However, the view that drug addiction represents a simple break with homeostasis is not sufficient to explain a number of key elements of addiction. Drug addiction, similar to other chronic physiological disorders such as high blood pressure, worsens over time, is subject to significant environmental influences, and leaves a residual neuroadaptive trace that allows rapid “re-addiction” even months and years after detoxification and abstinence. These characteristics of drug addiction imply more than simply a homeostatic dysregulation of hedonic function and executive function, but rather a dynamic break with homeostasis of these systems that represents more an allostatic-like change.
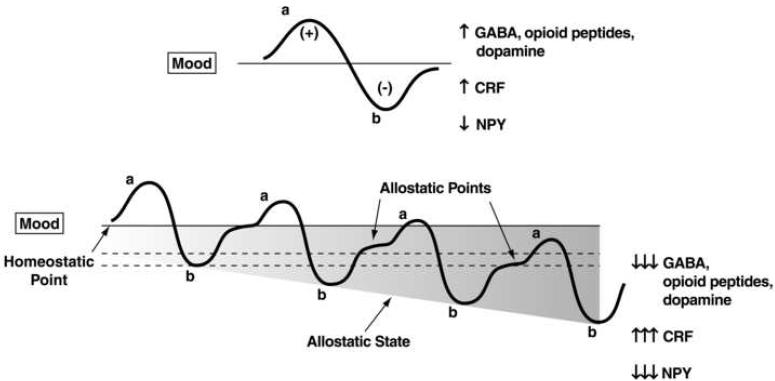
Allostasis, originally conceptualized to explain persistent morbidity of arousal and autonomic function, is defined as “stability through change” and a continuous readjustment of all parameters toward a new set point [52]. An allostatic state can be defined as a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level. Two components are hypothesized to adjust to challenges to the brain produced by drugs of abuse to produce an allostatic-like state: decreased activity of brain reward systems and recruitment of the brain anti-reward or brain stress systems (Figure 4). Repeated challenges, such as with drugs of abuse, lead to attempts of the brain via molecular, cellular, and neurocircuitry changes to maintain stability but at a cost. For the drug addiction framework elaborated here, the residual deviation from normal brain reward threshold regulation is termed an allostatic state. This neurochemical dysregulation in emotional systems in the extended amygdala outlined herein is hypothesized to persist into protracted abstinence and provide a motivational background for craving and relapse.
Figure 4.
Diagram illustrating an extension of Solomon and Corbit's opponent-process model of motivation to outline the conceptual framework of the allostatic hypothesis. Both panels represent the affective response to the presentation of a drug. (Top) This diagram represents the initial experience of a drug with no prior drug history. The a-process represents a positive hedonic or positive mood state, and the b-process represents a negative hedonic or negative mood state. The affective stimulus (state) has been argued to be the sum of both an a-process and a b-process. An individual experiencing a positive hedonic mood state from a drug of abuse with sufficient time between re-administering the drug is hypothesized to retain the a-process. In other words, an appropriate counteradaptive opponent-process (b-process) that balances the activational process (a-process) does not lead to an allostatic state. (Bottom) Changes in the affective stimulus (state) in an individual with repeated frequent drug use that may represent a transition to an allostatic state in the brain reward systems and, by extrapolation, a transition to addiction. Notice that the apparent b-process never returns to the original homeostatic level before drug-taking is reinitiated, thus creating a greater and greater allostatic state in the brain reward system. In other words, the counteradaptive opponent process (b-process) does not balance the activational process (a-process) but in fact shows a residual hysteresis. Although these changes are exaggerated and condensed over time in the present conceptualization, the hypothesis here is that even during post-detoxification (a period of “protracted abstinence”) the reward system is still bearing allostatic changes. In the nondependent state, reward experiences are normal, and the brain stress systems are not greatly engaged. During the transition to the state known as addiction, the brain reward system is in a major underactivated state while the brain stress system is highly activated. CRF, corticotropin-releasing factor; GABA, γ-aminobutyric acid; NPY, neuropeptide Y. The following definitions apply: allostasis, the process of achieving stability through change; allostatic state, a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level; allostatic load, the cost to the brain and body of the deviation, accumulating over time, and reflecting in many cases pathological states and accumulation of damage. (Modified with permission from [28].
Acknowledgments
The author would like to thank Michael Arends for his exemplary assistance with the preparation of this manuscript. Research was supported by National Institutes of Health grants AA06420 and AA08459 from the National Institute on Alcohol Abuse and Alcoholism, DA10072, DA04043, and DA04398 from the National Institute on Drug Abuse, and DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases. Research also was supported by the Pearson Center for Alcoholism and Addiction Research. This is publication number 19899 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 4.Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. European Journal of Pharmacology. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 6.Shippenberg TS, Koob GF. Recent advances in animal models of drug addiction and alcoholism. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 1381–1397. [Google Scholar]
- 7.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction Biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Federation Proceedings. 1979;38:2473–2476. [PubMed] [Google Scholar]
- 9.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koob GF, et al. Animal models of anxiety disorders. In: Schatzberg AF, Nemeroff CB, editors. Textbook of Psychopharmacology. 2nd edn American Psychiatric Press; Washington DC: 1998. pp. 133–144. [Google Scholar]
- 11.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 12.Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- 13.Wee S, et al. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. European Neuropsychopharmacology. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed SH, et al. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura O, et al. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 16.O'Dell LE, et al. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 17.George O, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed SH, et al. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature Neuroscience. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 19.Kenny PJ, et al. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. Journal of Neuroscience. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wee S, et al. Effect of aripiprazole, a partial D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged access. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebb DO. Textbook of Psychology. 3rd edn. W.B. Saunders; Philadelphia: 1972. [Google Scholar]
- 23.Solomon RL, Corbit JD. An opponent-process theory of motivation: 1. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 24.Van Dyke C, Byck R. Cocaine. Scientific American. 1982;246:128–141. doi: 10.1038/scientificamerican0382-128. [DOI] [PubMed] [Google Scholar]
- 25.Kenny PJ, et al. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. European Journal of Neuroscience. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacology Biochemistry and Behavior. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–28. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 29.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 30.Heimer L, Alheid G. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function. Plenum Press; New York: 1991. pp. 1–42. series title: Advances in Experimental Medicine and Biology, vol 295. [DOI] [PubMed] [Google Scholar]
- 31.Le Doux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Neugebauer V, et al. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 33.Melis M, et al. The dopamine hypothesis of drug addiction: hypodopaminergic state. International Review of Neurobiology. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- 34.Di Chiara G, North RA. Neurobiology of opiate abuse. Trends in Pharmacological Sciences. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- 35.Rossetti ZL, et al. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. European Journal of Pharmacology. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, et al. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behavioural Pharmacology. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends in Pharmacological Sciences. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Specio SE, et al. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwell TN, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addiction Biology. 2009a;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk CK, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delfs JM, et al. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 43.Walker BM, et al. α1-Noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwell TN, et al. The α1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacology Biochemistry and Behavior. 2009b;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 46.Pfeiffer A, et al. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 47.Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Land BB, et al. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Journal of Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valdez GR, et al. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. Journal of Pharmacology and Experimental Therapeutics. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- 50.Taylor CC, et al. κ-Opioid agonist, U50,488H, stimulates ovine fetal pituitary-adrenal function via hypothalamic arginine-vasopressin and corticotrophin-releasing factor. Journal of Pharmacology and Experimental Therapeutics. 1996;277:877–884. [PubMed] [Google Scholar]
- 51.Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proceedings of the National Academy of Sciences USA. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley; Chichester: 1988. pp. 629–649. [Google Scholar]
- 53.Basso AM, et al. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 54.Stinus L, et al. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- 55.Rassnick S, et al. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Research. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez de Fonseca F, et al. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 57.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 58.Weiss F, et al. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. In: Quinones-Jenab V, editor. The Biological Basis of Cocaine Addiction. New York Academy of Sciences; New York: 2001. pp. 1–26. series title: Annals of the New York Academy of Sciences, vol 937. [DOI] [PubMed] [Google Scholar]
- 59.Merlo-Pich E, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdez GR, et al. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]