Abstract
Background
Despite compelling evidence that aspirin reduces fatal and non-fatal vascular events among the overall population in various settings, women have frequently been underrepresented and their data underreported. We sought to evaluate the relationship between aspirin use, dose (81 or 325mg) and clinical outcomes among postmenopausal women with stable cardiovascular disease.
Methods
Women with cardiovascular disease (n=8928) enrolled in the Women’s Health Initiative Observational Study were used for this analysis. The primary outcome was the incidence of all-cause mortality and cardiovascular events (myocardial infarction, stroke and cardiovascular death).
Results
Among 8928 women with stable cardiovascular disease, 4101 (46%) reported taking aspirin, of whom 30% were on 81 and 70% were on 325mg. At 6.5 years of follow-up, no significant association was noted for aspirin use and all-cause mortality or cardiovascular events. However, after multivariate adjustment, aspirin use was associated with a significantly lower all-cause (adjusted HR 0.86, [0.75-0.99], P=0.04) and cardiovascular related mortality (adjusted HR 0.75, [0.60-0.95], P=0.01) compared with no aspirin. Aspirin use was associated with a lower risk of cardiovascular events (adjusted HR 0.90, [0.78-1.04], P=0.14) which did not meet statistical significance. Compared with 325mg, use of 81mg was not significantly different for all-cause mortality, cardiovascular events or any individual endpoint.
Conclusions
After multivariate adjustment, aspirin use was associated with significantly lower risk of all-cause mortality, specifically cardiovascular mortality, among postmenopausal women with stable cardiovascular disease. No significant difference was noted between 81 and 325mg of aspirin. Overall, aspirin use was low in this cohort of women with stable cardiovascular disease.
Keywords: Aspirin, Dose, Women, Cardiovascular Disease, Observational Study
Randomized studies of patients with cardiovascular disease provide compelling evidence that antiplatelet therapy reduces morbidity and mortality1, 2. Accordingly, evidence-based guidelines strongly advocate aspirin for the secondary prevention of cardiovascular events3, 4. However, the effect of aspirin in women with stable cardiovascular disease has not been fully evaluated. Among 278 trials included in the Antiplatelet Trialists’ Collaboration, 34 evaluated a population with stable cardiovascular disease (prior myocardial infarction, stroke/transient ischemic attack, or stable angina), and only 6 trials evaluated low-dose aspirin (50-325mg) versus placebo/control, some of which excluded or only included a minority of women5.
The dose of aspirin used for secondary prevention of adverse cardiac events varies6, 7. A meta-analysis found a similar reduction of cardiac adverse events for doses 75-150mg and 160-325mg1, 2. A more recent analysis of the same data among unstable patients documented a greater benefit with lower doses of aspirin8. Consistent with the uncertainty of optimal aspirin dose, guidelines tend to differ4, 9. The AHA/ACC guidelines for secondary prevention recommend aspirin (75-162mg) in all patients unless contraindicated. The recent AHA guidelines for cardiovascular disease prevention in women support aspirin (75-325mg) in women with established cardiovascular disease. Despite the increased recent attention to aspirin dose, little is known about aspirin dose in women with stable cardiovascular disease.
The purpose of this study was three-fold. First, to define the utilization of aspirin among post-menopausal women with cardiovascular disease in the Women’s Health Initiative (WHI) Observational Cohort. Second, to determine the association between aspirin use, all-cause mortality and other cardiovascular events. Finally, to evaluate the relationship between aspirin dose (81 vs. 325mg) and clinical outcomes.
Methods
Study Population
As described elsewhere10, the WHI has clinical trial and observational study (OS) components. The latter component is on ongoing, nationwide, prospective cohort study of post-menopausal women of diverse races and ethnicities and is designed to examine the association between clinical, socio-economic, behavioral, and dietary risk factors and the subsequent incidence of several health outcomes. The WHI-OS cohort consists of 93,676 women between 50-79 years of age enrolled at 40 clinical centers throughout the US between 1994-1998. The study was approved by the institutional review boards of the participating clinical centers, the coordinating center at the Fred Hutchinson Cancer Center, and the National Institutes of Health. Participants gave written informed consent. The design and reliability of baseline measures have been published in detail previously11.
Among WHI-OS participants, 8928 had a history of stable cardiovascular disease, defined by one or more of the following conditions at baseline: previous myocardial infarction, previous stroke/transient ischemic attack, previous or current angina, and a history of coronary revascularization.
Measurement of Exposure
Aspirin use was assessed from an interview-administered questionnaire. Each participant was asked: “Do you take aspirin pills or powders, ibuprofen pills or tablets, other nonsteroidal anti-inflammatory (NSAID) pain pills (including prescription drugs), or acetaminophen tablets or capsules?” Those individuals who reported using aspirin at least three times a week in each of the 2 weeks preceding the interview were considered aspirin users. Details of their aspirin use including type of compound and strength (in milligrams) were recorded. The medication data was validated by checking pill bottle labels and prescription records during the interview process. For the current analysis women reporting 81mg, 325mg and no aspirin (as the reference category) were included. Women who reported use of 120-300mg (n=86, 0.9%) or > 325mg (n=208, 2.2%) were excluded from this analysis.
Follow-up
As of February 2004, the mean duration of follow-up was 6.5 years (Standard Deviation, 1.6 years; Range, 0.1-9.3 years). Vital status was available for 98.2% of respondents. Participant fatalities were identified through communication with proxy respondents and through National Death Index searches. Deaths caused by coronary disease were confirmed on the basis of death certificates, autopsy reports, circumstances of death, electrocardiogram, laboratory test results, and reports from all relevant procedures. Participants are sent annual medical update forms to report the occurrence of any hospitalization and a wide variety of outcomes, including MI. Confirmation of self-reported nonfatal MI was based on adjudication by trained physicians of documentation of new chest pain syndromes accompanied by characteristic evolution of electrocardiographic changes or clear evidence of myocyte damage as evidenced by elevated creatine kinase-MB or troponin values. Stroke diagnosis was based on the rapid onset of a persistent neurological deficit attributable to an obstruction or rupture of the arterial system supported by imaging studies when available. The neurological deficit must have lasted more than 24 hours, unless death supervened or there was a demonstrable radiographic lesion compatible with acute stroke.
Statistical Analysis
Differences between aspirin users (81 and 325 mg) and nonusers were compared using X2 statistics for categorical variables and t test or ANOVA, as appropriate, for continuous variables. Aspirin use was related to all-cause mortality and cardiovascular endpoints using univariable and multivariable Cox proportional hazard regression analyses with inclusion of clinically plausible interactions. Outcome comparisons were made from Cox proportional hazards analyses and Kaplan-Meier curves.
Important subgroups were prespecified. To examine whether the effect of aspirin varied between subgroups, we constructed Cox models with a group of core variables (age, race, education, last medical visit within 1 year, insurance status, hormone replacement therapy, smoking status, body mass index, statin use, beta-blocker use and NSAID use, and history of myocardial infarction, transient ischemic attack, stroke, angina, peripheral artery disease, coronary revascularization, diabetes, and hypertension), treatment subgroup, and the interaction between subgroup and treatment; we then evaluated the interaction terms one at a time, for statistical significance.
Because aspirin use was not randomly assigned, potential confounding and selection biases were accounted for by developing a propensity score for aspirin use12, 13. The propensity for aspirin use was determined without regard to outcome, using multivariable logistic regression analysis. A full nonparsimonious model was developed that included 32 covariates. A propensity score for aspirin use was then calculated from the logistic equation for each patient. We then sought to match each aspirin user to a non-using patient who had a propensity score that was identical to 5 digits. If this could not be done, we then proceeded to a 4, 3, 2, or 1 digit match. We were able to match 2646 aspirin using patients to 2646 unique non-aspirin using patients.
A second propensity model to evaluate the effect of aspirin dose was created only among aspirin users. In this analysis we generated separate propensity scores for the use of 81 mg of aspirin. We then sought to match each user of 81mg to a user of 325mg. We were able to match 1036 users of 81mg to 1036 users of 325mg. Kaplan-Meier methods were then used in these cohorts to estimate the unadjusted event rates, and log-rank tests were used to formally compare the groups.
All analyses were conducted by use of Statistical Analysis Software (SAS).
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
Among 8928 post-menopausal women with cardiovascular disease, 4101 (46%) reported taking aspirin. Of the aspirin users, 1224 (30%) were on 81mg and 2877 (70%) were on 325mg. Among women with a history of myocardial infarction, transient ischemic attack, prior revascularization, stroke, and angina the rate of aspirin use was 54%, 43%, 50%, 71% and 44%, respectively. Baseline characteristics according to aspirin use and dose are summarized in Table 1.
Table 1. Baseline Differences for Women With Cardiovascular Disease According to Aspirin Dose.
| No aspirin (n=4,827) |
81mg (n=1,224) |
325 mg (n=2,877) |
P value | |
|---|---|---|---|---|
| Demographics (%) | ||||
| Age ± SD | 66 ± 7 | 68 ± 6 | 68 ± 7 | <0.001 |
| Age | <0.001 | |||
| ≤60 | 23 | 12 | 15 | |
| >60 | 77 | 88 | 85 | |
| Race/Ethnicity | <0.001 | |||
| White | 75 | 83 | 84 | |
| Black | 16 | 9.0 | 9.8 | |
| Other | 9.6 | 7.8 | 5.8 | |
| BMI (kg/m2) ± SD | 29 ± 6 | 28 ± 6 | 28 ± 6 | <0.001 |
| BMI | <0.001 | |||
| <25 | 28 | 34 | 38 | |
| 25-30 | 38 | 35 | 27 | |
| >30 | 31 | 30 | 32 | |
| Clinical History (%) | ||||
| Diabetes | 15 | 14 | 16 | 0.334 |
| Hypertension | 58 | 60 | 63 | <0.001 |
| Systolic BP ± SD | 132 ± 19 | 132 ± 19 | 133 ± 19 | 0.022 |
| Diastolic BP ± SD | 75 ± 10 | 72 ± 10 | 73 ± 10 | <0.001 |
| Hypercholesterolemia | 28 | 45 | 43 | <0.001 |
| Stroke | 16 | 13 | 15 | 0.052 |
| TIA | 24 | 27 | 25 | 0.032 |
| Angina | 63 | 60 | 57 | <0.001 |
| Myocardial infarction | 21 | 26 | 31 | <0.001 |
| Percutaneous revascularization |
6.3 | 17 | 21 | <0.001 |
| Bypass surgery | 5.1 | 13 | 16 | <0.001 |
| PAD | 8.3 | 8.1 | 9.3 | 0.301 |
| CHF | 6.4 | 5.3 | 5.8 | 0.250 |
| Hormone use ever | 0.006 | |||
| Past user | 27 | 27 | 26 | |
| Current user | 39 | 44 | 41 | |
| Social History | ||||
| Smoking | 0.013 | |||
| Never smoked | 48 | 47 | 46 | |
| Past smoker | 44 | 47 | 47 | |
| Current smoker | 7.9 | 5.7 | 7.0 | |
| >7 alcoholic drinks/wk | 8.0 | 10 | 10 | <0.001 |
| Last medical visit w/in 1 year |
89 | 93 | 92 | <0.001 |
| Any Insurance | 96 | 98 | 97 | 0.001 |
| Medicaid | 5.5 | 2.3 | 2.9 | <0.001 |
| Medicare | 54 | 63 | 62 | <0.001 |
| Military / VA | 2.9 | 2.2 | 3.0 | 0.389 |
| Region | <0.001 | |||
| Northeast | 22 | 26 | 25 | |
| South | 28 | 22 | 24 | |
| Midwest | 21 | 20 | 24 | |
| West | 29 | 32 | 28 | |
| Education | <0.001 | |||
| < high school diploma | 10 | 6.5 | 8.5 | |
| high school – college | 61 | 58 | 61 | |
| ≥ college graduate | 29 | 36 | 30 | |
| Medications | ||||
| NSAIDs | 22 | 19 | 18 | 0.001 |
| Beta blockers | 18 | 28 | 31 | <0.001 |
| Statins | 15 | 31 | 30 | <0.001 |
CVD, cardiovascular disease; BMI, body mass index; BP, blood pressure; TIA, transient ischemic attack; PAD, peripheral artery disease; CHF, congestive heart failure; VA, veterans affairs; NSAID, non-steroidal anti inflammatory drug.
Predictors of Aspirin Use and Dose
Clinical predictors of aspirin use included prior revascularization (3.08 [2.68-3.55]), hypercholesterolemia (1.27 [1.10-1.46]), treated hypertension (1.18 [1.05-1.33]), previous MI (1.18 [1.04-1.33]), prior transient ischemic attack (1.41 [1.23-1.61]), statin treatment (1.40 [1.19-1.65]) and beta blocker therapy (1.54 [1.36-1.74]). Demographic predictors of aspirin use included increasing age per year (1.02 [1.01-1.03]) and college education (1.25 [1.01-1.55]). Negative predictors of aspirin use included African American race (0.70 [0.59-0.83]), Medicaid insurance (0.59 [0.45-0.77]), increasing BMI (0.98 [0.98-0.99]) and NSAID use (0.86 [0.76-0.97]).
Women who were older and more educated were more frequently on 81mg. A history of myocardial infarction, prior revascularization, beta blocker use and increasing BMI were predictors for 325mg. Race and insurance type were not associated with aspirin dose.
Aspirin Use, All-Cause Mortality and Cardiovascular Outcomes
During an average of 6.5 years of follow-up, 956 participants (10.7%) died. Table 2 summarizes all outcomes based on aspirin use and dose. After multivariable adjustment, aspirin was associated with a 14% lowering in the risk of all cause mortality (HR 0.86 [0.75-0.99]) (Figure 1). A composite of adverse cardiovascular events occurred in 969 (10.8%) women during follow-up. After multivariable adjustment, aspirin was associated with a non-significant decrease in cardiovascular events (HR 0.90 [0.78-1.04]). However, aspirin was associated with a 25% significantly lower risk of cardiovascular mortality (HR 0.75 [0.60-0.95]).
Table 2. Clinical Outcomes According to Aspirin Use and Dose.
| No aspirin (n=4,827) |
75-81mg (n=1,224) |
325 mg (n=2,877) |
P value | |
|---|---|---|---|---|
| Clinical outcomes (%) | ||||
| All-cause mortality | 10.9 | 9.9 | 10.7 | 0.566 |
| Cardiovascular composite* | 10.3 | 11.0 | 11.7 | 0.186 |
| Myocardial infarction | 5.5 | 6.5 | 7.3 | 0.005 |
| Stroke | 4.3 | 4.1 | 4.5 | 0.818 |
| Cardiovascular mortality | 4.3 | 4.0 | 4.3 | 0.876 |
myocardial infarction/stroke/cardiovascular mortality
Figure 1. Adjusted Cox Proportional Hazards Analyses of Time to Adverse Outcomes among Women with Cardiovascular Disease (n=8,928).
Subgroup Analyses
Several characteristics of the participants were examined for possible interaction with the use of aspirin and the risk of all-cause mortality (Figure 2). No significant interaction was observed between age, race, smoking status, statin use, beta-blocker use and NSAID use. Women on current hormonal therapy appeared to have the greatest mortality benefit with aspirin therapy (P for interaction < 0.01). Possible interaction with aspirin use and the composite of adverse cardiovascular events were also assessed (Figure 2). For women between the ages 50 to 59, 60 to 69, and 70 to 79, the hazard ratios for the composite of adverse cardiovascular events associated with age were 1.09, 1.01, and 0.77, respectively (P for interaction = 0.02).
Figure 2. Subgroup Analyses According to Aspirin Use.
Aspirin Use in Propensity-Matched Patients
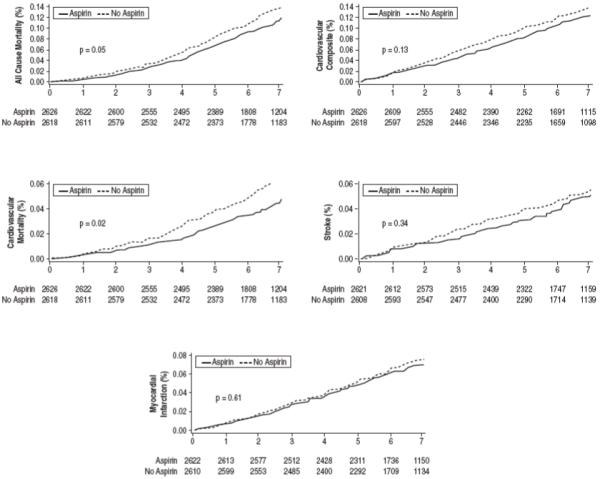
In order to better adjust for the baseline imbalances between groups, patients were matched on the basis of propensity score for aspirin use in a 1-to-1 fashion. This limited the analysis to 5,292 patients. These patients were well matched on the basis of baseline characteristics with no significant differences between users and non-users of aspirin (data not shown). Figure 3 illustrates survival curves in both groups. Overall, there were 565 deaths (10.7%). Aspirin use was associated with a significantly lower all-cause mortality (9.8% vs. 11.5%, P=0.045). Aspirin use was associated with a lower composite of cardiovascular adverse events, which was not statistically significant (10.1% vs. 11.3%, P=0.142). For the individual endpoints, aspirin therapy was associated with a significantly lower cardiovascular mortality with no significant lowering in the risk of myocardial infarction or stroke.
Figure 3. Clinical Outcomes According to Aspirin Use.
Aspirin Dose in Propensity-Matched Patients
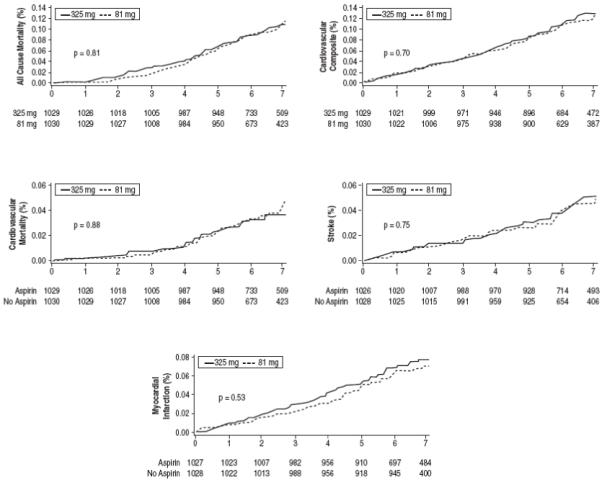
To investigate aspirin dose, a second propensity analysis was performed. We matched 1036 users of 81mg to 1036 users of 325mg. Baseline variables were similar between groups (data not shown). Figure 4 illustrates survival curves in both groups. No significant difference in mortality or cardiovascular events was noted between 81mg and 325mg patients.
Figure 4. Clinical Outcomes According to Aspirin Dose.
Discussion
This analysis produced 3 major findings. First, is the observation that only 46% of women with preexisting cardiovascular disease reported aspirin use. A regression model identified clinical, demographic and socioeconomic factors as positive and negative predictors for use of therapy. Second, aspirin use is associated with a reduction in all-cause and cardiovascular mortality. Third, no significant difference in any clinical outcome is noted between 81 and 325mg of aspirin.
Aspirin Use
In total, 46% of postmenopausal women with cardiovascular disease were on aspirin. Thus, a considerable percentage of women remain at an increased risk for adverse outcomes. Our findings are consistent with previous reports of underutilization of aspirin among patients with cardiovascular disease13-22.
Prior studies14-23 demonstrated a wide range of aspirin utilization (25-80%). Studies in community settings such as this one have demonstrated lower rates21, 22 compared to studies in the hospitalized or post-hospitalization period14, 16-20. The present study is unique because it focuses on postmenopausal women with cardiovascular disease. Because the WHI-OS population is large and diverse, and assesses the use of medication in the primary care setting, it provides information of adherence patterns across the US.
As with other studies14-16, 18-22, aspirin use was not uniform across subpopulations. Positive predictors of aspirin use included increasing age and college education. Independent negative predictors of aspirin use were African American race and Medicaid insurance. Although past studies have noted aspirin use was lower in older patients15, 22, this study found a positive association between age and aspirin utilization. Consistent with prior studies on educational status and cardiovascular disease 24, 25, higher education was associated with use of aspirin. The lower utilization of aspirin among African Americans and patients with Medicaid insurance is consistent with the observation that minorities and socio-economically disadvantaged populations receive less aggressive treatment of cardiovascular disease26, 27. However, one must be mindful that many differences in treatment may result from unmeasured issues such as differences in patient preferences, communication patterns between clinicians and patients, or clinician practice bias, all of which may influence patterns of care.
Efficacy of Aspirin
Previous analyses demonstrated that anti-platelet therapy prevents adverse events across many patients subgroups 1, 2, 5. Women and men had a 33% and 37% reduction in cardiovascular events, respectively. However, these results were found when combining all anti-platelet medications and when grouping stable and unstable cardiovascular disease. There is little data on aspirin in women with stable cardiovascular disease.
In unadjusted analyses, no significant difference was detected between aspirin use and mortality or a composite of cardiovascular events. However, this null effect appeared to be due to the fact that those receiving treatment were at substantially higher risk for recurrence; after full adjustment for confounding variables, we demonstrated that aspirin use was associated with a significantly lower risk of all-cause mortality among women with stable cardiovascular disease. Subsequently, we performed a propensity analysis to further adjust for potential confounders and selection biases12, 13, which demonstrated a similar lowering in the risk of death. No subgroup of women except those on current HRT had evidence of a risk of all-cause mortality with aspirin that differed significantly from that observed for all women, and the findings related to HRT may have been due to chance. Alternatively, by inhibiting the post menopausal hormone-induced increase in C-reactive protein28 or thrombosis risk29, aspirin may be additionally protective in this population.
This study also noted a non-significant decrease in the composite of cardiovascular adverse events. In subgroup analyses, older women were noted to have the greatest benefit with aspirin use (P for interaction = 0.02), a finding consistent with the Women’s Health Study30. Although aspirin use was associated with a significantly lower cardiovascular mortality, no association was noted for myocardial infarction or stroke. Several possible explanations exist: First, aspirin may exert its greatest effect on fatal vascular events7. Second, data from primary prevention studies suggest that aspirin may not be effective in reducing the risk of myocardial infarction in women30, 31. Third, our data may be consistent with the non significant effect of aspirin for preventing cardiovascular events in women without cardiovascular disease as noted in the Women’s Health Study30. Finally, several studies have suggested a reduced effect of aspirin among women compared to men32, 33.
Our study is unique because it focused on a population of postmenopausal women with cardiovascular disease. Previous randomized trials of aspirin that included women34, 35 were unable to find any significant effect of aspirin use on mortality. However, women were under-represented in trial enrollment and therefore the studies were underpowered. Two previous observational analyses36, 37 demonstrated reduced mortality rates with aspirin in women with known or suspected coronary disease. The current study extends these previous findings in several respects. First, we included all postmenopausal women with stable cardiovascular disease, not just women with coronary disease. Second, women enrolled in our study were older than in prior studies. This is important because many studies have noted that older women are less likely to receive standard-of-care level treatment18, 19, 38. Of note, our study demonstrated that older women had the greatest benefit with aspirin treatment. Third, our cohort was drawn from 40 clinical centers across the US, representing diverse community practice.
Aspirin Dose
The ideal dose of aspirin for the prevention of vascular events has been the subject of much debate6, 7. Studies comparing the dose effect of aspirin noted increased bleeding complications with higher doses while observing no differences in effectiveness6, 39-41. Other reports have presented conflicting results. Quinn et al. demonstrated a decreased rate of myocardial infarction with a higher dose (325mg) of aspirin42 while other studies found that a lower dose (81mg) is associated with a lower risk of cardiovascular complications7, 43. No previous study evaluated the optimal aspirin dose among women. In the current study, we observed no significant difference in any clinical outcome among women reporting 81 or 325mg of aspirin use.
Limitations
When interpreting the results of our study, several limitations need to be kept in mind. First, aspirin use was not determined by randomized assignment and therefore is subject to the inherent limitations in any observational study design. However, recent work has suggested that observational studies, if properly done, may expand the evidence base for therapy44. Moreover, we used a propensity analysis to minimize the potential for residual confounding around aspirin use12, 13. Second, there is a potential for aspirin use to be under reported by participants because of its availability at low cost without a prescription. However, this was unlikely a major problem because all women were asked to bring in prescription, non-prescription and all over the counter medications at study enrollment. Third, there is a potential that participants were on other anti-platelet or anti-thrombotic medications that we did not assess. Fourth, all women studied were postmenopausal and self-enrolled in a cohort study, which may not be generalizable to premenopausal women or women who would not enroll in a clinical study. Other limitations of our study include lack of information about aspirin allergy, duration of treatment, contraindications and side effects.
Conclusions
While the influence of unmeasured confounders cannot be ruled out, we found that aspirin therapy was associated with a significantly lower risk of all-cause mortality and cardiovascular mortality raising the hypothesis that aspirin may improve survival in postmenopausal women with stable cardiovascular disease. No significant difference in dose (81 vs. 325mg) was noted for any of the clinical outcomes measured. In addition, aspirin use was very low among women with cardiovascular disease. This under-utilization was most pronounced in African Americans and women with Medicaid insurance.
Acknowledgements
A special note of appreciation to Dr’s Adrienne Fleckman, Stephen Baum and Roslyn Schneider from the Department of Internal Medicine at Beth Israel Medical Center. We would also like to thank Dr Paul Marantz and the Clinical Research Training Program at Albert Einstein College of Medicine, Victor Kamensky for the statistical programming, and Jill Gregory for her illustrative efforts.
Funding Sources
The WHI-OS was funded by the National Heart, Lung, and Blood Institute (NHLBI), the NIH Department of Health and Human Services. Jeffrey Berger is funded by an American Heart Association Fellow to Faculty Award - 0775074N.
Footnotes
Conflict of Interest Disclosures
None
- Among postmenopausal women with stable cardiovascular disease, aspirin use was reported in 46% of the population.
- Among aspirin users, 30% were on 81mg and 70% were on 325mg.
- Under-utilization was most pronounced in African Americans and women with Medicaid insurance.
- After multivariate adjustment, aspirin use was associated with a significantly lower risk of all-cause (HR 0.86, [0.75-0.99], P=0.04) and cardiovascular related mortality (HR 0.75, [0.60-0.95], P=0.01).
- Aspirin use was not associated with a significant lowering of the composite (myocardial infarction, stroke or cardiovascular death) (HR 0.90, [0.78-1.04], P=0.14).
- Compared with 325mg, use of 81mg was not significantly different for all-cause mortality, cardiovascular events or any individual endpoint.
Clinical Summary
Among 8928 postmenopausal women with stable cardiovascular disease enrolled in the Women’s Health Initiative Observational Study, we sought to evaluate the relationship between aspirin use, dose (81 or 325mg) and clinical outcomes. In this cohort, aspirin use was low in this cohort of women with stable cardiovascular disease. Forty six percent reported taking aspirin, of whom 30% were on 81mg and 70% were on 325mg. This under-utilization was most pronounced in African Americans and women with Medicaid insurance. After multivariate adjustment, aspirin use was associated with a significantly lower risk of all-cause (HR 0.86, [0.75-0.99], P=0.04) and cardiovascular related mortality (HR 0.75, [0.60-0.95], P=0.01), but no significant lowering in a composite of myocardial infarction, stroke or cardiovascular death (HR 0.90, [0.78-1.04], P=0.14). Compared with 325mg, use of 81mg was not significantly different for all-cause mortality, cardiovascular events or any individual endpoint.
References
- 1.anonymous, Antiplatelet Trialists’ Collaboration Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists C Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr., Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr., Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, American College of C. American Heart Association Task Force on Practice G. American College of Emergency P. Society for Cardiovascular Angiography and I. Society of Thoracic S. American Association of Cardiovascular and Pulmonary R. Society for Academic Emergency M ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Jr., Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA, Aha. Acc. National Heart LaBI AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 5.Berger JS, Brown DL, Becker RC. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. American Journal of Medicine. 2008;121:43–49. doi: 10.1016/j.amjmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297:2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- 7.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 8.Kong DF, Hasselblad V, Kandzari DE, Newby LK, Califf RM. Seeking the optimal aspirin dose in acute coronary syndromes. American Journal of Cardiology. 2002;90:622–625. doi: 10.1016/s0002-9149(02)02566-3. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr., Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK, Expert Panel/Writing G. American Heart A. American Academy of Family P. American College of Obstetricians and G. American College of Cardiology F. Society of Thoracic S. American Medical Women’s A. Centers for Disease Control and P. Office of Research on Women’s H. Association of Black C. American College of P. World Heart F. National Heart LaBI. American College of Nurse P Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 10.anonymous, The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clinical Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of Epidemiology. 2003;13:S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. American Journal of Epidemiology. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 14.Becker RC, Terrin M, Ross R, Knatterud GL, Desvigne-Nickens P, Gore JM, Braunwald E, The Thrombolysis in Myocardial Infarction Investigators Comparison of clinical outcomes for women and men after acute myocardial infarction. Annals of Internal Medicine. 1994;120:638–645. doi: 10.7326/0003-4819-120-8-199404150-00003. see comment. [DOI] [PubMed] [Google Scholar]
- 15.Califf RM, DeLong ER, Ostbye T, Muhlbaier LH, Chen A, LaPointe NA, Hammill BG, McCants CB, Kramer JM. Underuse of aspirin in a referral population with documented coronary artery disease. American Journal of Cardiology. 2002;89:653–661. doi: 10.1016/s0002-9149(01)02335-9. [DOI] [PubMed] [Google Scholar]
- 16.Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Archives of Internal Medicine. 1998;158:981–988. doi: 10.1001/archinte.158.9.981. see comment. [DOI] [PubMed] [Google Scholar]
- 17.Eagle KA, Kline-Rogers E, Goodman SG, Gurfinkel EP, Avezum A, Flather MD, Granger CB, Erickson S, White K, Steg PG. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. American Journal of Medicine. 2004;117:73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Krumholz HM, Radford MJ, Ellerbeck EF, Hennen J, Meehan TP, Petrillo M, Wang Y, Jencks SF. Aspirin for secondary prevention after acute myocardial infarction in the elderly: prescribed use and outcomes. Annals of Internal Medicine. 1996;124:292–298. doi: 10.7326/0003-4819-124-3-199602010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Radford MJ, Ellerbeck EF, Hennen J, Meehan TP, Petrillo M, Wang Y, Kresowik TF, Jencks SF. Aspirin in the treatment of acute myocardial infarction in elderly Medicare beneficiaries. Patterns of use and outcomes. Circulation. 1995;92:2841–2847. doi: 10.1161/01.cir.92.10.2841. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin TJ, Soumerai SB, Willison DJ, Gurwitz JH, Borbas C, Guadagnoli E, McLaughlin B, Morris N, Cheng SC, Hauptman PJ, Antman E, Casey L, Asinger R, Gobel F. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Archives of Internal Medicine. 1996;156:799–805. [PubMed] [Google Scholar]
- 21.Shahar E, Folsom AR, Romm FJ, Bisgard KM, Metcalf PA, Crum L, McGovern PG, Hutchinson RG, Heiss G. Patterns of aspirin use in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal. 1996;131:915–922. doi: 10.1016/s0002-8703(96)90173-8. [DOI] [PubMed] [Google Scholar]
- 22.Stafford RS. Aspirin use is low among United States outpatients with coronary artery disease. Circulation. 2000;101:1097–1101. doi: 10.1161/01.cir.101.10.1097. [DOI] [PubMed] [Google Scholar]
- 23.Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. British Journal of Clinical Pharmacology. 2003;55:604–608. doi: 10.1046/j.1365-2125.2003.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. American Journal of Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manor O, Eisenbach Z, Friedlander Y, Kark JD. Educational differentials in mortality from cardiovascular disease among men and women: the Israel Longitudinal Mortality Study. Annals of Epidemiology. 2004;14:453–460. doi: 10.1016/j.annepidem.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Alter DA, Iron K, Austin PC, Naylor CD, Group SS. Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–1107. doi: 10.1001/jama.291.9.1100. [DOI] [PubMed] [Google Scholar]
- 27.Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation. 2003;108:286–291. doi: 10.1161/01.CIR.0000079164.95019.5A. [DOI] [PubMed] [Google Scholar]
- 28.Eidelman RS, Lamas GA, Hennekens CH, Ridker PM. Aspirin, postmenopausal hormones, and C-reactive protein. Archives of Internal Medicine. 2002;162:480–481. doi: 10.1001/archinte.162.4.480. [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M, Women’s Health Initiative I Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. New England Journal of Medicine. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 31.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 32.Escolar G, Bastida E, Garrido M, Rodriguez-Gomez J, Castillo R, Ordinas A. Sex-related differences in the effects of aspirin on the interaction of platelets with subendothelium. Thrombosis Research. 1986;44:837–847. doi: 10.1016/0049-3848(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 33.Spranger M, Aspey BS, Harrison MJ. Sex difference in antithrombotic effect of aspirin. Stroke. 1989;20:34–37. doi: 10.1161/01.str.20.1.34. [DOI] [PubMed] [Google Scholar]
- 34.anonymous A randomized, controlled trial of aspirin in persons recovered from myocardial infarction. JAMA. 1980;243:661–669. [PubMed] [Google Scholar]
- 35.Elwood PC, Sweetnam PM. Aspirin and secondary mortality after myocardial infarction. Lancet. 1979;2:1313–1315. doi: 10.1016/s0140-6736(79)92808-3. [DOI] [PubMed] [Google Scholar]
- 36.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. JAMA. 2001;286:1187–1194. doi: 10.1001/jama.286.10.1187. see comment. [DOI] [PubMed] [Google Scholar]
- 37.Harpaz D, Benderly M, Goldbourt U, Kishon Y, Behar S, Israeli BIP Study Group Effect of aspirin on mortality in women with symptomatic or silent myocardial ischemia. American Journal of Cardiology. 1996;78:1215–1219. doi: 10.1016/s0002-9149(96)00598-x. [DOI] [PubMed] [Google Scholar]
- 38.Higashi T, Shekelle PG, Solomon DH, Knight EL, Roth C, Chang JT, Kamberg CJ, MacLean CH, Young RT, Adams J, Reuben DB, Avorn J, Wenger NS. The quality of pharmacologic care for vulnerable older patients. Annals of Internal Medicine. 2004;140:714–720. doi: 10.7326/0003-4819-140-9-200405040-00011. [DOI] [PubMed] [Google Scholar]
- 39.anonymous, The Dutch TIA Trial Study Group A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. New England Journal of Medicine. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 40.Berger JS, Stebbins A, Granger CB, Ohman EM, Armstrong PW, Van de Werf F, White HD, Simes RJ, Harrington RA, Califf RM, Peterson ED. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation. 2008;117:192–199. doi: 10.1161/CIRCULATIONAHA.107.729558. [DOI] [PubMed] [Google Scholar]
- 41.Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, Diaz R, Commerford PJ, Valentin V, Yusuf S, Clopidogrel in Unstable angina to prevent Recurrent Events Trial I Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108:1682–1687. doi: 10.1161/01.CIR.0000091201.39590.CB. [DOI] [PubMed] [Google Scholar]
- 42.Quinn MJ, Aronow HD, Califf RM, Bhatt DL, Sapp S, Kleiman NS, Harrington RA, Kong DF, Kandzari DE, Topol EJ. Aspirin dose and six-month outcome after an acute coronary syndrome. Journal of the American College of Cardiology. 2004;43:972–978. doi: 10.1016/j.jacc.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 43.Taylor DW, Barnett HJ, Haynes RB, Ferguson GG, Sackett DL, Thorpe KE, Simard D, Silver FL, Hachinski V, Clagett GP, Barnes R, Spence JD, ASA and Carotid Endarterectomy (ACE) Trial Collaborators Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. Lancet. 1999;353:2179–2184. doi: 10.1016/s0140-6736(99)05388-x. [DOI] [PubMed] [Google Scholar]
- 44.Radford MJ, Foody JM. How do observational studies expand the evidence base for therapy? JAMA. 2001;286:1228–1230. doi: 10.1001/jama.286.10.1228. [DOI] [PubMed] [Google Scholar]




