Abstract
Background
Very few data are available on the determinants of PSA testing in Canada, and it is a matter of debate whether prostate-specific antigen (PSA) screening in asymptomatic men age 50 and older with no risk factors for prostate cancer is useful. If PSA screening is introduced into the periodic health examination, it will be important to know what factors influence its use.
Objectives
The purpose of this study is to determine the factors associated with PSA testing among asymptomatic men age 50 and older participating in the Tomorrow Project in Alberta.
Methods
The Tomorrow Project is a population-based cohort study with over 11,000 participants accrued in Alberta since February 2003. Information was collected on medical history, sociodemographic factors, health status and lifestyle characteristics. This analysis includes 2136 men 50 years of age and older. The independent association between various factors and recent PSA screening is estimated using logistic regression.
Results
Approximately 50% of of the study group had received one or more PSA tests in their lifetime. Of these, 58% were asymptomatic for prostate disease at the time of their most recent PSA test. Variables independently associated with recent PSA screening for prostate cancer in this population include older age (≥ 65 versus < 55 years: adjusted odds ratio [OR] 2.60; 95% confidence interval [CI] 1.77–3.83), higher income (≥ $80,000 versus < $20,000, OR 1.97; 95% CI 1.09–3.55), region of health care delivery, perception of health status (good versus excellent health status; OR 0.65, CI 0.43–0.96], increased number of chronic health conditions (OR 1.73, 95% CI 1.10–2.71), and history of colorectal cancer screening with fecal occult blood test (OR 2.21; 95% CI 1.73–2.83).
Conclusions
An increasing proportion of men in Alberta are receiving a PSA test. A number of significant predictors of having a PSA test were identified, suggesting that factors other than having a clinical indication for prostate disease can influence decisions about PSA screening.
Introduction
Evidence for the benefit of early prostate cancer screening using prostate-specific antigen (PSA) and digital rectal examination (DRE) is inconclusive. Although screening can result in earlier detection of prostate cancer, there is insufficient high-quality evidence to suggest a reduction in mortality (the most reliable measure of benefit from a screening program).1,2-6 As such, prostate cancer screening guidelines remain less definitive than other cancer screening guidelines. The Canadian Task Force on the Periodic Health Exam has concluded that there is insufficient evidence to recommend PSA screening in asymptomatic men over the age of 50.7 However, both the American Cancer Society and the American Urology Association recommend PSA and DRE testing in men over 50 years or at high risk.8, 9 Similarly, the Canadian Cancer Society recommends that all men over the age of 50 years discuss with their doctor the potential benefits and risks of early detection of prostate cancer using a PSA test and DRE so they can make informed decisions about the use of the tests.1 Provincial differences in the population prevalence of reported PSA tests10 suggest that physician practices and public awareness with regard to the use of the test may vary widely across Canada.11
Some studies have examined factors associated with prostate cancer screening practices in defined populations such as medical clinics,12, 13 but very few that have done so in general population samples.14, 15 The presence of symptoms is strongly related to prostate cancer testing;16-18 however, because screening by definition applies only to asymptomatic individuals, it can not be said to predict prostate cancer screening. Other frequently cited factors related to PSA testing include increasing age,14, 19, 20 being married,15, 21 a family history of prostate cancer, having a physical illness,14 having a regular physician14, 19 and having medical insurance.15, 22 However, none of these studies have identified predictors for PSA testing for asymptomatic and symptomatic men separately. It is important for health care decision-makers to understand the dynamics of the increasing widespread “acceptance” of a test that is not currently recommended for asymptomatic men. Furthermore, if guidelines do change to recommend PSA screening for the general population it may be of interest to know who is not being screened in order to better target and inform men about prostate cancer and PSA testing.
Therefore, the purpose of this study was to identify factors associated with prostate cancer screening among participants in the Tomorrow cohort study in Alberta who are aged 50 or over and have no clinical indication for PSA testing. Our hypothesis was that men with a recent PSA test and with no clinical indication of prostate disease are different with respect to sociodemographic and health-related factors from men of a similar age who have never had a PSA test.
Methods
The Tomorrow Project is a research initiative of the Alberta Cancer Board, Division of Population Health and Information. This population-based cohort study began in October 2000, and recruited participants aged 35–69 years prior to Feb. 20, 2003, from households in over 583 cities, towns, villages and rural areas throughout the province of Alberta. A two-stage sampling design was used to identify eligible individuals without a history of cancer. The first stage used a random digit dial procedure to select households in the 17 regional health authorities extant in Alberta in 2000, and the second stage selected one eligible adult within each household.23 Of the 77,327 randomly selected households, one individual from each of the 47,169 households engaged in a screening interview. Approximately 50% of those interviewed were ineligible to participate, primarily because they were outside the target age range. Of the 22,652 men and women who were eligible for the study, 11,865 (52.4%) were enrolled, representing 84% of Alberta communities. Limited information on sociodemographic factors was available for interviewees who were eligible but did not join the study. The Tomorrow cohort (n = 11,865) was comparable with the Canadian Community Health Survey (CCHS) sample in Alberta with respect to marital status, annual household income below and above $50,000, and completion of postsecondary education.23 Study enrolment included completion of a detailed consent form and a set of self-administered questionnaires asking about: (1) baseline health and lifestyle factors; (2) physical activity; (3) habitual diet. Included in this analysis are 2136 consenting male participants in the Tomorrow Project who were 50 years of age or older and had completed all three questionnaires.
For the purposes of this study, only baseline data collected from the health and lifestyle questionnaire were used. This questionnaire is a composite of items used in other large studies relating to personal health and reproductive history, psychosocial factors, anthrometric measures, use of cancer screening services, smoking behaviour, sun exposure and sociodemographic characteristics.23 The items concerning the PSA test originated from the Canadian Community Health Survey (CCHS 2000/01). Men were asked the following questions: “Have you ever had a PSA test for prostate cancer?”; “When was the last time you had a PSA test?”; “Why did you have the last PSA test?”. For this last question, there were 6 possible responses: (a) Family history of prostate cancer; (b) Part of regular check-up/routine screening; (c) Age; (d) Signs or symptoms of possible problem; (e) Follow-up of previous problem; (f) Other (and specify).
Statistical analyses
The main dependent variable of interest in this analysis was PSA screening. Therefore, those men who reported they had their last PSA test because of their age or because it was part of their regular check-up were classified as asymptomatic and were compared with men who had never had a PSA examination (reference group), to better identify predictors of recent PSA screening.
Potential factors associated with recent PSA screening included: sociodemographic characteristics such as age, education, employment status, income, marital status, ethnicity, and health region; health characteristics such as self-reported health status, health conditions, and personal or family history of cancer (not including prostate cancer); cancer screening practices, including a history of endoscopic examinations (colonoscopy or sigmoidoscopy) for colorectal cancer and precancer detection; and male reproductive health and other lifestyle factors, such as history of vasectomy, smoking status and body mass index. The 17 different geographic health regions were classified into the following 5 health care delivery regions: Calgary, Capital, Central, South and North.
The association between various factors and recent PSA screening was estimated using unconditional logistic regression.24 Independent variables for which at least one of their categories yielded a p value of 0.20 or less for the Wald test in the univariate analysis were evaluated in multivariable models. The independent effect of these potential predictors on PSA screening was assessed separately using a backward stepwise regression technique (removed from model if p value ≥ 0.10). All analyses were conducted using SPSS version 12.0.
Results
Of the 2136 men in the Tomorrow Project who were 50 years of age or older, 171 (8%) did not know whether they had ever had a PSA test and were excluded from the analyses. Of the remaining 1965, 949 (48%) had never had a PSA test, while 1016 (52 %) had received one or more PSA tests in their lifetime. Of those 1016, there were 426 (42%) who had at least one PSA test because of specific indications that may be related to prostate cancer risk (i.e., possible urological symptoms, enlarged prostate or surgery of the prostate, family history of prostate cancer, or follow-up of a previous problem)(see Table 1). The remaining 590 (58%) responded that they had their most recent PSA test because of their age or because it was part of their regular check-up with their physician.
Table 1.
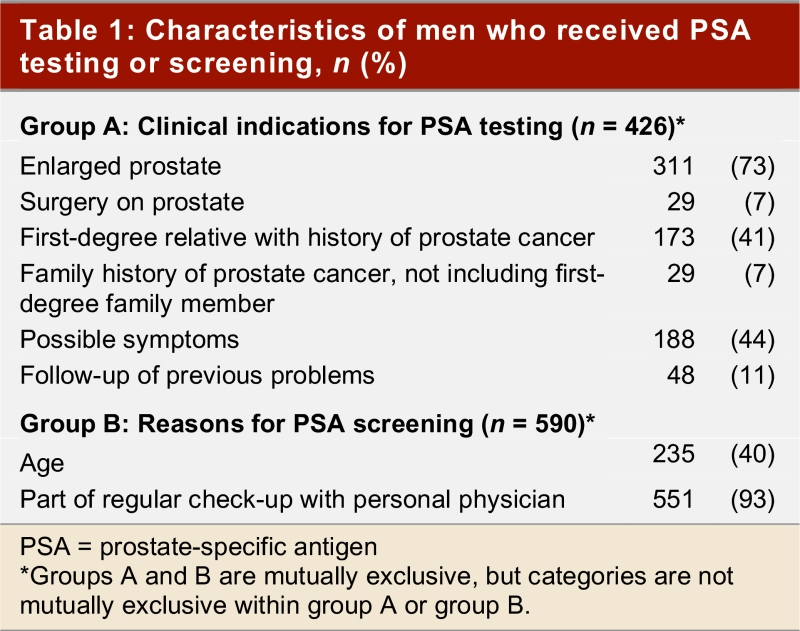
Characteristics of men who received PSA testing or screening, n (%)
Table 2, Table 3, and Table 4 show the frequency distribution of sociodemographic and health-related characteristics stratified by men with different PSA testing status. There were no striking differences in the distribution of these factors between the PSA screening and PSA testing groups. However, men who were tested for PSA with a clinical indication tended to be older than men in the PSA screening group. Furthermore, the distribution of men attending the 5 main health care delivery regions differed between the two groups of men who had received PSA tests. The vast majority of men in the cohort received at least one DRE in their lifetime, and the time of the last DRE was highly correlated with the time of the last PSA test (Spearman r = 0.619, p < 0.001; data not shown). The most striking difference in the non-PSA tested group was the greater number of men who had never had a digital rectal examination compared to the other groups of men.
Table 2.
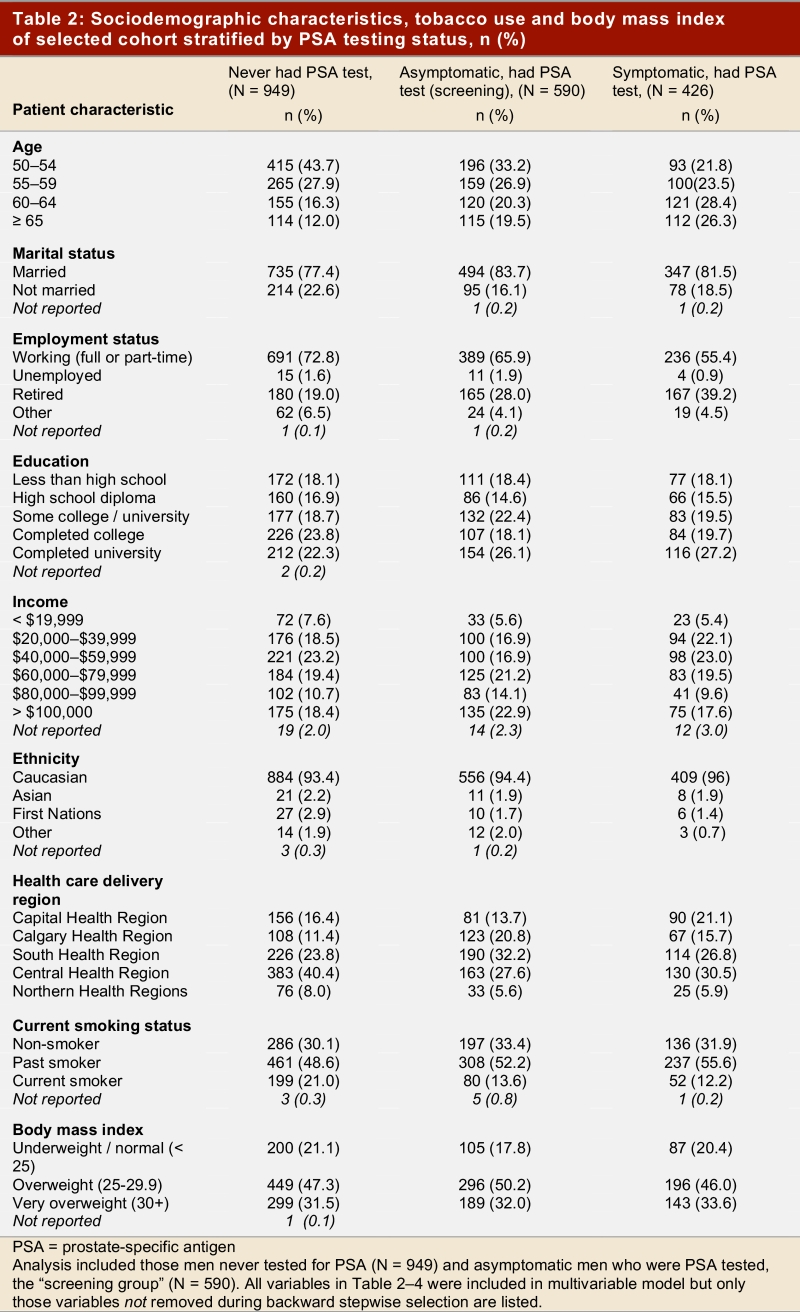
Sociodemographic characteristics, tobacco use and body mass index of selected cohort stratified by PSA testing status, n (%)
Table 3.
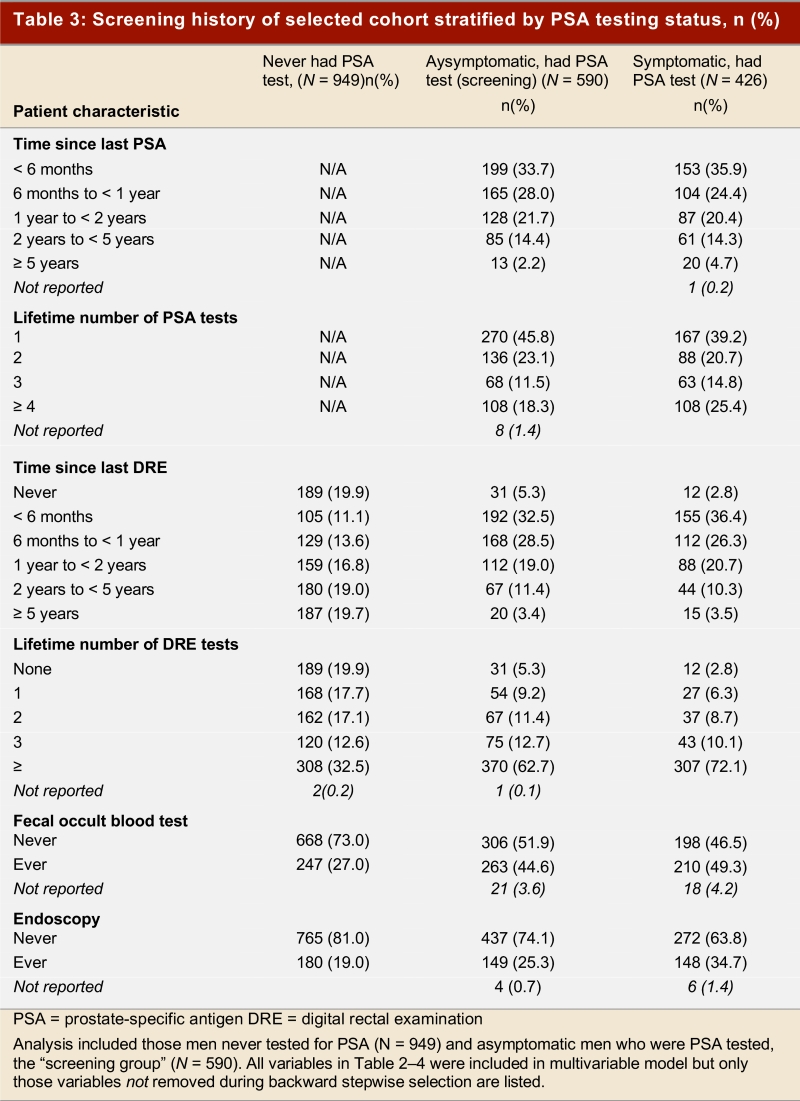
Screening history of selected cohort stratified by PSA testing status, n (%)
Table 4.
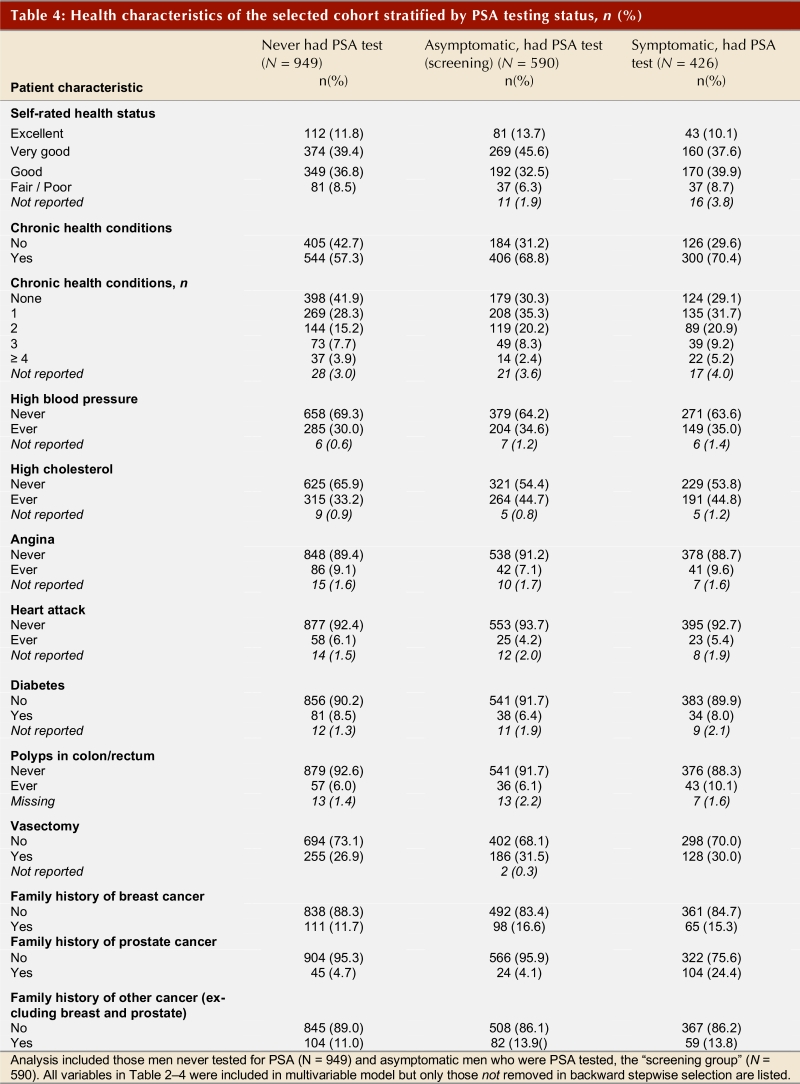
Health characteristics of the selected cohort stratified by PSA testing status, n (%)
Table 5 (part 1; part 2) shows the crude and adjusted odds ratios for sociodemographic and health-related factors in association with PSA screening among men without recent clinical indications for a test. There is a clear trend with age; successively older men (65 or older) are more likely to have had a PSA test compared with men younger than 55 years of age (adjusted odds ratio [OR] 2.14; 95% CI 1.57–2.97). Higher income and place of health care delivery are also significant predictors of PSA screening. Men served by the South and Calgary Health Region are more than twice as likely to have had a recent PSA test compared with men served by the Capital Health Region (OR 2.20, CI 1.50–3.2 and OR 2.81, CI 1.83–4.32, respectively) despite an absence of symptoms. Other factors significantly associated with PSA screening included the presence of at least one chronic health condition and having had a fecal occult blood test for colorectal cancer screening (OR 2.21, 95% CI 1.73–2.83).
Table 5 - Part 1.
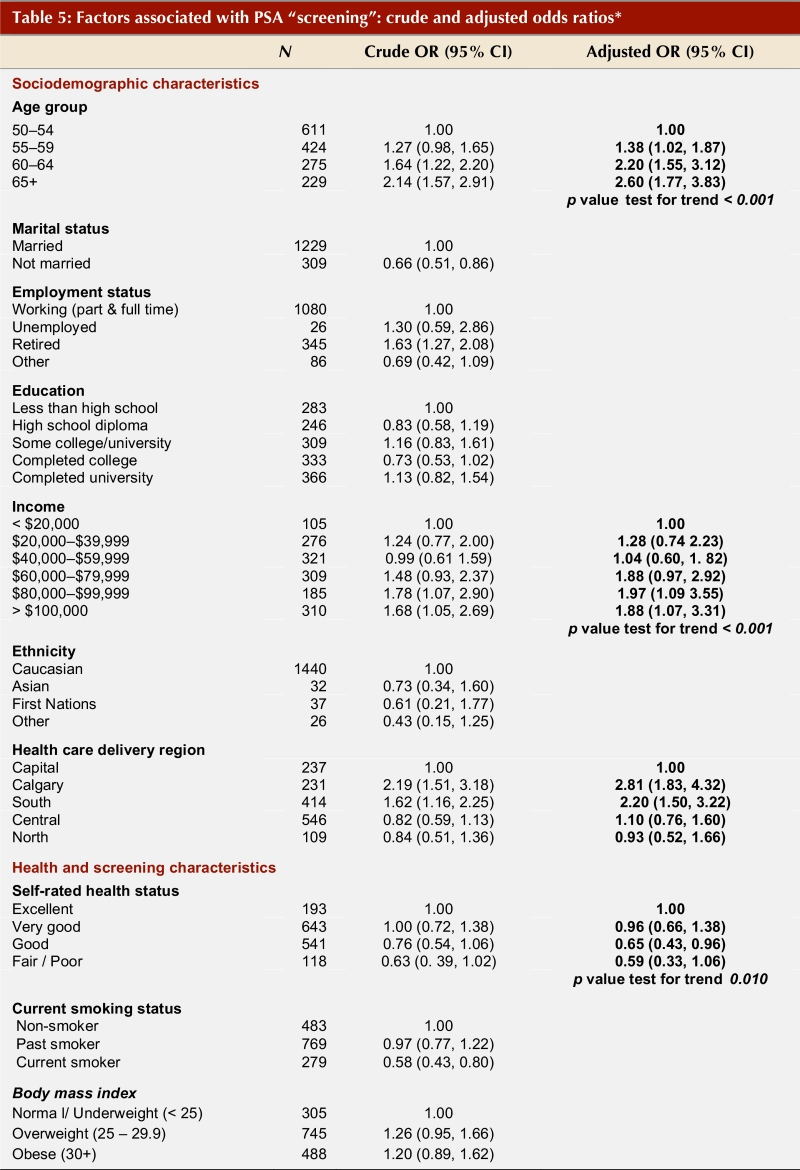
Factors associated with PSA "screening": crude and adjusted odds ratios*
Table 5 - Part 2.
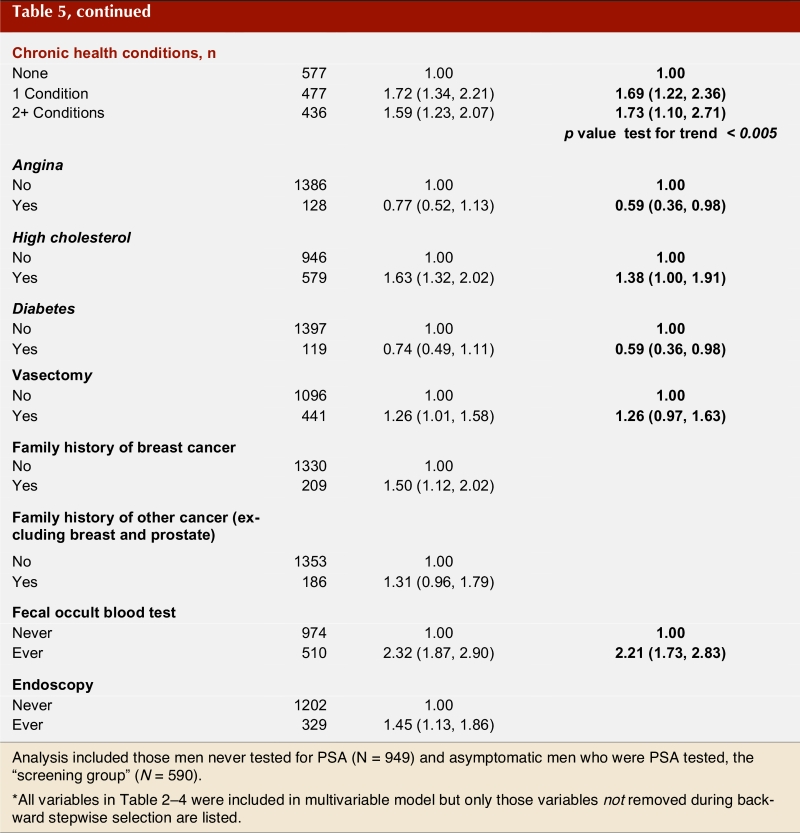
Factors associated with PSA "screening": crude and adjusted odds ratios*
Discussion
About half of all men 50 years and older in the Tomorrow Project had received one or more PSA tests in their lifetime. This prevalence is consistent with data collected in 2001 from national databases, which indicated that the average proportion of men who had received one or more PSA tests in their lifetime was 43% among those 40 years or age or older10 and 47.5% among those over the age of 50.19 This was a substantial increase from the 9% who, in 1995, reported having had had a PSA test in their lifetime, in a Canada-wide cross-sectional telephone survey of 662 men over 40 years of age.25 The proportion of men receiving PSA tests has also been increasing systematically over the last decade in the United States, and recent PSA test rates are reported to be greater than 40% for men over 40 attending regular health care facilities.15, 22
Clinical indications for ordering a PSA test can include lower urinary tract symptoms (symptoms of prostatism), history of benign prostate hyperplasia, a recent abnormal DRE, and a history of first-degree relatives diagnosed with prostate cancer. Some surveys conducted within clinical settings have observed that PSA testing rates are much higher among men with benign prostatic hyperplasia or moderate or severe urinary tract symptoms compared with asymptomatic men.17 McGregor and colleagues16 also observed that the majority of men in a 1996 population-based Alberta survey who had received a PSA test had a clinical indication for the test. This group represented those men at a possibly higher risk for prostate cancer and who were eligible for a PSA test under the Alberta provincial health insurance reimbursement plan. Conversely, the majority of the PSA tests in the Alberta Tomorrow cohort were in asymptomatic men (58%), suggesting a possible shift toward opportunistic screening in clinical practice.
In our study population we observed that the PSA screening rates differed significantly by household income. Higher income was not identified as a predictor of PSA testing in a number of cross-sectional studies conducted in the United States,15, 26 but was associated with PSA testing in a cohort of American veterans who were interviewed on two separate occasions in 1992 and 1995.14 Interestingly, all these studies observed a positive association between access to health insurance and PSA testing.14, 15, 26 Although access to health care insurance is an important determinant of screening in the United States,18, 22, 27 this is not the case in Canada, given our universal health care coverage. However, measuring access to care by socioeconomic status can be important to help determine if provincial health care systems are delivering access to services in an equitable manner. At least two Canadian surveys have observed that high-income earners are more likely to have a family physician,28, 29 and regular visits with a family doctor appears to influence PSA testing rates.19, 30 Interestingly, the men in this cohort study who had ever undergone screening for colon cancer with a fecal occult blood test were significantly more likely to have a PSA test despite an absence of symptoms, and the frequency fecal occult blood testing also appeared to increase with increasing income (data not shown). Therefore, it is possible that, in our cohort, a higher income is related to increased health care access or an increased awareness of the availability of PSA testing and other cancer screening options by patient or health care provider.
An increase in physician awareness of PSA testing may also explain part of the increased prevalence of testing. Among men with family physicians, the use of PSA testing varies depending on the insistence of the patient and on the physician’s views on the wisdom of using PSA tests for early detection of prostate cancer.11, 12, 31 According to a study in Ontario, family physicians were twice as likely as urologists to use PSA tests for screening purposes.30 The higher rates of PSA screening observed in the cohort of men from health care regions in Calgary and the South of Alberta may also reflect differences in practice patterns among family physicians and/or urologists in those regions.
As has been seen in a few other studies,15, 32 we observed that men who perceived their health status to be poor were less likely to have a PSA test compared with men who classified themselves to be in excellent health. This observation suggests that good health may be a marker for preventive health practices. Paradoxically, men who were diagnosed with one or more chronic health conditions, notably high cholesterol, were significantly more likely to have a PSA test compared to men without any chronic health problems. Eisen and colleagues14 also observed that physical health problems significantly influenced the likelihood of PSA testing, although no specific conditions were identified, suggesting that there may be selective screening on the part of health care practitioners.
Our study has several limitations. Our objective was to study predictors of prostate cancer screening in asymptomatic men in the Tomorrow Project cohort. Ideally, we would have compared asymptomatic men who had a history of PSA screening (or received a PSA test) with a reference group of asymptomatic men who have never had a PSA test. However, it was not possible to ascertain whether the population of untested men in the cohort was also asymptomatic, since questions about clinical indications for their last PSA test were only asked of men who reported ever having a PSA test. As such, the reference group is likely to contain a small proportion of men who were never tested but may have had clinical indications for a PSA test. If this group of symptomatic but untested men is very different from the asymptomatic and untested group, combining them into one reference group could lead to biased measures of effect. Secondly, some men in the screened group may have had a previous PSA test because of a clinical indication. In this case, the identified predictors of PSA screening (in an asymptomatic population) may also be determinants of PSA testing (among men with a past history of clinical indications).
In summary, an increasing proportion of men in Alberta are being tested for prostate cancer despite the absence of clinical symptoms or of a familial history of prostate cancer. A number of significant predictors of having a PSA test were identified in this study, including higher income, good health status, and variation in the regional health care delivery facilities, suggesting that factors other than having a clinical indication for prostate disease can influence PSA testing rates.
Whether this increase in PSA testing among asymptomatic men translates into a net benefit with longer survival and reduced mortality rates remains to be answered by ongoing long-term randomized clinical trials.33, 34 However, if PSA screening is found to be beneficial, public health strategies will need to identify how best to educate and screen certain groups, including men of low socioeconomic status and perceived poor health status.
Additional research to identify the main factors that motivate physicians in Canada to recommend screening is warranted. Future research may also want to address the apparent variation in regional health care delivery services and study factors that may explain differences in the administration of cancer control programs.
Acknowledgments
We thank all the participants in the Tomorrow Project who have been so generous with their time and commitment to the project.
Biographies
Harriet Richardson is an Assistant Professor in the Department of Community Health & Epidemiology, Queen’s University, and Project Coordinator for the National Cancer Institute of Canada Clinical Trials Group, Kingston, Ont.
Kristan J. Aronson is a Professor in the Department of Community Health & Epidemiology, Queen’s University, Kingston, Ont.
Alison James is Research Manager in the Division of Cardiology, QEII Health Sciences Centre, Halifax, NS.
Elizabeth S. McGregor is a Research Scientist with Population Health & Information, Alberta Cancer Board, Calgary, Alta.
Heather Bryant is Vice President and Chief Information Officer, Alberta Cancer Board, and Director, Population Health & Information, Alberta Cancer Board, Calgary, Alta.
Footnotes
Funding Source: Alberta Cancer Board
Competing interests: None declared.
References
- 1.Canadian Cancer Society Early detection and screening for prostate cancer. 2006. [accessed 2007 Mar 9]. http://www.cancer.ca/ccs/internet/standard/0,3182,3172_10175_74550606_langId-en,00.html.
- 2.Coldman Andrew J, Phillips Norman, Pickles Thomas A. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ. 2003 Jan 7;168(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Kopec Jacek A, Goel Vivek, Bunting Peter S, Neuman Jan, Sayre Eric C, Warde Padraig, Levers Peter, Fleshner Neil. Screening with prostate specific antigen and metastatic prostate cancer risk: a population based case-control study. J Urol. 2005 Aug;174(2):495–9. doi: 10.1097/01.ju.0000165153.83698.42. discussion 499. [DOI] [PubMed] [Google Scholar]
- 4.Labrie Fernand, Candas Bernard, Cusan Lionel, Gomez Jose Luis, Bélanger Alain, Brousseau G, Chevrette Eric, Lévesque Jacques. Screening decreases prostate cancer mortality: 11-year follow-up of the 1988 Quebec prospective randomized controlled trial. Prostate. 2004 May 15;59(3):311–318. doi: 10.1002/pros.20017. [DOI] [PubMed] [Google Scholar]
- 5.Horninger Wolfgang, Berger Andreas, Pelzer Alexandre, Klocker Helmut, Oberaigner Wilhelm, Schönitzer Dieter, Severi Gianluca, Robertson Chris, Boyle Peter, Bartsch Georg. Screening for prostate cancer: updated experience from the Tyrol study. Can J Urol. 2005 Feb;12 Suppl 1:7–13. discussion 92-3. [PubMed] [Google Scholar]
- 6.Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;3:CD004720. doi: 10.1002/14651858.CD004720.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Feightner J W. Screening for prostate cancer. 1994. pp. 811–823. http://www.phac-aspc.gc.ca/publicat/clinic-clinique/pdf/s10c67e.pdf.
- 8.Smith R A, Mettlin C J, Davis K J, Eyre H. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2000 Jan;50(1):34–49. doi: 10.3322/canjclin.50.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Alberta Medical Association Guidelines for the use of PSA and screening for prostate cancer: The Alberta Clinical Practice Guidelines Program. 1999. [accessed 2007 Mar 9]. http://www.topalbertadoctors.org/NR/rdonlyres/20429283-ADAE-4BD0-9977-DC23715A230D/0/prostate_brochure.pdf.
- 10.Gibbons Laurie, Waters Chris. Prostate cancer--testing, incidence, surgery and mortality. Health Rep. 2003 May;14(3):9–20. [PubMed] [Google Scholar]
- 11.Tudiver Fred, Guibert Remi, Haggerty Jeannie, Ciampi Antonio, Medved Wendy, Brown Judith Belle, Herbert Carol, Katz Alan, Ritvo Paul, Grant Bill, Goel Vivek, Smith Philip, O'Beirne Maeve, Williams J Ivan, Moliner Peter. What influences family physicians' cancer screening decisions when practice guidelines are unclear or conflicting? J Fam Pract. 2002 Sep;51(9):760. [PubMed] [Google Scholar]
- 12.Bunting Peter S. Has there been a change in practice of screening for prostate cancer with prostate-specific antigen in Ontario? Clin Biochem. 2004 Oct;37(10):898–903. doi: 10.1016/j.clinbiochem.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Williams R B, Boles M, Johnson R E. Use of prostate-specific antigen for prostate cancer screening in primary care practice. Arch Fam Med. 1995 Apr;4(4):311–315. doi: 10.1001/archfami.4.4.311. [DOI] [PubMed] [Google Scholar]
- 14.Eisen S A, Waterman B, Skinner C S, Scherrer J F, Romeis J C, Bucholz K, Heath A, Goldberg J, Lyons M J, Tsuang M T, True W R. Sociodemographic and health status characteristics with prostate cancer screening in a national cohort of middle-aged male veterans. Urology. 1999 Mar;53(3):516–522. doi: 10.1016/S0090-4295(98)00545-7. [DOI] [PubMed] [Google Scholar]
- 15.Merrill R M. Demographics and health-related factors of men receiving prostate-specific antigen screening in Utah. Prev Med. 2001 Dec;33(6):646–652. doi: 10.1006/pmed.2001.0940. [DOI] [PubMed] [Google Scholar]
- 16.McGregor S Elizabeth, Bryant Heather E, Brant Rollin F, Corbett Peter J. Prevalence of PSA testing and effect of clinical indications on patterns of PSA testing in a population-based sample of Alberta men. Chronic Dis Can. 2002;23(3):111–119. [PubMed] [Google Scholar]
- 17.Perkins J J, Sanson-Fisher R W, Clarke S J, Youman P. An exploration of screening practices for prostate cancer and the associated community expenditure. Br J Urol. 1998 Oct;82(4):524–529. doi: 10.1046/j.1464-410x.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman R M, Gilliland F D. A population-based survey of prostate cancer testing in New Mexico. J Community Health. 1999 Dec;24(6):409–419. doi: 10.1023/A:1018790421714. [DOI] [PubMed] [Google Scholar]
- 19.Beaulac Jennifer A, Fry Richard N, Onysko Jay. Lifetime and recent prostate specific antigen (PSA) screening of men for prostate cancer in Canada. Can J Public Health. 2006;97(3):171–176. doi: 10.1007/BF03405578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter F, Graham E, Pal N, Gonzalez E, Roetzheim R. Prostate cancer screening in primary care. South Med J. 1999 Mar;92(3):300–304. doi: 10.1097/00007611-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Livingston P, Cohen P, Frydenberg M, Borland R, Reading D, Clarke V, Hill D. Knowledge, attitudes and experience associated with testing for prostate cancer: a comparison between male doctors and men in the community. Intern Med J. 2002;32(5-6):215–223. doi: 10.1046/j.1445-5994.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Moran W P, Cohen S J, Preisser J S, Wofford J L, Shelton B J, McClatchey M W. Factors influencing use of the prostate-specific antigen screening test in primary care. Am J Manag Care. 2000 Mar;6(3):315–324. [PubMed] [Google Scholar]
- 23.Bryant Heather, Robson Paula J, Ullman Ruth, Friedenreich Christine, Dawe Ursula. Population-based cohort development in Alberta, Canada: a feasibility study. Chronic Dis Can. 2006;27(2):51–59. [PubMed] [Google Scholar]
- 24.Breslow NE, Day NE. Statistical Methods in Cancer Research: Volume I: The Analysis of Case-Control Studies. Lyon: International Agency for Research on Cancer; 1980. [PubMed] [Google Scholar]
- 25.Mercer S L, Goel V, Levy I G, Ashbury F D, Iverson D C, Iscoe N A. Prostate cancer screening in the midst of controversy: Canadian men's knowledge, beliefs, utilization, and future intentions. Can J Public Health. 1997;88(5):327–332. doi: 10.1007/BF03403900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDavid K, Melnik T A, Derderian H. Prostate cancer screening trends of New York State men at least 50 years of age, 1994 to 1997. Prev Med. 2000 Sep;31(3):195–202. doi: 10.1006/pmed.2000.0709. [DOI] [PubMed] [Google Scholar]
- 27.McIsaac W J, Fuller-Thomson E, Talbot Y. Does having regular care by a family physician improve preventive care? Can Fam Physician. 2001 Jan;47:70–76. [PMC free article] [PubMed] [Google Scholar]
- 28.Health Canada Statistical report on the health of Canadians. 2006. [accessed 2007 Mar 9]. http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=reports_statistical_e.
- 29.Klein Douglas. Short report: adolescents' health. Does having a family physician make a difference? Can Fam Physician. 2003 Aug;49:1000–1002. [PMC free article] [PubMed] [Google Scholar]
- 30.Bunting P S, Goel V, Williams J I, Iscoe N A. Prostate-specific antigen testing in Ontario: reasons for testing patients without diagnosed prostate cancer. CMAJ. 1999 Jan 12;160(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbons L, Waters C, Mao Y, Ellison L. Trends in colorectal cancer incidence and mortality. Health Rep. 2001;12(2):41–55. [PubMed] [Google Scholar]
- 32.Close D R, Kristal A R, Li S, Patterson R E, White E. Associations of demographic and health-related characteristics with prostate cancer screening in Washington State. Cancer Epidemiol Biomarkers Prev. 1998 Jul;7(7):627–630. [PubMed] [Google Scholar]
- 33.Gohagan J K, Prorok P C, Hayes R B, Kramer B S. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000 Dec;21(6 Suppl):251–72. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 34.Schröder Fritz H, Habbema Dik F, Roobol Monique J, Bangma Chris H. Prostate cancer in the Swedish section of ERSPC--evidence for less metastases at diagnosis but not for mortality reduction. Eur Urol. 2007 Mar;51(3):588–590. doi: 10.1016/j.eururo.2006.07.013. [DOI] [PubMed] [Google Scholar]


