Abstract
Restoration of cardiovascular function is the ultimate goal of stem cell-based therapy. In principle, cardiovascular stem cells can improve cardiac function via de novo cardiomyogenesis, enhanced myocardial neovascularization, and prevention of post-infarct remodeling. Stem cell transplantation to improve cardiac function has received mixed results in human clinical trials. These early data suggest that a critical reassessment of the scientific basis to stem cell-based therapy is needed in order to bring this highly promising treatment modality to mainstream clinical care.
Introduction
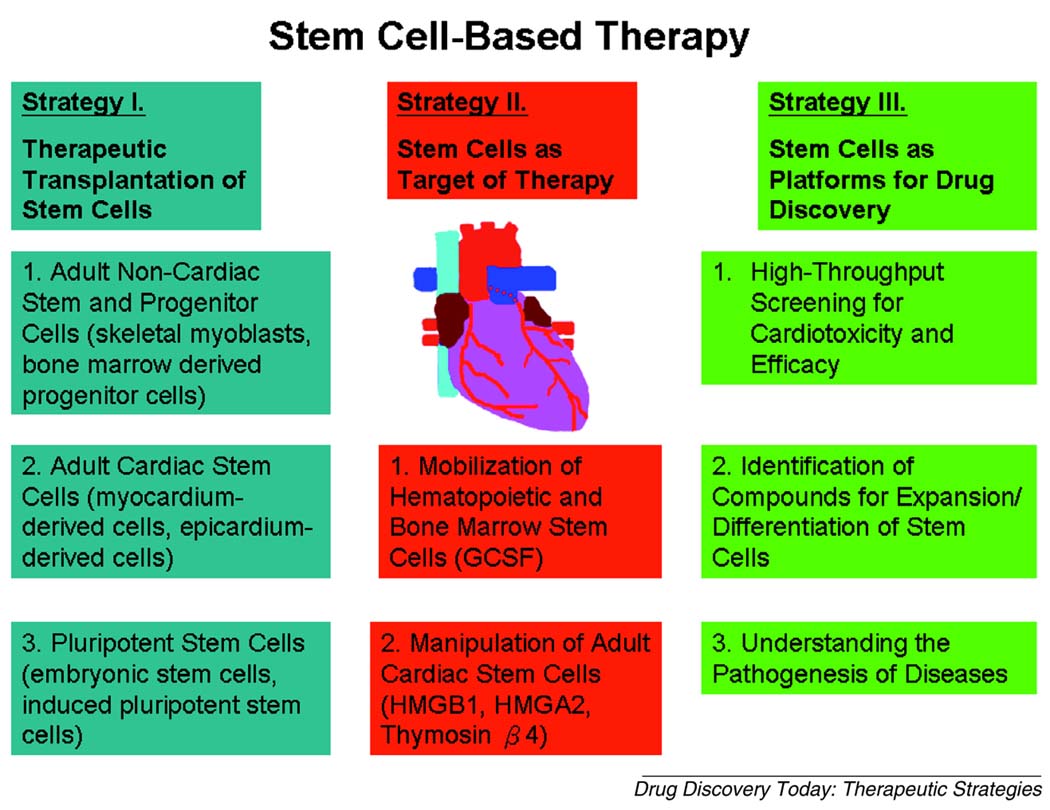
Cardiovascular disease is a major cause of morbidity and mortality worldwide. Loss of cardiomyocytes leads to decreased cardiac performance that current pharmacological therapies can only modestly improve. Although there is evidence for limited cell division in adult cardiomyocytes [1], this capacity is inadequate to compensate for the massive myocyte loss (~1.0×109 cells) following acute myocardial infarction (MI). Recently, basic and clinical scientists have quickly embraced the possibility that stem/progenitor cells can be exploited as a means for myocardial repair. Although promising results have been reported in early animal studies, clinical translation has proved more difficult. Here, we review stem cell-based approaches (Table 1) that have been used in recent cardiac regenerative studies and address methods by which cardiac stem cells may be incorporated into platforms for drug screening and toxicity assays. A summary of the strategies and cell types discussed is shown in Figure 1.
Table 1.
Potential strategies of stem cells in cardiovascular medicine
| Cell Type | Pros | Cons | Latest developments | Who is working on this strategy? | Refs |
|---|---|---|---|---|---|
| Strategy 1 - Cells as therapy | |||||
| 1. Skeletal myoblast |
Ischemia-resistant | Arrhythmogenesis due to lack of electromechanical coupling |
Connexin 43-expressing cell engraftment prevent post-infarct arrhythmia |
Fleischmann, B.K. bernd.fleischmann@uni-bonn.de |
[5,6] |
| 2. Bone marrow stem cell |
Autologous Safe |
Mixed results Paracrine effects? |
Lin−/c-Kit+ cells | Zeiher, A.M. zeiher@em.uni-frankfurt.de |
[15] |
| 3. Resdient cardiac stem cell |
Endogenous | Paracrine effect? Limited proliferating capacity? |
Myocardium-derived cells Epicardium-derived cells |
Marban, E. marban@jhmi.edu Gittenberge Groot, A.C. acgitten@lumc.nl |
[29] [33] |
| 4. Embryonic stem cell |
Highly proliferative |
Ethics Long-term benefits? Poor survival after transplantation |
Embryonic cell-derived cardiac cells |
Murry, C.E. murry@u.washington.edu Mummery, C. christin@niob.knaw.nl |
[39] |
| 5. Induced pluripotent stem cell |
Patient-specific | Viral infection | Reprogramming with different types of cells and factors |
Yamanaka, S. yamanaka@frontier.kyoto-u.ac.jp Hochedlinger, K. khochedlinger@helix.mgh.harvard.edu |
[49] |
| Strategy 2 - Cells as target of therapy | |||||
| 1. GCSF | Safe | Lack of cardiomyocyte transdifferentiation from hematopoietic stem cells |
Disappointing in clinical trials |
Schomig, A. aschoemig@dhm.mhn.de |
[54] |
| 2. HMG (B1, A2) |
No rejection | Limited clinical data |
Activation of c-Kit+ cardiac stem cells |
Capogrossi, M.C. capogrossi@idi.it Kumuro, I. komuro-tky@umin.ac.jp |
[57] [58] |
| 3. Thymosin β4 |
No rejection | Limited clinical data |
Promoting coronary neovascularization |
Riley, P.R. P.Riley@ich.ucl.ac.uk |
[36] |
| Strategy 3 - Cells as platform for drug screening | |||||
| 1. Toxicity screening |
High throughput Less expensive |
Need in vivo validation |
Expanding library of chemicals and assays |
Rubin, Lee L lee_rubin@harvard.edu |
[59] |
| 2. Efficacy screening |
High throughput Less expansive |
Need in vivo validation |
Use target compound to probe disease mechanism |
Zon, L.I. zon@enders.tch.harvard.edu |
[60] |
| 3. Disease modeling |
Patient-specific | Need in vivo validation |
Validate disease pathogenesis in patient-specific ESCs and iPSCs |
Eggan, K. eggan@mcb.harvard.edu |
[62] |
Figure 1.
A summary of the three different categories of strategies that are currently being investigated for the use of stem cells in regenerative cardiac therapy.
Strategy I: Therapeutic Transplantation of Stem Cells
Adult Non-cardiac Stem and Progenitor Cells
Skeletal myoblasts
Skeletal myoblasts were among the first cell types used for post-infarction cell therapy because they can be obtained autologously, rapidly amplified, and are relatively resistant to ischemia [2]. Initial animal studies with skeletal myoblasts showed remarkable improvements in post-infarct ventricular function following transplantation [3]. These promising early results led to several small clinical trials. While a benefit in ventricular function was observed, some patients developed episodes of ventricular tachycardia requiring the placement of defibrillators or the use of prophylactic amiodarone [4]. Genetically engineered skeletal myoblasts expressing the main gap junction protein, connexin-43, were shown to abrogate pacing-induced ventricular tachycardia in post-infarct animals [5]. Subsequent trials have documented more modest gains in ventricular function following skeletal myoblast transplantation and concluded that the functional benefits are unlikely to out-weigh the risks involved [6].
Bone marrow derived progenitor cells
The adult marrow harbors a variety of cells with stem/progenitor-like activity. Early animal studies demonstrated the capacity for these cells to reconstitute the hematopoietic system in sublethally-irradiated animals upon transplantation. More recent studies raised the intriguing possibility that these cells may transdifferentiate into other cell types [7]. Follow up studies employing murine models of myocardial infarction have supported the ability of bone marrow-derived Lin−/c-Kit+ cells to transdifferentiate into cardiomyocytes within the infarct region and enhance left ventricular function [8,9]. Based in part on these and other studies, clinical trials have been undertaken to assess the effect of intracoronary injection of bone marrow mononuclear cells (BMMC) in patients following acute myocardial infarction. Of the major studies published recently, the results have been mixed [10–15]. Follow up meta-analyses demonstrated a small but statistically significant positive effect (2–5%) on post-infarct left ventricular function at 3 months after treatment [16,17]. While the clinical significance of such effect is being debated, larger trials are on going to determine if the observed functional benefit can translate into favorable clinical end-points such as improved survival and reduced hospitalization.
Results from clinical trials using BMMC raise many questions. The first and foremost is the issue of safety. Although no major adverse events have been reported in the small studies completed thus far (100–300 patients), cases of patients developing intractable ventricular tachycardia [18], aggravation of in-stent restenosis [19], or luminar loss of the infarct-related artery [20] after cell infusion have been described. It remains to be determined in larger clinical trials whether these issues can be attributed directly to the infusion of bone marrow cells, however, microvascular obstruction by bone marrow stromal cells has been shown to complicate cell transplantation in canine model of myocardial infarction [21].
As with any experimental therapy, the likelihood of benefit must outweigh the risk for harm. Given the modest clinical improvement with bone marrow cell therapy, our tolerance for adverse events must be extremely low. The highly publicized failure of gene therapy trials serves as a sobering reminder for us that one catastrophic incident that occurs during a bone marrow stem cell study will result in enormous repercussion for all stem cell research.
Beyond safety, the next most important issue is the mechanism for the observed clinical benefit. Although the effect of BMMC’s was originally thought to be transdifferentiation into cardiomyocytes, subsequent animal studies have refuted this hypothesis [22,23]. Paracrine factors, neoangiogenesis, or cell fusion may account for the observed effect of BMMC’s [24,25]. In addition it appears that patients who suffer the greatest cardiomyocytes loss may preferentially benefit the most from cell transplantation (Stefan Janssen, abstract from Cell and Gene Therapy IV Meeting, New York, 2008). This suggests that cell infusion in general may exert a favorable change in ventricular remodeling. Since less than 5% of the transplanted cells survive and engraft, the beneficial effect of cell infusion is likely to be a consequence of the release of paracrine factor(s) by the transplanted cells. If so, an important target for future investigation will be the identification of soluble factors responsible for the clinical improvement seen in BMMC trials.
At present, the clinical niche for BMMC therapy remains unclear. An important point raised by these early studies is the need for a commonly accepted standard to move promising animal-based preclinical studies to human clinical trials in order to minimize the frequent losses of time, money, and patient safety in failed clinical studies.
Adult Cardiac Stem Cells
Myocardium-derived Cells
For decades, the prevailing dogma in cardiovascular biology has been that adult cardiomyocytes are terminally-differentiated without capacity for endogenous replication. Since then, a number of groups have reported the isolation and differentiation of cardiac stem cells from adult murine and human hearts [26,27]. Lin−/c-kit+ or Sca-1+ cells in the adult heart have been shown to differentiate into functional cardiomyocytes in vitro and engraft infarcted heart in vivo [26,27]. Similarly, the ability for cardiac side population (SP) cells (based on their ability to exclude Hoechst dye) to differentiate into cardiomyocytes has been documented [28].
The inter-relationship of these different endogenous cardiac progenitor cells and their relative cardiomyogenic potential compared to BMMC’s remains unclear. So far, only Lin−/c-kit+, Sca-1+, and cardiosphere cells have been shown to improve left ventricular function following transplantation into a post-infarct rodent heart [26,27,29]. In the case of Sca-1+ cells, the mechanism of action is due, in part, to cell fusion between transplanted cells and resident cardiomyocytes [27]. Additional studies will be needed to determine whether neo-cardiomyogenesis or paracrine effects are responsible for the reported functional improvement after adult cardiac stem cell treatment.
Epicardium-derived Cells
The embryonic epicardium undergoes epithelial to mesenchymal transformation to give rise to cardiac fibroblasts, smooth muscle cells, and endothelial cells during cardiogenesis. Very recently, epicardium-derived myocardial and vascular precursor cells have been reported in embryonic mouse and adult human hearts [30–32]. Interestingly, adult human epicardial cells appear to harbor the capacity for myocardial repair when transplanted in a mouse model of infarction [33]. This raises the possibility that an endogenous myocardial repair/regeneration response could be elicited from epicardial progenitor cells if the appropriate signals are present in the setting of myocardial infarction. A recent report from cardiac regeneration studies in zebrafish appears to support this hypothesis [34]. Paracrine factors that enhance myocardial survival and function after infarction may exert their effects via cells in the epicardium [35,36]. Hence, epicardial progenitor cells may represent an exciting and novel target for cardiac cell therapy in the years to come.
Pluripotent Stem Cells
Pluripotent stem cells such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) share the ability to self-renew indefinitely and contribute to cells of all three germ-layers in a chimeric assay. Despite their bona fide capacity to differentiate into functional beating cardiomyoytes in vitro, these cells have been difficult to translate from animal to human studies due to the risk of teratoma formation by undifferentiated cells. In addition, the ethical issues surrounding the fetal origin of human ESCs also make this cell type difficult to be widely accepted for clinical applications.
Embryonic stem cells
The use of mouse ES cell-derived cardiomyocytes (ES-CM) for cardiac regeneration has been evaluated recently [37]. By isolating highly purified cardiomocytes using a drug-selection strategy, investigators have found evidence of mouse ES cell-derived cardiomyocyte (ES-CM) engraftment into an immunodeficient rat heart without teratoma formation [38]. The engrafted cardiomyoyctes improved ejection fraction, end-systolic and diastolic dimensions, and contractile force generation compared with control cell injections [38]. This study provided the first proof-of-principle that ES-CM transplantation is technically feasible. Shortly after, two studies using human ES-CM were reported [39,40]. Laflamme et al co-administered ES-CM with a pro-survival cocktail to improve the efficiency of cell engraftment. These transplanted ES-CM prevented ventricular wall dilation and decline in fractional shortening in infarcted rat hearts four weeks after transplantation. Interestingly, although engrafted cells were present twelve weeks after treatment, functional improvement was no longer evident [40]. While these preclinical studies are encouraging, the efficacy of human ES-CM transplantation in large animal models remains to be determined [41].
Since differentiated cardiomyocytes are unlikely to expand significantly after transplantation, we and others have recently isolated mouse ES-derived cardiac progenitors cells that are multipotent and highly proliferative [42–44]. These cells, in principle, should maintain their commitment to cardiac lineages while undergoing rapid cell division. The isolation of human ESC-derived cardiac progenitor cells has also been described recently [45]. None of these cell populations, thus far, have been tested in a post MI setting pre-clinically.
Induced pluripotent stem cells
Until recently, pluripotency was thought to be a unique property reserved for germline stem cells and derivatives of cells within the inner cell mass of a developing embryo. However it has been shown that somatic cells can be reprogrammed into pluripotent stem cells by either cell fusion or somatic cell nuclear transfer (i.e. therapeutic cloning) (for review, see [46]). A remarkable breakthrough was made in 2006 when Takahashi et al discovered that by introducing only four factors (Oct 3/4, Sox2, c-Myc, and Klf4), somatic cells can be reprogrammed into pluripotent cells (iPSCs) [47]. Subsequent refinement of this technique resulted in the generation of iPSCs with as few as two factors from mouse fibroblasts [48]. Generation of human iPSCs using the same [49] or similar factors [50] has also been reported. While iPSCs appear similar to ESCs in morphology, proliferative capacity, epigenetic state, and gene expression, they have not been evaluated in detail regarding their ability to generation functional differentiated cells. Despite the remarkable demonstration of the ability of HoxB4 immortalized hematopoietic cell derived from iPSC to reverse sickle cell anemia phenotype in mice [51], we found that iPSC-derived cardiomyocytes have impaired ability to mature into well differentiated, functional cardiomyocytes compared with ESCs (Wu, S.M. unpublished data). This difference may be due to the use of retro- or lentiviral vectors that are known to cause insertional mutagenesis. Another possibility is the incompleteness of reprogramming with only four factors. Hence, efforts are currently underway to determine which factors are most likely to give rise to fully reprogrammed iPSCS and to replace the retro- and lentiviral gene delivery system with non-integrating viruses or non-viral delivery systems [52,53]. While highly promising for cell-based therapy and as platforms for drug screening, the clinical and pharmacological use of iPSCs cells will have to wait until these issues are resolved. As the technology for derivation and tissue-specific differentiation of iPSCs improves over the next few years, we envision that future therapy using iPSC-derived cardiac cells may involve either transplantation of functional 3-dimensional myocardium that has been generated via tissue engineering approaches and/or direct intramyocardial injection of iPSC-derived cardiac progenitor cells in end-stage heart failure patients awaiting cardiac transplantation at the time of their ventricular assist device implantation.
Strategy II: Stem Cells as Target of Therapy
Rather than using stem cells directly as therapy, strategies that target the mobilization, expansion, activation, or differentiation of endogenous stem cells by the introduction of drugs or biological molecules may be more promising. Manipulation of endogenous stem cells for clinical benefit is easier to implement and evaluate for their therapeutic efficacy in preclinical studies and clinical trials.
Mobilization of hematopoietic and bone marrow stem cells
GCSF infusion to mobilize bone marrow-derived stem cells has been hypothesized to enhance the repair of an infarcted heart. The rationale behind such approach was based largely on the reported capacity for bone marrow-derived stem cells to transdifferentiate into cardiomyocytes. Since GCSF is an FDA approved treatment for patients with febrile neutropenia, several clinical trials using GCSF following MI were conducted shortly after these initial publications. Thus far, the results from these studies have been disappointing. Two large clinical trials and a recent meta-analysis of smaller trials showed no benefit to post-infarct ventricular function despite a robust increase in circulating bone marrow-derived stem cells [54–56]. Given the lack of cardiomyocyte transdifferentiation following hematopoietic and bone morrow stem cell transplantation, it appears unlikely for growth factor-based mobilization of the same cells to achieve a cardiac functional benefit.
Manipulation of Adult Cardiac Stem Cells
Since bone marrow-derived stem cells may only be itinerant in the heart, strategies that directly target endogenous cardiac stem cells appear attractive for therapeutic cardiac regeneration. Recent studies have identified candidate molecules that may either stimulate the mobilization of endogenous cardiac stem cells or protect the survival of cardiomyocytes that are at risk after infarction. One of such molecules is a non-histone chromatin architectural protein, high-mobility group box 1 (HMGB1). This molecule is typically intracellular but released into the extracellular space upon cardiomyocyte injury/inflammation when cells become necrotic. HMGB1 is a chemoattractant for vascular smooth muscle cells and fibroblasts, but also activates mesangioblasts and adult c-Kit+ cardiac stem cells to proliferate and transmigrate when introduced directly into the peri-infarct region of experimentally injured heart [57]. A related molecule, HMGA2, has been reported to promote cardiomyocyte differentiation by activating cells to express Nkx2.5, the key cardiac transcription factor driving cardiogenesis during early embryonic development [58]. The exact mechanism responsible for the observed effect of HMGB1 and HMGA2 treatment is unknown, but their role as chemoattractants suggest a plasma membrane receptor may be involved to relay these extracellular signals to the nuclear transcriptional machinery.
Molecules that regulate epicardial-derived progenitor cells have also been described recently. Thymosin β4, a G-actin sequestering peptide, is required for cell motility and organogenesis via actin-cytoskeleton organization. Intraperitoneal infusion of thymosin β4 improves cardiac contractility and survival by activating Akt pathway via integrin-linked kinases [35]. These biological effects of thymosin β4 may be due, at least in part, to its ability to promote coronary neovascularization by inducing migration of adult epicardial progenitor cells [36]. Given the recent successes in identifying molecular factors that promote endogenous adult cardiac stem/progenitor cell expansion and/or migration, a systematic effort to identify small molecules to achieve similar effects may prove extremely valuable.
Strategy III: Stem Cells as Platforms for Drug Discovery
Recent advancements in both adult and pluripotent stem cell biology are poised create a new era of drug discovery (for review, see [59]). Traditionally, drug development for cardiovascular diseases has been hampered by our incomplete understanding of the disease mechanism and the high frequency of unanticipated toxicity. The use of stem cell derived cardiac cells may offer some advantages compared with conventional drug screening.
As an example, purified cell populations such as cardiomyocytes that are typically inaccessible from normal individuals could be derived from human ESCs or iPSCs for toxicity or efficacy assays in a high-throughput fashion. A significant proportion of drugs under development fail due to their unexpected cardiotoxicity profile. If this becomes apparent in early clinical, or worse, phase III trials, the results can be costly. No reliable assay has been developed thus far to predict the likelihood that a candidate drug will induce arrhythmia or depressed myocardial function in humans. The availability of human ESC or iPSC-derived cardiomyocytes combined with a reliable phenotypic readout could make ES-CM-based high-throughput screening an attractive approach for cardiotoxicity assay in the near future.
Another approach where stem cells may be used as a drug discovery platform is to screen for compounds that induce self-renewal or differentiation of stem cells for therapeutic purposes. An elegant example of this was described recently where prostaglandin E2 (PGE2) was found to promote the self-renewal of hematopoietic stem cells ex vivo by screening known bioactive compounds in the developing zebrafish [60]. Treatment of zebrafish with PGE2 in vivo expanded the pool of endogenous hematopoietic stem cells. A similar approach may also be adopted for resident or ESC-derived cardiac stem/progenitor cells to determine a drug or a combination of drugs could be used to expand these cell populations ex vivo prior to transplantation [61].
Finally, with the discovery of human iPSCs, there has been a great deal of excitement over the creation of patient-specific iPSCs for in vitro human disease modeling. By capturing the entire genetic repertoire of an individual with the disease, one may be able to recapitulate the pathogenesis of the individual’s disease “in a dish”. For example, a recent study demonstrated that mouse ES cells modified with a mutant form of superoxide dismutase could be used to study the onset of amyotrophic lateral sclerosis [62]. As more patient-specific iPSC lines become available and validated for disease modeling, the introduction of drug screening in this system may accelerate the discovery of promising compounds capable of reversing or even preventing the onset of disease.
Conclusions
Evidence is emerging that stem/progenitor cells from a variety of sources may play a role in regenerating the injured heart. However, many questions remain before these promising approaches can be translated into human trials. In general, strategies involving transplanting autologous bone marrow stem cells into the injured myocardium will require a more detailed analysis to determine the appropriate clinical setting, the cell type, the route of delivery, and the safety profile. For all stem cells, the mechanism of benefit derived from cell therapy must be rigorously assessed. If the release of paracrine factors is found to be the primary benefit from cell transplantation, efforts to determine whether these factors act to stimulate the expansion/mobilization of endogenous cardiac progenitors or to enhance myocardial survival after ischemic insult may prove extremely useful. For example, a number of animal studies have shown significant improvement in myocardial function after systemic infusion or direct injection of factors (e.g. thymosin β4, HMG proteins, etc) that regulate pathways important for cardiac stem/progenitor cell biology. Since these factors can be easily manufactured, one may see clinical studies involving the use of these agents in the near future.
Finally, stem cells may ultimately make their greatest impact on science and medicine when their biology is combined with high-throughput drug screening. At present, few reliable human cell-based in vitro models for cardiovascular disease exist. With the availability of patient-specific iPSC’s, the prospect for stem cell-based drug screening may herald a new era of pharmaceutical development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltrami AP, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50(1):7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4(8):929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 4.Siminiak T, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148(3):531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Roell W, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450(7171):819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 6.Menasche P, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 7.Brazelton TR, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 11.Meyer GP, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 12.Janssens S, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 13.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 14.Lunde K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 15.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Latif A, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski MJ, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50(18):1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 18.Villa A, et al. Ventricular arrhythmias following intracoronary bone marrow stem cell transplantation. Europace. 2007;9(12):1222–1223. doi: 10.1093/europace/eum190. [DOI] [PubMed] [Google Scholar]
- 19.Kang HJ, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 20.Mansour S, et al. Intracoronary delivery of hematopoietic bone marrow stem cells and luminal loss of the infarct-related artery in patients with recent myocardial infarction. J Am Coll Cardiol. 2006;47(8):1727–1730. doi: 10.1016/j.jacc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Vulliet PR, et al. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 22.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 23.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 24.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 25.Nygren JM, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 26.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 27.Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin CM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Smith RR, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 30.Limana F, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101(12):1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 31.Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter EM, et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116(8):917–927. doi: 10.1161/CIRCULATIONAHA.106.668178. [DOI] [PubMed] [Google Scholar]
- 34.Lepilina A, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127(3):607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 35.Bock-Marquette I, et al. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 36.Smart N, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 37.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Kolossov E, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infracted myocardium. J Exp Med. 2006;203(10):2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 40.van Laake LW, et al. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102(9):1008–1010. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 41.Menard C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366(9490):1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 42.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 43.Moretti A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Kattman SJ, et al. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 46.Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18(5):467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008 doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 51.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 52.Stadtfeld M, et al. Induced Pluripotent Stem Cells Generated Without Viral Integration. Science. 2008 doi: 10.1126/science.1162494. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okita K, et al. Generation of Mouse Induced Pluripotent Stem Cells Without Viral Vectors. Science. 2008 doi: 10.1126/science.1164270. In press. [DOI] [PubMed] [Google Scholar]
- 54.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295(9):1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 55.Engelmann MG, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48(8):1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 56.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51(15):1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 57.Limana F, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97(8):e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 58.Monzen K, et al. A crucial role of a high mobility group protein HMGA2 in cardiogenesis. Nat Cell Biol. 2008;10(5):567–574. doi: 10.1038/ncb1719. [DOI] [PubMed] [Google Scholar]
- 59.Rubin LL. Stem cells and drug discovery: the beginning of a new era? Cell. 2008;132(4):549–552. doi: 10.1016/j.cell.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 60.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadek H, et al. Cardiogenic small molecules that enhance myocardial repair by stem cells. Proc Natl Acad Sci U S A. 2008;105(16):6063–6068. doi: 10.1073/pnas.0711507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Giorgio FP, et al. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10(5):608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]