Over the past quarter-century, prescription drug manufacturers in the United States have increasingly invested in direct-to-consumer advertising (DTCA) designed to build brand recognition and to foster patients' belief in the quality of their products. Policy-makers in Canada, where limited DTCA is permitted, and in countries that do not permit DTCA are under increasing pressure to allow such marketing activities. In this article I will review recent trends in DTCA and expenditures on prescription drugs in the United States to illustrate the significant impact that brand-oriented, consumer-targeted marketing activities could have on the Canadian health care system.
There are essentially 3 types of DTCA. The first type consists of disease-awareness advertisements, which provide information about a medical condition and encourage people to talk to their physician about available treatments. Such advertisements are permitted in both Canada and the United States. The second type of DTCA consists of reminder advertisements, which may state the name of a product and may provide information about strength, dosage, form and price but may not mention the product's indication or make claims about effectiveness. With relatively few exceptions, reminder advertisements are also permitted in both countries. Product-claim advertisements are the third type of DTCA. These advertisements combine the brand name with claims about indication and effectiveness. This form of DTCA is permitted in the United States but not in Canada.
For-profit pharmaceutical manufacturers invest in DTCA to generate profits.1 Product-claim advertising is important to manufacturers because it allows them to associate claims with their particular brands. Disease-awareness advertising, in contrast, may prompt consumers to talk to their physicians about treatment but may not result in an expression of brand preferences. The distinction between these 2 types of DTCA is important because, as with other types of products, the ability to build brand loyalty is a potentially valuable means by which drug manufacturers can increase market share.2 Competing firms may capture some of the demand induced by brand-specific advertisements, but the intent of investing in the advertisements is unquestionably to generate a financial return.3
The first US product-claim DTCA was a series of print campaigns that began in 1982 and 1983.4, 5 Among the first products advertised (in outlets such as Readers Digest and the Washington Post) were Oraflex (benoxaprofen), Pneumovax (pneumococcal vaccine) and Zorvirax (acyclovir). These DTCA advertisements were permitted under US law provided that product labelling information was presented with the advertisement. (This is similar to the requirement for medical journals to publish the product monograph for prescription drugs advertised in their pages.) Shortly after the first product-claim advertisements were launched, the US Food and Drug Administration (FDA) asked the pharmaceutical industry for a voluntary moratorium while consultations on DTCA took place. Limited DTCA occurred during the moratorium, which was lifted in September 1985.5 It is estimated that, by 1987, firms were spending US$35 million annually on DTCA in the United States.4
US law permitted broadcast product-claim advertisements that contained information about major side effects and contraindications (the "major statement") and a brief summary of product labelling information, or that contained the major statement and made "adequate provision" to give consumers detailed labelling information in connection with the broadcast advertisement.6 Use of broadcast product-claim advertising was limited in the early days of DTCA. However, DTCA spending accelerated in the mid-1990s as manufacturers began to use television reminder advertisements to reinforce product-claim advertisements placed in other media.5 Spending on DTCA reached US$380 million in 1995 and more than doubled to US$790 million in 1996.
Then, in August 1997, the FDA introduced new guidelines about what constituted adequate provision for labelling information with broadcast DTCA.6 In addition to the requirement to include a major statement about risk, the advertisement would have to refer consumers to 4 sources for further information: a toll-free telephone service, concurrently running print advertisements or brochures, the consumer's health care provider and a Web site.6 Spending on DTCA grew at a rapid pace after the publication of these guidelines, with increasing emphasis on broadcast advertising. In 2005, firms spent an estimated US$4.24 billion on DTCA — 11 times the amount spent in 1995.
From 1996 to 2004, DTCA grew from 9% to 16% of total expenditures on pharmaceutical promotion, including the retail value of professional samples.7-9 Excluding professional samples, DTCA grew from 19% of expenditures on pharmaceutical promotion in 1996 to 37% in 2005.8 If promotional spending by target continues to grow at the rates seen from 1996 to 2005, consumer-targeted promotional expenditures will exceed professional-targeted expenditures in 2011.
It is important to note, however, that DTCA is not a substitute for promotions that target health professionals. For a DTCA campaign to be successful, the advertiser must also invest in complementary marketing activities targeted at professionals.5, 10, 11 Professional detailing ensures that prescribers are prepared for DTCA-induced patient visits (so that the prescriber–manufacturer relationship is not strained by such visits), and increased distribution of samples ensures that prescribers have the advertised product at hand (so that competing firms do not benefit excessively from DTCA-induced demand). It is therefore not surprising that while DTCA expenditures in the United States increased by 408% from 1996 to 2004, spending on sales representative contacts and drug samples increased 144% and 224% respectively in the same period.
As mentioned earlier, DTCA and other promotions are intended to increase sales of advertised brands. On the basis of an analysis of 49 brands that were the subject of DTCA between 1998 and 2003, IMS Management Consulting concluded that the return on investment from DTCA is "nearly unprecedented in terms of the positive sales response generated."10 DTCA can also affect sales of competing products positively or negatively. An estimate of the overall impact of DTCA on prescription drug expenditures in North America can be obtained by considering US and Canadian expenditure levels before and after the increase in US DTCA. If DTCA has had a significant impact on total prescription drug expenditures in the United States, then it is expected that the difference between expenditure levels in the United States and Canada will have changed.
Figure 1 illustrates inflation-adjusted per capita expenditures on prescription drugs in the United States and Canada from 1975 to 2005. This figure, which takes general inflation and population growth into consideration, shows that the past decade was one of particularly rapid growth in prescription drug expenditures in both countries. Inflation-adjusted prescription drug expenditures per capita doubled in Canada from 1995 to 2005 and increased even more rapidly in the United States.
Figure 1.
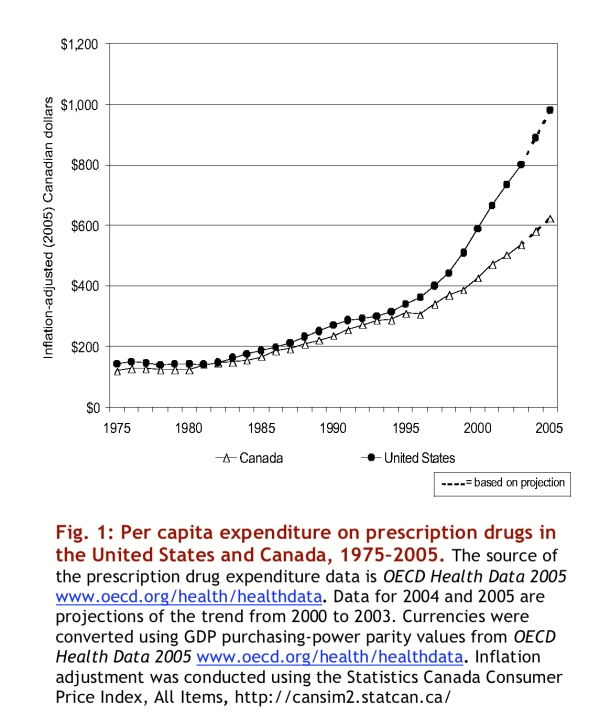
Per capita expenditure on prescription drugs in the United States and Canada, 1975-2005
The difference in per capita expenditures on prescription drugs in the United States and Canada began to increase at almost exactly the same time that DTCA began to flourish in the United States (Figure 2). From 1975 to 1994, the difference in inflation-adjusted expenditures on prescription drugs between the United States and Canada was never more than $36 per capita (measured in year 2005 Canadian dollars). Over the same period, spending on DTCA in the United States was never more than $2 per capita (year 2005 Canadian dollars). Inflation-adjusted per capita spending on DTCA in the United States grew from just over $2 in 1995 to just under $18 in 2005 (year 2005 Canadian dollars). Over the same period, the difference in inflation-adjusted per capita expenditures on prescription drugs between the 2 countries grew from approximately $31 to approximately $356 (year 2005 Canadian dollars).
Figure 2.
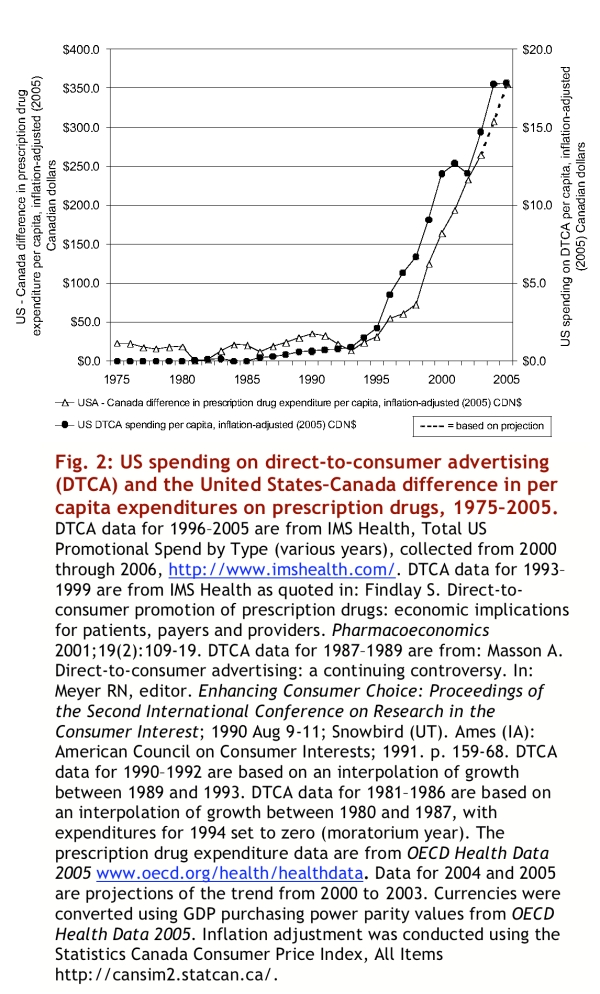
US spending on direct-to-consumer advertising (DCTA) and the United States-Canada difference in per capita expenditures on prescription drugs, 1975-2005
Some have suggested that the recent growth in pharmaceutical expenditures in the United States has been driven in part by the fact that the proportion of pharmaceutical purchases paid for out of pocket is falling.12 However, out-of-pocket spending has represented a steadily declining share of US expenditures on prescription drugs since 1960, with the most rapid decline occurring between 1989 and 1996, before the major changes illustrated in Figure 2(data provided in the appendices).
That the difference in prescription drug expenditures per capita between Canada and the United States would start to rise in the mid-1990s in apparent lockstep with the new phenomenon of spending on DTCA in the United States, after 20 years of relative stasis, would be a rather remarkable coincidence. There have been no other policy, demographic or economic changes that could explain the direction, magnitude and timing of the recent divergence between the 2 countries' per capita expenditures on prescription drugs.
The recent divergence in per capita expenditures between Canada and the United States gives an indication of the potential impact of increased DTCA in Canada and possibly of the introduction of DTCA in countries where it is currently not permitted. If, over the last decade, Canada had followed a path of DTCA similar to that taken by the United States and if per capita expenditures on prescription drugs had risen as much in Canada as they have in the United States, Canadian expenditures on prescription drugs would be approximately $10 billion higher per year than they currently are. This amount would be sufficient to pay annual salaries of $250,000 to 40,000 physicians.
The DTCA-associated increased spending on prescription drugs may be of value if it is on treatments that are appropriate and cost-effective. However, after reviewing studies published to 2004, Gilbody and colleagues concluded that, while DTCA is associated with increased requests for and use of advertised products, no health benefits have been established.13 A more recent study involving standardized patients randomly assigned to make no request, brand-specific requests or general requests for treatment of adjustment disorder or major depression found that general and brand-specific requests resulted in better quality of care (defined as receiving some form of treatment for their condition).14 Not surprisingly, patients who request a specific brand are more likely to receive that specific brand rather than available alternatives.14
It is certainly desirable to make better use of prescription drugs in Canada, although doing so may result in increased pharmaceutical expenditures. However, to promote safe, effective and efficient medicine use, policy-makers would be well advised to maintain and enhance restrictions on product-claim (brand-specific) DTCA, because such advertisements are designed to instill product preferences in people who often do not have the information, training or incentive to compare the risks, benefits and costs of the available treatment options.
If, owing to a lack of economic incentive for nonbranded advertising, manufacturers fail to promote awareness of conditions that are critical to the health of the population, the appropriate public policy response would be to invest in publicly sponsored campaigns to promote better use of prescription drugs, not to relax restrictions on product-claim DTCA and thereby give manufacturers the opportunity to instil brand preferences in patients. The potential impact of product-claim DTCA on the Canadian health system is simply too large to accept such advertising before other ways of better use of prescription drugs have been thoroughly explored.
Acknowledgments
I thank Gillian Hanley, Devon Greyson and Morris Barer for comments on an earlier draft of this article.
Biography
Steven G. Morgan, PhD, is Assistant Professor, Health Care and Epidemiology, and Research Lead, Program in Pharmaceutical Policy, Department of Health Care and Epidemiology, Centre for Health Services and Policy Research, University of British Columbia, Vancouver, BC
Appendices
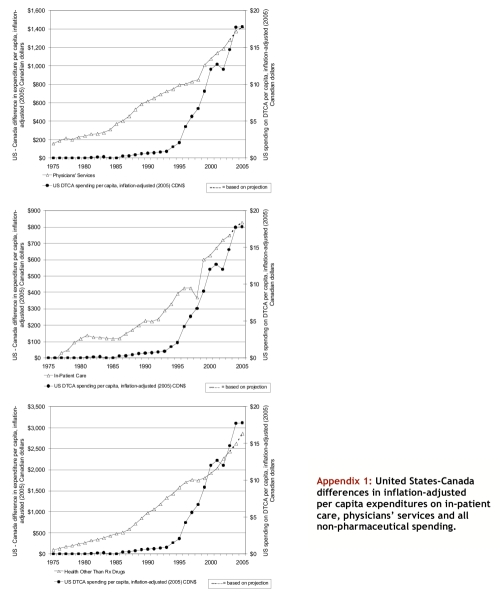
Appendix 1.
United States-Canada differences in inflation-adjusted per capita expenditures on in-patient care, physicians' services and all non-pharmaceutical spending
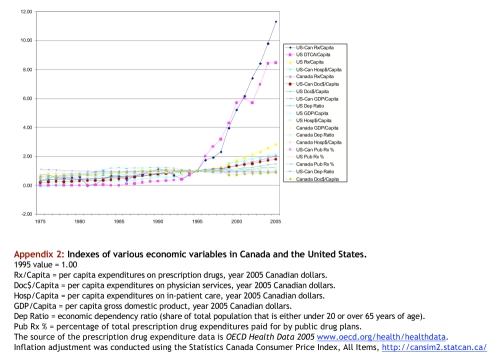
Appendix 2.
Indexes of various economic variables in Canada and the United States
Appendix 3.
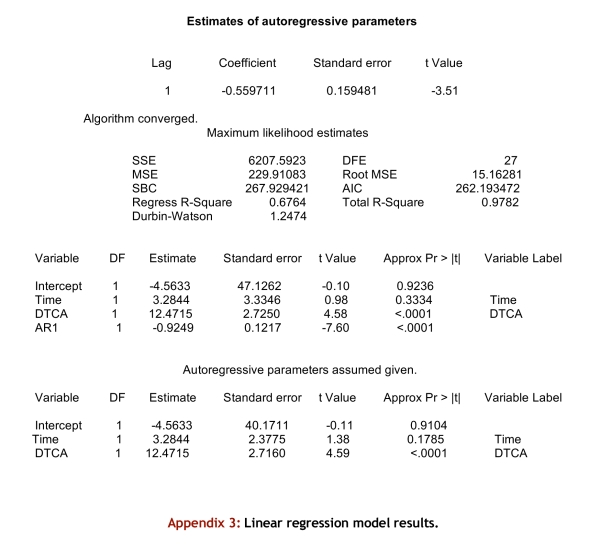
Linear regression model results
Appendix 4.
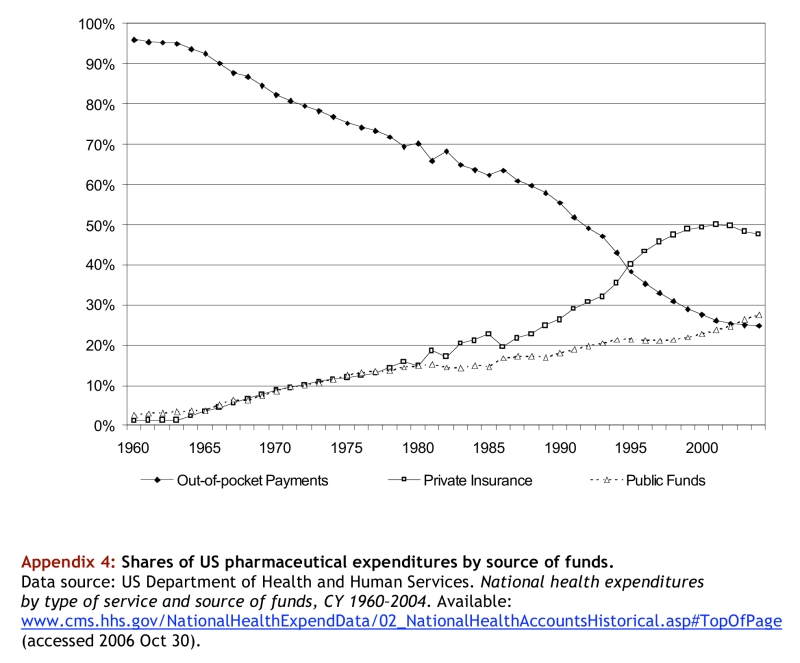
Shares of US pharmaceutical expenditures by source of funds
Appendix 5.
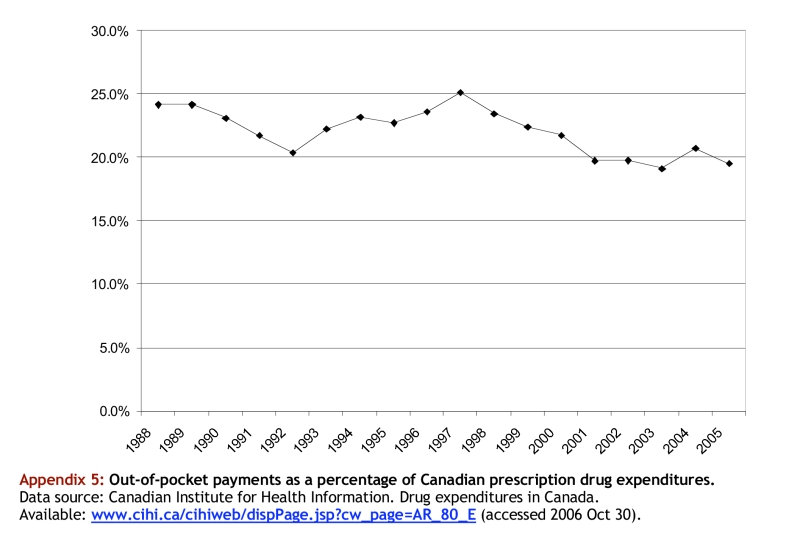
Out-of-pocket payments as a percentage of Canadian prescription drug expenditures
Footnotes
Competing interests: The author is supported, in part, by funding from the Canadian Institutes of Health Research (CIHR) and the Michael Smith Foundation for Health Research.
References
- 1.Morgan Steven, Mintzes Barbara, Barer Morris. The economics of direct-to-consumer advertising of prescription-only drugs: prescribed to improve consumer welfare? J Health Serv Res Policy. 2003 Oct;8(4):237–244. doi: 10.1258/135581903322403317. [DOI] [PubMed] [Google Scholar]
- 2.Tirole J. The theory of industrial organization. Cambridge, MA: MIT Press; 1988. [Google Scholar]
- 3.Kaldor N. The economic aspects of advertising. Rev Econ Stud. 1950;18(1):1–27. [Google Scholar]
- 4.Gilbert D. London, UK: PJB Publications Ltd.; 1998. "Direct to Consumer Advertising of Prescription Medicines" Script Reports, Industry Alert. http://www.pjbpubs.com/cms.asp?pageid=287&reportid=136. [Google Scholar]
- 5.Mertens G. Direct to consumer advertising: global drug promotion. London, UK: FT Healthcare; 1998. [Google Scholar]
- 6.US Food and Drug Administration . Rockville, MD: US Food and Drug Administration; 1999. [accessed 2006 Oct 24]. Guidance for industry: consumer-directed broadcast advertisements. http://www.fda.gov/cder/guidance/1804fnl.htm. [Google Scholar]
- 7.IMS Health Inc . Total US promotional spend by type, 2003. Fairfield, CT: IMS Health Inc.; 2004. [accessed 2006 Oct 24]. http://www.imshealth.com/ims/portal/front/articleC/0,2777,6599_44304752_44889690,00.html. [Google Scholar]
- 8.IMS Health Inc . Total US promotional spend by type, 2005. Fairfield, CT: IMS Health Inc.; 2006. [accessed 2006 Oct 24]. http://www.imshealth.com/ims/portal/front/articleC/0,2777,6599_78084568_78152318,00.html. [Google Scholar]
- 9.IMS Health Inc . Total US value of free product samples, 2004. Fairfield, CT: IMS Health Inc.; 2005. [accessed 2006 Oct 24]. http://www.imshealth.com/ims/portal/front/articleC/0,2777,6599_78152267_78152297,00.html. [Google Scholar]
- 10.Gascoigne D. DTC at the crossroads: a "direct" hit ... or miss? Plymouth Meeting, PA: IMS Management Consulting; 2004. [accessed 2006 Oct 24]. http://www.imshealth.com/vgn/images/portal/cit_40000873/58/47/75805929DTC.pdf. [Google Scholar]
- 11.Gascoigne D. The 'science' of promotional planning: evidence-based analyses optimize promotional returns. Fairfield, CT: IMS Health Inc.; 2006. [accessed 2006 Oct 24]. http://us.imshealth.com/content/SciencePromoPlgarticle.pdf. [Google Scholar]
- 12.Henry J. Kaiser Family Foundation . Washington, DC: The Foundation; 2006. [accessed 2006 Oct 24]. Prescription drug trends factsheet -- June 2006 update. http://www.kff.org/rxdrugs/3057.cfm. [Google Scholar]
- 13.Gilbody S, Wilson P, Watt I. Benefits and harms of direct to consumer advertising: a systematic review. Qual Saf Health Care. 2005 Aug;14(4):246–250. doi: 10.1136/qshc.2004.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kravitz Richard L, Epstein Ronald M, Feldman Mitchell D, Franz Carol E, Azari Rahman, Wilkes Michael S, Hinton Ladson, Franks Peter. Influence of patients' requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. JAMA. 2005 Apr 27;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]