Summary
The accumulation of CD28− T cells, particularly within the CD8 subset, is one of the most prominent changes during T cell homeostasis and function associated with aging in human. CD28, a major co-stimulatory receptor, is responsible for the optimal antigen-mediated T cell activation, proliferation, and survival of T cells. CD28− T cells exhibit reduced antigen receptor diversity, defective antigen-induced proliferation, and a shorter replicative lifespan while showing enhanced cytotoxicity and regulatory functions. Gene expression analyses reveal profound changes of CD28− T cells in comparison to their CD28+ counterparts and support their functional differences. Here we review the recent advance of our understanding of CD28− T cells and their role in age-associated decline of immune function.
Introduction
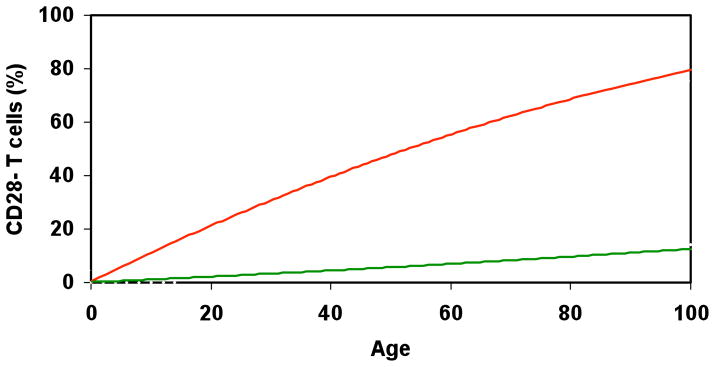
The effectiveness of the immune response declines with age particularly in the latter stages of life [1–3]. Among the multiple complex factors that contribute to the age-associated changes of T cells in human, the accumulation of CD28− T cells is one of the most profound and consistent [4,5]. At birth virtually all human T cells express CD28, however by age 80 and above about 10–15% of CD4 and 50–60% of CD8 T cells lack CD28 expression [6] (Figure 1). The increase of circulating CD28− T cells with age is observed in human and primates but not in mouse [7]. Whether this is related to the difference of microorganism environment they interact and/or their lifespan between human/primate and mouse remains to be elucidated.
Figure 1.
Age associated accumulation of CD28− T cells. A schematic presentation of the accumulation of CD28− T cells with age for both CD4 and CD8 T cells. The ordinate indicates the frequency of CD28− CD4 or CD28− CD8 T cells. CD28− CD8 T cells show a dramatic increase in number with age whereas the increase of CD28− CD4 T cells is much more modest.
CD28, an extensively studied co-stimulatory molecule, plays multiple roles during T cell activation, proliferation, and survival [8,9]. T cells that lose expression of CD28 display several striking features including reduced diversity of T cell receptor (TCR) [10] and defects in antigen-induced proliferation [11]. However, at the same time, CD28− T cells have enhanced cytotoxicity [11] and display suppressive functions [12]. Furthermore, the accumulation of CD28− T cells is associated with the reduced overall immune response to pathogens and vaccines in the elderly [13,14].
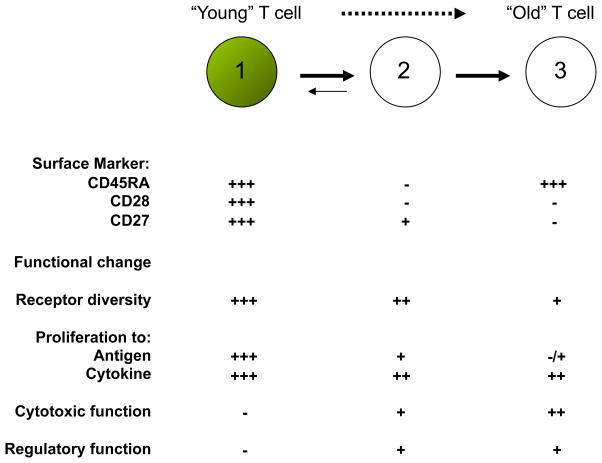
CD28− T cells are heterogenous populations and can be further divided into different subsets based on the expression of CD27, CD57 and other markers [15–17] (Figure 2). In CD8 T cells, the CD28−CD27− subset is considered to be close to terminal differentiation [18]. There is considerable interest in identifying the unique features of CD28− T cells as an important first step to understand age-associated changes in T cells and to subsequently develop potential intervention strategies to improve immune functions in the elderly.
Figure 2.
CD28− T cell differentiation and functional changes. In vitro and probably in vivo, CD28− T cells arise from CD28+ naïve and memory T cells (1) that have undergone repeated stimulation by antigen and/or by cytokines, particularly homeostatic cytokines (e.g IL-2, IL-7, IL-15). The differentiation path of CD28− T cells can be further divided into two main stages based on the expression of CD45RA and CD27. Appropriate triggers (e.g. certain cytokines) might reverse the differentiation of CD45RA−CD28−CD27+ T cells (2) back to CD45RA+CD28+CD27+ T cells but CD45RA+CD28−CD27− T cells (3) are considered to be terminally differentiated CD8 T cells. The antigen-induced proliferation of CD28− T cells is profoundly impaired but their proliferative response to homeostatic cytokines appears normal. The acquisition of cytolytic function is one of the major features of CD28− T cells and in addition immunoregulatory abilities have also been recently reported.
Origin of CD28− T cells
Virtually all naïve T cells in umbilical cord blood express CD28 [19] however a dominant fraction of peripheral blood T cells become CD28− during ageing [6]. The cause of loss of CD28 expression in T cells with age has been attributed to repeated antigenic stimulation which can also be observed in CD28+ T cells after repeated antigen stimulation in vitro [20,21]. This activation-induced progressive loss of CD28 culminates in a T cell population that is entirely CD28− in vitro [21,22]. The activation and differentiation-induced loss of CD28 is supported by the observations that CD28− T cells have shorter telomeres than their CD28+ counterparts, even when studied within the same clonal population [23–25]. The corollary of this is that CD28− T cells found in vivo have experienced past episodes of activation and cell cycling [2]. It is important to note that CD28 loss only occurs in human and non-human primate T cells [20] and not normally in murine systems [26]. This has limited the study of CD28− T cells to primates in vitro [27].
The loss of CD28 is not only a result of repeated TCR activation. Recent studies show that cytokines using the common γ-chain (e.g. IL-2, IL-7, and IL-15) accelerate the loss of CD28 in CD8 T cells that have been activated by the TCR [28,29]. IL-7 and IL-15 are critically important for the homeostatic maintenance of T cells in the absence of antigenic stimulation, particularly for CD8 memory T cells [30]. Loss of CD28 expression in CD8 memory T cells in the presence of IL-15 alone without TCR stimulation in vitro suggests that homeostatic proliferation can be another cause for age-associated loss of CD28 expression [29]. Interestingly, CD28− CD8 T cells do not display obvious proliferative defects in response to IL-15 in vitro, suggesting a significant role of homeostatic cytokines in accumulation of CD28− CD8 T cells with age [29].
In addition, the presence of type I interferons (IFN-α and IFN-β) during TCR activation also increases the proportion of CD8 CD28− [28] and CD4 CD28− [31] T cells in culture. Furthermore, TNFα induces a significant quantitative reduction of CD28 molecules on the cell surface that leads to the emergence of CD28− T cells [32]. This suggests that these cells may be generated in proinflammatory environments e.g. during viral infections and that persistent infections in particular, may drive the accumulations of these cells [31]. In fact, the loss of CD28 that is induced by IL-15 occurs in part by the induction of TNF-α in IL-15 cultured CD8 T cells [29]. As a higher level of TNF-α is present in the elderly than in young adults [33], TNF-α may play a prominent role in age-associated accumulation of CD28− CD8 T cells.
Regulation of CD28 expression
CD28 down-regulation with T cell activation involves transcriptional repression and increased protein turnover and has been interpreted as a negative feedback mechanism [34,35]. During normal antigenic exposure, CD28 expression is reduced but rapidly returns to the same level as that before stimulation. However, with sustained T cell stimulation and turnover, CD28 expression decreases and is eventually lost. CD28 can be initially re-induced by IL-12 [36] but once firmly established, CD28 loss is irreversible in the majority of CD28− T cells suggesting active transcriptional silencing.
Among the various epigenetic silencing mechanisms, CpG DNA methylation has a prominent role in fate decisions during T cell differentiation and is also known to change with age in proliferating tissues [37,38]. Cumulative CpG demethylation associated with proliferation and aging has been associated with the overexpression of a number of genes in T cells of the elderly including killer-cell immunoglobulin-like receptors (KIRs), leukocyte function receptor (LFA-1), CD70, and perforin [39–42]. While most of these genes are overexpressed in CD28− cells, the mechanism underlying the loss of CD28 appears to be fundamentally different. Reporter gene assays using the minimal CD28 promoter have shown that transcriptional activation is defective in CD28− T cells. This defect has been mapped to a unique transcriptional initiator element in the CD28 promoter that lacks sequence homology with the consensus initiator. T cells that have lost CD28 are not able to support CD28 initiator-dependent reporter gene constructs [43]; and they lack protein complexes able to bind to the CD28 initiator [44]. These protein complexes have only been partially characterized and include the ubiquitous proteins nucleolin and isoform A of heterogeneous ribonucleoprotein [45].
DNA binding complexes are rapidly lost in proliferating CD8 cells in parallel with declining CD28 expression while they persist in CD4 T cells which may explain the resistance of CD4 T cells to lose CD28 in vivo with aging [46]. Stimuli other than proliferation that control CD28 expression also exert their effect by regulating the CD28 initiator. For instance, in a negative feedback mechanism ligation of CD28 by CD80 represses the CD28 initiator function, likely mimicking the down-regulation seen with T cell activation [47]. Similarly, TNF-α suppresses initiator-driven transcription, while IL-12 restores it [47]. How transient CD28 repression is permanently imprinted is unknown and needs to be elucidated to develop strategies to either prevent CD28 loss with aging or to re-express CD28 in elderly T cells.
As described in the previous paragraph, CD28− T cells express high levels of the adhesion molecule LFA-1 and of effector molecules (perforin, granzyme B) and exhibit enhanced cytotoxicity. The high level of LFA-1 has the additional effect of lowering the T cell activation threshold [48] and predisposing to defective tolerance. In addition, it provides signals to ICAM (LFA-1 receptor) expressing somatic cells, controlling their proliferation and survival [49]. Expression of NK cell receptors (KLRs and KIRs) in CD28− T cells fundamentally influences how these cells sense signals from their environment because the ligands for these receptors are not limited to antigen-presenting cells. In contrast to inhibitory KIRs on NK cells, their presence on CD28− T cells only inhibits complex cellular functions such as proliferation while leaving certain effector functions, such as cytotoxicity, essentially intact, likely because they are initially excluded from the T cell receptor activation complex and therefore do not inhibit the transmission of early activation signals [50].
CD8 CD28− T cells appear to negatively impact immune responses through a variety of mechanisms and significantly contribute to the immune defect that is characteristic of the elderly. CD28− T cells are frequently oligoclonally expanded and constrain the adaptive immune system by competing for available space. In addition, they also exert direct negative effects on adaptive immune responses. In two vaccination studies, the frequency of CD8 CD28− T cells was a good predictor for defective humoral responses to the influenza vaccine, suggesting that CD8 CD28− cells exert suppressive function and, therefore, hinder CD4 vaccine responses [51]. In support of this interpretation, a study has linked CD28 loss from CD8 T cells in vitro with the development of the ability to tolerize dendritic cells and to suppress CD4 responses [52]. One possible mechanism for this is that CD8 CD28− T cells induce the expression of the negative regulatory receptors ILT3 and ILT4 on dendritic cells that prevent the upregulation of co-stimulatory ligands.
Molecular features of CD28− T cells
The completion of the human genome sequence and the advance of microarray technology in gene expression analysis allow one to directly examine the genome-wide gene expression features of CD28− T cells [53–56]. These analyses have revealed a small set of genes that significantly altered their expression levels between CD28− and their CD28+ counterparts. These differentially expressed genes are shared to a great degree between CD4 and CD8 T cells, and appear to be associated with the differentiation states of these cells rather than with the age of their donors per se [55]. Here we discuss the four groups of differentially expressed functional genes: 1) co-stimulatory receptors, 2) NK cell receptors, 3) cytokines/chemokines and their receptors, and 4) effector molecules.
Co-stimulatory receptors
The CD28 family of receptors consists of CD28, CTLA-4 (CD152), inducible co-stimulator (ICOS), program death-1 (PD-1, PDCD-1), and B and T lymphocyte attenuator (BTLA) [9]. In addition to CD28, the expressions of ICOS and CTLA4 are also down regulated in CD28− CD8 T cells. CD27-CD70 is another co-stimulatory receptor-ligand pair involved in T cell activation [57]. While the expression of CD27 decreases, the level of CD70 appears to be increased in CD28− CD4 and CD8 T cells. While the altered expressions of these co-stimulatory receptors provide more details and reason for the proliferative deficiency of CD28 T cells in response to antigen, it also reflects the adaptation of CD28− T cells to their changed environments and conditions.
NK cell receptors
A striking feature of CD28− T cells is the acquired expression of several receptors more commonly associated with the nature killer (NK) cells. These NK cell receptors include KIR2DL2 (CD158B1), KIR3DL2 (CD158K), KLRD1 (CD94), KLRF1, KLRK1 (NKG2D), NCR1 (CD355), and CD244 (2B4). KLRD1 forms a dimer with KLRC1 (NKG2A) [58] binds to HLA-E and inhibits cytotoxicity whereas with KLRD1 (NKG2C) or KLRK1 can deliver a stimulatory function when expressed on CD8 T cells [59]. In contrast, NCR1, and CD244 are activation receptors. The level of CD244 expression is elevated in cytomegalovirus (CMV)-specific effector CD8 T cells [60] and induces elevated effector function. The identification of elevated NK cell receptor expression in CD28− T cells provides evidence that CD28− T cells are capable of carrying out some NK cell functions such cytotoxic killing.
Chemokine/cytokine and their receptors
Profound altered expression of several chemokines, cytokines and their receptors have been observed in CD28− CD8 T cells. While enhanced expression of genes including IL-12A (IL12A), chemokine (C-C motif) ligand 4 (CCL4 or MIP1-β), and chemokine (C-X3-C motif) receptor 1 (CX3CR1 or CCRL1) are observed, interleukins (IL-3 and IL-23A), interleukin 12 receptor β2 (IL12RB2), and chemokine receptor (CCR6, and CCR7) transcription are decreased in CD28− T cells. Some of these changes might be co-regulated as either positive or a negative feedback loops. IL-12 facilitates Th1 differentiation through inducing production of IFN-γ [61] and also enhances CD28 transcription in CD28− CD4 T cells in vitro [36]. The co-existence of an increased expression of IL-12 and a decreased expression of its own receptor (IL12RB2) in CD28− T cells might suggest that increased IL-12 expression is a consequence of decreased expression of IL-12 receptor. Functionally, elevated expression of IL-12 also contributes to the enhanced cytotoxicity of CD28− T cells. Chemokines and their receptors are the key mediators of lymphocyte migration. How the elevated expression of CCL4 and CX3CR1 [62,63] along with the diminished expression of CCR6 and CCR7 contributes to the migration pattern and ultimately function of CD28− T cells will require additional study.
Effector molecules
The gain of cytolytic function of CD28− T cells is supported by elevated expression of the key cytolytic molecules including perforin (PRF1), granzyme B (GZMB), and granzyme H (GZMH). In CD28+ CD8 T cells, the levels of PRF1 and GZMB are very low and increase only after activation [64] while the GZMH level is low in both freshly isolated and activated T cells [65]. Interestingly, CD28− CD8 T cells express high levels of both GZMB and GZMH, presenting a unique feature that is found in both activated/effector T cells and NK cells.
Functional characteristics of CD28− T cells
Early studies showed that the loss of CD28 was associated with a change of cellular function in T cells including decreased anti-CD3 stimulation, decreased ability to secrete IL-2 after activation [66], decreased ability to up-regulate telomerase after activation [67,68] and increased susceptibility to activation-induced cell death [28]. In addition, the shorter telomeres in CD28− compared to CD28+ T cells [23,69] might be due to the fact that CD28 signaling is required for optimal telomerase induction [70]. Amongst the various CD28− T cell sub-populations, CD28−CD27− T cells were shown to have reduced capacity to survive and proliferate after TCR activation in vitro compared to CD28−CD27+ T cells [28]. Interestingly, the addition of either IL-2 or IL-15 enabled the CD28−CD27− T cells to survive and proliferate to levels observed in the other populations [15]. However the dependence on these cells on IL-2 and related cytokines for activation cannot explain all their functional defects after activation.
The most reproducible activation induced defect in CD28− T cells, in particular those that have differentiated to a CD28−CD27− state, is their lack of telomerase activity that is not reversed by the addition of IL-2 or the provision of alternative co-stimulatory signals [15]. The differentiation-induced down-regulation of telomerase in human T cells does not correlate with decreased transcription of hTERT, the catalytic component of the enzyme [15,71,72]. It has been shown that the kinase Akt (protein kinase B, PKB) signaling is crucial for cell proliferation, survival [73], and telomerase induction [74]. Phosphorylation of Akt at two different sites (Ser473 and Thr308) is associated with activation [75,76] and a progressive decline in Akt phosphorylation at the Ser473 site was observed as T cells differentiated from CD28+CD27+ to CD28−CD27+ to CD28−CD27− and this could not be reversed by the addition of IL-2 [15]. As telomerase activity in T cells requires hTERT phosphorylation [77] and since hTERT is a substrate for Akt [74], one prediction of the impact of decreased Akt phosphorylation in CD28−CD27− T cells is that these cells would also have decreased hTERT phosphorylation. Indeed, decreased hTERT phosphorylation has been found in these cells compared to CD28−CD27+ and CD28+CD27+ T cells. However it has been shown that other signaling events such as that via the mitogen activated protein (MAPK) ERK might have a more prominent role than Akt in regulating telomerase activity in T cells [78]. The changes in cell signaling that occurs during differentiation, some of which are associated with loss of CD28− expression during T cell differentiation require further study to determine which of these are reversible.
As described above, CD28− T cells express high levels of effector molecules (perforin, granzyme B) and exhibit enhanced cytotoxicity. The high level of LFA-1 lowers the T cell activation threshold [48] and predisposes to defective tolerance, but also provides signals to ICAM (LFA-1 receptor) expressing somatic cells, controlling their proliferation and survival [49]. Expression of NK cell receptors (KLRs and KIRs) in CD28− T cells fundamentally influences how these cells sense signals from their environment because the ligands for these receptors are not limited to antigen-presenting cells. In contrast to inhibitory KIRs on NK cells, their presence on CD28− T cells only inhibits complex cellular functions such as proliferation while leaving certain effector functions, such as cytotoxicity, essentially intact, likely because their recruitment to the T cell receptor synapse does not occur immediately after activation [50]. Consequently, some effector functions are not affected while proliferative responses and IFN-γ production that require early and sustained signaling are clearly inhibited.
CD8 CD28− T cells appear to negatively impact immune responses through a variety of mechanisms and significantly contribute to the immune defect that is characteristic of the elderly. CD28− T cells are frequently oligoclonally expanded and constrain the adaptive immune system by competing for available space. In addition, they also exert direct negative effects on adaptive immune responses. In two vaccination studies, the frequency of CD8 CD28− T cells was a good predictor for defective humoral responses to the influenza vaccine, suggesting that CD8 CD28− cells exert suppressive function and, therefore, hinder CD4 vaccine responses [51]. In support of this interpretation, a study has linked CD28 loss from CD8 T cells in vitro with the development of the ability to tolerize dendritic cells and to suppress CD4 responses [52]. One possible mechanism for this is inhibiting the upregulation of the co-stimulatory molecule CD154 on helper CD4+ T cells that prevents their interaction and activation with CD40 the dendritic cells.
CD28− T cells in disease
Increased frequencies of CD28− T cells are common features of immune aging and chronic viral infections (Box 1). Expansion of CD4 CD28− T cells with age is usually minute and infrequent while CD8 CD28− T cells predominate. In otherwise healthy elderly, CD8 CD28− T cells are frequently specific for CMV and EBV antigens. As discussed above, these cells have a negative impact on the adaptive immune system through a variety of mechanism. It has therefore been suggested that the immune exhaustion and immune system remodeling associated with chronic CMV infection is in part responsible for the immune defects seen in the elderly [79]. CD4 CD28− T cells reach high frequencies in some individuals. Persistent infection with CMV in particular has been shown to drive has been shown to drive the accumulation of CD28− T cells within both the CD4 and CD8 compartments, in part by the induction of INF-α synthesis by plasmacytoid dendritic cells (PDC) [31]. It has been hypothesized that the accumulation of these CD28− T cells cells may be detrimental during ageing as they occupy vital immunological niches and render them inaccessible to protective T cells of other specificities [79].
Box 1. CD28− T cells and clinical manifestations.
CD4+CD28− T cells
are infrequent in most elderly individuals
are expanded in patients with autoimmune diseases (multiple sclerosis, rheumatoid arthritis, Wegener’s granulomatosis) and in patients at risk for inflammatory vascular complications (plaque rupture, acute coronary syndrome, stroke).
have increased effector functions and may amplify autoimmune and inflammatory responses.
CD8+CD28− T cells
accumulate in most individuals with normal aging
in part reflect the infectious burden from chronic viral infections (CMV, EBV. HIV)
can, depending on the setting, exhibit increased effector functions or display signs of lymphocyte exhaustion with dominant inhibitory receptors and loss of cytotoxic ability.
are directly or indirectly responsible for the immune defects in the elderly by competing for resources and compromising T cell homeostasis or by having suppressor activity
It is notable that many of individuals who have elevated number of CD28− T cells have an autoimmune disease, e.g. rheumatoid arthritis (RA), multiple sclerosis, Wegener’s granulomatosis, Graves’ disease, or ankylosing spondylitis [80–84] further suggesting that chronic inflammation may drive the accumulation of these cells. In addition, CD4 CD28− T cells play a role in the vascular inflammation in atherosclerotic diseases that lead to plaque instability, acute coronary syndromes and stroke [85,86]. Whether the frequency of CD8 CD28− T cells in these settings is age-inappropriately increased has been less well studied. It is possible that the higher frequency of CD4 CD28− T cells is the consequence of chronic immune stimulation by an autoantigen. The contribution of autoantigen-specific responses to CD4 CD28− T cells expansion is difficult to estimate because for the majority of these systemic diseases, the nature of the autoantigen has not been defined. In most studies CD4 CD28− T cells do not respond to the usual suspects such as collagen in rheumatoid arthritis or myelin basic protein in multiple sclerosis, but can be stimulated by rather ubiquitous antigens such as heat shock proteins or viral antigens [87,88]. This finding resonates with the clinical interpretation that expansion of CD4 CD28− T cells represent an important amplification mechanism leading to more severe and systemic disease rather than being involved in the initial break of tolerance.
Most efforts to understand the contribution of CD4 CD28− T cells to the inflammatory response have suggested that they are readily triggered by inflammation and thereafter perpetuate and amplify the inflammatory process [88]. Chemokine receptors and effector molecules such as perforin and granzyme B uniquely expressed on CD4 CD28− T cells may enable these cells to infiltrate tissue and to cause tissue damage [89]. Expression of stimulatory receptors lowers the activation threshold for antigen-specific stimulation and, in selected cases renders cell activation completely independent of the recognition of the appropriate antigenic peptide [90]. NKG2D expressed by CD4 CD28− T cells can provide a costimulatory signal and increase the promiscuity of these cells towards antigenic peptide stimulation. NKG2D expression on CD4 CD28−T cells has been identified in patients with RA, as well as in patients with Wegener’s granulomatosis [91].
It is perplexing that aged individuals who are becoming increasingly immune incompetent in terms of vaccine and pathogen responses have at the same time an increased risk of developing certain autoimmune diseases. Only lately have we started to understand that the age-dependent immune degeneration also includes a propensity for increased activation of the innate immune system and breaches of tolerance [92]. CD28− T cells represent a remarkable example of how the same mechanisms lead to autoimmunity and immune deficiency. The increased frequencies of CD28− effector T cells occur at the expense of naïve and central memory T cells and this accumulation predisposes to tissue injury while compromising responses to novel antigens. These CD28− effector T cells acquire the expression of negative and positive regulatory receptors that are evolutionarily selected to regulate NK cell function. Expression of inhibitory receptors is aimed at containing unwanted effector cell activities, but also carries the risk of dampening appropriate immune responses. On the contrary, co-stimulatory receptors lower the T cell activation threshold. As most of the ligands for these receptors are expressed on somatic cells, activation of CD8 and, more importantly CD4 T cells is no longer under the control of professional antigen-presenting cells with the consequence of increased tissue inflammation or autoimmunity.
Conclusion
Substantial progress has been made in understanding the molecular, cellular, and functional features of CD28− T cells since their initial identification in early 1990s. From the characterization of the loss of function (oligoclonality, antigen mediated proliferation deficiency, and reduced replicative lifespan) to the gain of function (enhanced cytotoxicity and immunoregulation), the multi-faceted nature of CD28− T cells has become increasingly clear. The current challenges are to elucidate the differentiation process of CD28− T cell generation, differentiation, and maintenance, and to understand the networks that down- and up- regulate expression of CD28 and its associated related genes. Further characterization and better understanding of the molecular changes of CD28− T cells will open new avenues to explore the potential targets that can restore or reverse the changes in CD28− T cell function during ageing.
Acknowledgments
N-P Weng was supported by the Intramural Research Programs of the National Institute on Aging, National Institutes of Health (NIH). A. Akbar is supported by British Biotechnological and Biological Sciences Research Council. J. Goronzy is supported by RO1-AR41974, RO1-AG15043 and U19-AI57266.
References
- 1.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effros RB, et al. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 5.Vallejo AN, et al. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Fagnoni FF, et al. Expansion of cytotoxic CD8+CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz-Suarez A, Miller RA. A subset of CD8 memory T cells from old mice have high levels of CD28 and produce IFN-gamma. Clin Immunol. 2002;104:282–292. doi: 10.1006/clim.2002.5221. [DOI] [PubMed] [Google Scholar]
- 8.Lenschow DJ, et al. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 9.Riley JL, June CH. The CD28 Family: a T Cell Rheostat for Therapeutic Control of T Cell Activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 10.Batliwalla F, et al. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68–76. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 11.Azuma M, et al. CD28− T lymphocytes: antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 12.Ciubotariu R, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+ CD28− Regulator T cells. J Immunol. 1998;161:5193–5202. [PubMed] [Google Scholar]
- 13.Saurwein-Teissl M, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 14.Goronzy JJ, et al. Prognostic markers of radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2004;50:43–54. doi: 10.1002/art.11445. [DOI] [PubMed] [Google Scholar]
- 15.Plunkett FJ, et al. The loss of telomerase activity in highly differentiated CD8+CD28−CD27− T cell is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 16.Appay V, et al. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 17.Sze DM, et al. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(−) compartment. Blood. 2001;98:2817–2827. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 18.Van Lier RA, et al. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 19.Azuma M, et al. CD28 co-stimulation of T-cell-mediated cytotoxicity. Int J Cancer Suppl. 1992;7:33–35. [PubMed] [Google Scholar]
- 20.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 22.Vallejo AN, et al. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–6579. [PubMed] [Google Scholar]
- 23.Monteiro J, et al. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 24.Colombatti A, et al. Age-related persistent clonal expansions of CD28(−) cells: phenotypic and molecular TCR analysis reveals both CD4(+) and CD4(+)CD8(+) cells with identical CDR3 sequences. Clin Immunol Immunopathol. 1998;89:61–70. doi: 10.1006/clin.1998.4580. [DOI] [PubMed] [Google Scholar]
- 25.Posnett DN, et al. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+CD28− cytotoxic effector clones. Int Immunol. 1999;11:229–241. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz-Suarez A, Miller RA. A subset of CD8 memory T cells from old mice have high levels of CD28 and produce IFN-gamma. Clin Immunol. 2002;104:282–292. doi: 10.1006/clim.2002.5221. [DOI] [PubMed] [Google Scholar]
- 27.Plunkett FJ, et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech Ageing Dev. 2005;126:855–865. doi: 10.1016/j.mad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Borthwick NJ, et al. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 29.Chiu WK, et al. Generation and Growth of CD28nullCD8+ Memory T Cells Mediated by IL-15 and Its Induced Cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher JM, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 32.Bryl E, et al. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 33.Fagiolo U, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 34.Linsley PS, et al. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J Immunol. 1993;150:3161–3169. [PubMed] [Google Scholar]
- 35.Swigut T, et al. Mechanism for down-regulation of CD28 by Nef. EMBO J. 2001;20:1593–1604. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warrington KJ, et al. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003;101:3543–3549. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 37.Wilson CB, et al. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–119. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Golbus J, et al. Quantitative changes in T cell DNA methylation occur during differentiation and ageing. Eur J Immunol. 1990;20:1869–1872. doi: 10.1002/eji.1830200836. [DOI] [PubMed] [Google Scholar]
- 39.Oelke K, et al. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–1860. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 40.Lee WW, et al. Unchecked CD70 expression on T cells lowers threshold for T cell activation in rheumatoid arthritis. J Immunol. 2007;179:2609–2615. doi: 10.4049/jimmunol.179.4.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan HW, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197:245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, et al. Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech Ageing Dev. 2002;123:1257–1268. doi: 10.1016/s0047-6374(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 43.Vallejo AN, et al. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- 44.Vallejo AN, et al. Functional disruption of the CD28 gene transcriptional initiator in senescent T cells. J Biol Chem. 2001;276:2565–2570. doi: 10.1074/jbc.M005503200. [DOI] [PubMed] [Google Scholar]
- 45.Vallejo AN, et al. Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem. 2002;277:46940–46949. doi: 10.1074/jbc.M207352200. [DOI] [PubMed] [Google Scholar]
- 46.Vallejo AN, et al. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–6579. [PubMed] [Google Scholar]
- 47.Bryl E, et al. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 48.Yung R, et al. Mechanisms of drug-induced lupus. II T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866–2871. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh K, et al. Synoviocyte stimulation by the LFA-1-intercellular adhesion molecule-2-Ezrin-Akt pathway in rheumatoid arthritis. J Immunol. 2008;180:1971–1978. doi: 10.4049/jimmunol.180.3.1971. [DOI] [PubMed] [Google Scholar]
- 50.Henel G, et al. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 2006;107:4449–4457. doi: 10.1182/blood-2005-06-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saurwein-Teissl M, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 52.Ciubotariu R, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+ CD28− Regulator T cells. J Immunol. 1998;161:5193–5202. [PubMed] [Google Scholar]
- 53.Fann M, et al. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol Rev. 2005;205:190–206. doi: 10.1111/j.0105-2896.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 54.Godlove J, et al. Gene expression and generation of CD28−CD8 T cells mediated by interleukin 15. Exp Gerontol. 2007;42:412–415. doi: 10.1016/j.exger.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazuardi L, et al. Microarray analysis reveals similarity between CD8(+)CD28 (−) T cells from young and elderly persons, but not of CD8 (+)CD28 (+) T cells. Biogerontology. 2008 doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- 56.Czesnikiewicz-Guzik M, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 58.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 59.Guma M, et al. The CD94/NKG2C killer lectin-like receptor constitutes an alternative activation pathway for a subset of CD8+ T cells. Eur J Immunol. 2005;35:2071–2080. doi: 10.1002/eji.200425843. [DOI] [PubMed] [Google Scholar]
- 60.Speiser DE, et al. The activatory receptor 2B4 is expressed in vivo by human CD8+ effector alpha beta T cells. J Immunol. 2001;167:6165–6170. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 61.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka Y, et al. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 63.Imai T, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 64.Clement MV, et al. Granzyme B-gene expression: a marker of human lymphocytes “activated” in vitro or in renal allografts. Hum Immunol. 1990;28:159–166. doi: 10.1016/0198-8859(90)90013-f. [DOI] [PubMed] [Google Scholar]
- 65.Sedelies KA, et al. Discordant regulation of granzyme H and granzyme B expression in human lymphocytes. J Biol Chem. 2004;279:26581–26587. doi: 10.1074/jbc.M312481200. [DOI] [PubMed] [Google Scholar]
- 66.Topp MS, et al. Restoration of CD28 expression in. J Exp Med. 2003;198:947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akbar AN, Vukmanovic-Stejic M. Telomerase in T lymphocytes: use it and lose it? J Immunol. 2007;178:6689–6694. doi: 10.4049/jimmunol.178.11.6689. [DOI] [PubMed] [Google Scholar]
- 68.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 69.Effros RB, et al. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Weng NP, et al. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu K, et al. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci U S A. 1999;96:5147–5152. doi: 10.1073/pnas.96.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dagarag M, et al. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 73.Song G, et al. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang SS, et al. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 75.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 76.Jacinto E, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 77.Weng NP, et al. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 78.Fauce SR, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pawelec G, et al. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 80.Sun Z, et al. Association of Graves’ disease and prevalence of circulating IFN-gamma-producing CD28(−) T cells. J Clin Immunol. 2008;28:464–472. doi: 10.1007/s10875-008-9213-4. [DOI] [PubMed] [Google Scholar]
- 81.Schirmer M, et al. Circulating cytotoxic CD8(+) CD28(−) T cells in ankylosing spondylitis. Arthritis Res. 2002;4:71–76. doi: 10.1186/ar386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komocsi A, et al. Peripheral blood and granuloma CD4(+)CD28(−) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener’s granulomatosis. Am J Pathol. 2002;160:1717–1724. doi: 10.1016/s0002-9440(10)61118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markovic-Plese S, et al. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–1194. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmidt D, et al. CD4+ CD7−CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nadareishvili ZG, et al. Elevated pro-inflammatory CD4+CD28− lymphocytes and stroke recurrence and death. Neurology. 2004;63:1446–1451. doi: 10.1212/01.wnl.0000142260.61443.7c. [DOI] [PubMed] [Google Scholar]
- 86.Liuzzo G, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 87.Zal B, et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109:1230–1235. doi: 10.1161/01.CIR.0000118476.29352.2A. [DOI] [PubMed] [Google Scholar]
- 88.Thewissen M, et al. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol. 2007;179:6514–6523. doi: 10.4049/jimmunol.179.10.6514. [DOI] [PubMed] [Google Scholar]
- 89.Nakajima T, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 90.Yen JH, et al. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–1167. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Groh V, et al. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]