Abstract
We sought to determine, retrospectively, whether obesity was associated with adverse renal outcomes in 17,630 patients who underwent cardiac surgery from January 1995 through December 2006. Obesity was defined as a body mass index ≥30 kg/m2. The primary outcome was any episode of postoperative renal insufficiency (requiring or not requiring dialysis) before hospital discharge. Outcomes were evaluated in the entire cohort and in subgroups undergoing isolated coronary artery bypass grafting (CABG), isolated valve surgery, and combined CABG and valve surgery.
The final analysis included 16,429 patients, 5,124 (31%) of whom were obese. In the entire cohort, obesity was associated both with increased risk of any postoperative renal insufficiency (odds ratio [OR], 1.37; 95% confidence interval [CI], 1.21–1.55) and with increased risk of renal insufficiency not requiring dialysis (OR, 1.41; 95% CI, 1.23–1.62). Obesity was associated with an increased risk of postoperative renal insufficiency in patients undergoing isolated CABG (OR, 1.38; 95% CI, 1.18–1.61), isolated valve surgeries (OR, 1.39; 95% CI, 1.05–1.85), and combined CABG and valve surgeries (OR, 1.35; 95% CI, 0.99–1.83; statistically nonsignificant). Development of postoperative renal insufficiency was associated with a significantly higher mortality rate (P <0.0001) and with a significantly longer hospital stay (23 vs 10.5 days; P <0.0001).
We conclude that obesity is associated with a significant increase in postoperative renal insufficiency in cardiac surgery patients, an effect that we attribute to an increase in postoperative renal failure that does not require dialysis.
Key words: Body mass index, cardiac surgical procedures/adverse effects, cardiopulmonary bypass/adverse effects, inflammation/complications, kidney failure/etiology, obesity/complications, oxidative stress, postoperative complications, retrospective studies, risk factors, systemic inflammatory response syndrome, treatment outcome
Obesity is associated with the development of chronic kidney disease.1–6 It has also been shown to be associated, in patients who have chronic kidney disease, with increased oxidative stress and inflammation,1 impairment of renal endothelial function,2,3 and proteinuria.4,5 Inflammation appears to be an important mediator in the development of postoperative renal failure.6,7 Although epidemiologic data indicate that obesity might be associated with the development of chronic kidney disease in the general population,8–10 there are very few studies pertaining to obesity as an independent predictor of poor renal outcomes in patients undergoing cardiac surgeries. In most of these studies,6,7,11–15 the occurrence of postoperative renal insufficiency has not been the primary outcome; moreover, the results have been inconsistent. We lack firm data on whether obesity is associated with an increase in the severity of postoperative renal failure.
The aim of this study was to determine whether obesity is an independent predictor of postoperative renal insufficiency in patients undergoing cardiac surgeries, including patients undergoing isolated coronary artery bypass grafting (CABG), isolated valve surgeries, and combined CABG and valve surgeries. We also investigated the possible association between obesity and increased severity of postoperative renal failure (that is, postoperative renal failure requiring in-hospital dialysis).
Patients and Methods
We conducted a retrospective cohort analysis of 17,630 consecutive patients who underwent surgical procedures (CABG, valve surgery, or both) at St. Luke's Episcopal Hospital from 1 January 1995 through 31 December 2006. Of these, we excluded from the analysis 1,038 patients who underwent any associated procedures, including left ventricular aneurysm repair and ascending aortic repairs, and another 163 patients who experienced preoperative renal failure requiring dialysis. The study protocol was approved by the Institutional Review Board.
The remaining 16,429 patients were divided into 2 groups (obese and nonobese) on the basis of body mass index (BMI). Obesity was defined as a BMI of ≥30 kg/m2 (weight in kilograms divided by the square of height in meters). Patients' weights at admission to the hospital were used to calculate BMI. Patients' baseline characteristics and intraoperative variables were obtained from the Texas Heart Institute Research Database (THIRDBase). This database had written documentation for each field coded. Data from patient charts were abstracted by 3 trained abstractors, with 95% agreement among abstractors. Monthly electronic quality-control checks were conducted to catch logical errors. When verification coding (recoding the same admission) was performed on a minimum of 10 admissions per month, it found that 95% of the data collected was correct.
The variables used for our analysis included age, sex, history of hypertension, diabetes mellitus, prior myocardial infarction, preoperative renal insufficiency, hyperlipidemia, unstable angina, peripheral vascular disease, transient ischemic attacks, cerebrovascular accident, low left ventricular ejection fraction (defined as <0.35), need for urgent or repeat surgery, need for an intra-aortic balloon pump, New York Heart Association class at the time of surgery, and aortic cross-clamp time.
The primary outcome of interest was the occurrence of any postoperative renal insufficiency up to the time of the patient's discharge from index hospitalization for cardiac surgery. Postoperative renal insufficiency was defined as the occurrence of acute tubular necrosis, acute interstitial nephritis, postoperative anuria, or any increase in serum creatinine deemed clinically significant by the treating physician. Postoperative renal insufficiency was subdivided into renal failure requiring any dialysis session during the hospitalization and renal failure not requiring dialysis.
Statistical Analysis
All statistical analyses were performed using SAS statistical software version 9.1 (SAS Institute, Inc.; Cary, NC). Baseline characteristics and intraoperative variables were compared in both patient groups. Differences between groups were evaluated by the c2 test for discrete variables and the t test for continuous variables. A P value <0.05 was considered statistically significant. A multivariate logistic regression model was used to control for potential confounders and to ascertain which variables were independently associated with outcome. Variables used in the multivariate logistic regression model included all the preoperative and intraoperative variables.
Because it can be argued that obese patients on average were sicker and had a higher risk of developing postoperative renal insufficiency when compared with nonobese patients, we attempted to minimize this selection bias by performing propensity-score matching.16 Propensity scores were estimated using unconditional logistic regression to determine the predicted probability of obesity for each patient. The variables used in the model after propensity-score matching are shown in Table I. Obese patients and nonobese patients were then matched 1-to-1 on the basis of these variables, resulting in successful matching of 6,866 patients (3,433 patients in each group). All predictor variables were then entered into a multivariate stepwise logistic model to determine whether obesity was independently associated with the risk of renal insufficiency in propensity-score-matched subjects.
TABLE I. Preoperative and Intraoperative Characteristics of Obese (n = 3,433) versus Nonobese (n = 3,433) Patients after Propensity-Score Matching
Results
The final study population consisted of 16,429 patients. Of these, 11,830 (72%) patients had isolated CABG, 3,062 (19%) had isolated valve surgeries, and 1,537 (9%) had combined CABG and valve surgeries.
A total of 5,124 patients (31%) had a BMI ≥30 kg/m2. In this obese cohort, 3,414 patients (63%) had a BMI between 30 and 34.99 kg/m2, 1,189 patients (23%) had a BMI between 35 and 39.99 kg/m2, and 521 patients (10%) had a BMI ≥40 kg/m2.
Preoperative characteristics of obese and nonobese patients are compared in Table II. In the obese group, there were more women than men. Obese patients had a higher prevalence of hypertension, diabetes, smoking, hyperlipidemia, and unstable angina, and had longer total bypass times. Nonobese patients were older, had a higher prevalence of peripheral vascular disease and transient ischemic attacks, and underwent more repeat operations when compared with obese patients.
TABLE II. Preoperative and Intraoperative Characteristics of Obese versus Nonobese Patients Included in Retrospective Chart Review
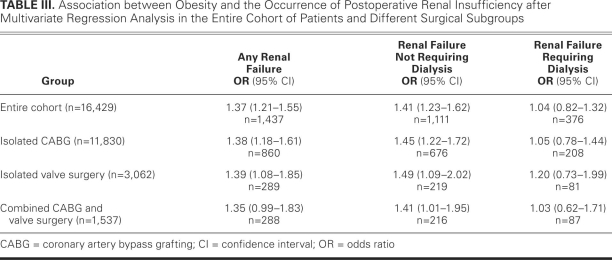
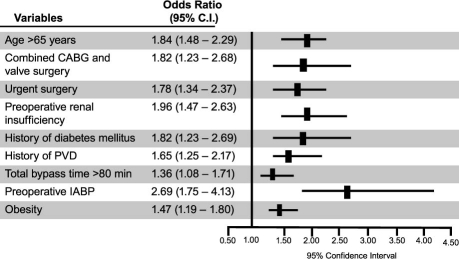
In the entire cohort, 1,437 patients (9%) had the primary outcome of postoperative renal insufficiency. Variables independently associated with the occurrence of postoperative renal insufficiency in the entire cohort are shown in Figure 1. As shown in Table III, obesity in the entire cohort was independently associated with a 37% increase in the risk of developing any postoperative renal insufficiency (odds ratio [OR], 1.37; 95% confidence interval [CI], 1.21–1.55), and this risk was mostly attributable to an increase in renal insufficiency not requiring dialysis (OR, 1.41; 95% CI, 1.23–1.62). Subgroup analysis revealed that obesity was associated with a statistically significant increased risk of renal insufficiency in patients undergoing isolated CABG (OR, 1.38; 95% CI, 1.18–1.61) or isolated valve surgeries (OR, 1.39; 95% CI, 1.05–1.85) and with a statistically nonsignificant increased risk of postoperative renal insufficiency in patients undergoing combined CABG and valve surgeries (OR, 1.35; 95% CI, 0.99–1.83), as shown in Table III. Within these subgroups, the increased risk was attributable to the increase in renal insufficiency not requiring dialysis.
Fig. 1. Characteristics independently associated with postoperative renal insufficiency during hospitalization after cardiac surgery in our entire study population of 16,429 patients.*
CABG = coronary artery bypass grafting; CI = confidence interval; CVA = cerebrovascular accident; IABP = intra-aortic balloon pump; PVD = peripheral vascular disease
*Includes variables significantly associated with postoperative renal insufficiency after multivariate regression analysis.
TABLE III. Association between Obesity and the Occurrence of Postoperative Renal Insufficiency after Multivariate Regression Analysis in the Entire Cohort of Patients and Different Surgical Subgroups
Patients who developed postoperative renal failure had a significantly higher 30-day mortality rate (25% vs 2.9%; P <0.001) and significantly prolonged length of hospital stay (mean, 23 days), when compared with patients who did not develop any postoperative renal insufficiency (mean, 10.5 days; P <0.0001).
A higher risk for the development of postoperative renal insufficiency was associated with increasing severity of obesity. For the BMI categories of 30 to 34.99 kg/m2, 35 to 39.99 kg/m2, and ≥40 kg/m2, the percentages of patients who developed postoperative renal failure were 9.2%, 11.1%, and 13.1%, respectively (P = 0.001 for the trend).
Patient characteristics independently associated with the risk of postoperative renal insufficiency after propensity-score matching for obesity status are shown in Figure 2. Obesity was associated with a 47% increase in the likelihood of developing postoperative renal insufficiency after propensity matching (OR, 1.47; 95% CI, 1.19–1.80; P = 0.0003).
Fig. 2. Characteristics independently associated with postoperative renal insufficiency after propensity-score matching in 6,866 patients (3,433 obese and 3,433 nonobese).*
CABG = coronary artery bypass grafting; CI = confidence interval; IABP = intra-aortic balloon pump; PVD = peripheral vascular disease
*Results obtained by multivariate regression analysis after propensity-score matching (n = 6,866 patients). Please see text in Methods section as well as Table I for list of variables matched for propensity-score analyses.
Discussion
In this retrospective cohort analysis of 16,429 consecutive cases, we found that obesity was associated with a significant increase in postoperative renal insufficiency in patients undergoing cardiac surgeries. This effect—seen in the entire cohort, as well as in patients undergoing isolated CABG or isolated valve surgeries—was attributable mostly to an increase in postoperative renal failure that did not require dialysis. The risk was graded with the increasing risk that is associated with an increase in the severity of obesity. The development of postoperative renal insufficiency was associated with a significantly higher mortality rate and with a longer length of stay.
Obesity is associated with oxidative stress and endothelial dysfunction.1 Obese patients are at a higher risk of developing hypertension17 and diabetes,18 which are also associated with elevated inflammatory response and impaired endothelial function.16,17 The use of cardiopulmonary bypass for cardiac surgeries has been shown to be associated with an up-regulation of the inflammatory cascade.19,20 This, together with the prevalence of risk factors associated with the development of postoperative renal insufficiency and the risk associated with obesity itself, means that obese patients may have a higher incidence of postoperative renal insufficiency.
Although epidemiologic studies have shown that obesity is independently associated with the development of chronic kidney disease,8–10,21–23 studies of the association between obesity and the development of postoperative renal insufficiency have yielded disparate results.6,7,11–15,24,25 Moulton and colleagues13 did not find an association between obesity and postoperative renal failure, but an analysis of data from the Society of Thoracic Surgeons' database by Prabhakar and associates12 showed that moderate obesity (BMI, 35–39.9 kg/m2) and severe obesity (BMI ≥40 kg/m2) were independently associated with the development of renal insufficiency in the postoperative period. The reasons for these varying results may include the small sample sizes of some studies. In addition, the development of renal insufficiency in surgical subgroups (especially isolated valve surgery and combined CABG and valve surgery) has not been well studied. Our study is one of the first to specifically evaluate the association between obesity and postoperative renal outcomes in a very large cohort of patients who underwent cardiac surgeries.
The risk estimates we obtained are comparable with those obtained in prior studies.12,25 The risk estimate obtained by Prabhakar and associates12 for patients with moderate obesity, defined as a BMI of 35 to 39.9 kg/m2, was 1.58 (1.50–1.67). We show that the presence of even mild forms of obesity (a BMI of 30 to 34.99 kg/m2) was independently associated with the occurrence of postoperative renal insufficiency and that patient cohorts with higher BMIs have higher incidences of postoperative renal insufficiency—which suggests a dose–response relationship. Most of the postoperative renal failure attributable to obesity did not require dialysis, but it was associated with a significantly longer hospital stay. Our findings extend the findings from earlier studies12,25 to show that obesity is associated also with an increased risk of renal insufficiency in subgroups that include patients undergoing isolated CABG or isolated valve surgeries. These subgroups have not been well studied before.
The development of postoperative renal insufficiency is an important clinical event, because it is associated with an increase in death26 and with prolonged stays in the intensive care unit after cardiac surgery.27 Our results support some of these earlier findings and show that patients who develop renal failure in the postoperative setting have, on average, an 8-fold higher incidence of early postoperative death and twice the length of hospital stay, when compared with patients who do not develop postoperative renal insufficiency.
Limitations
Our study had limitations. Because we did not have postoperative serum creatinine measurements in our database, we used a clinical definition of renal failure as our outcome measure, which may have caused us to underestimate the true incidence of postoperative renal failure. However, the incidence of postoperative renal insufficiency in our cohort was comparable with those reported in prior studies of patients who had undergone cardiac surgery.11 Furthermore, although we performed propensity-score matching to account for differences between the obese and nonobese groups, it is possible that some unmeasured differences between the 2 groups are responsible for observed differences in outcomes. Moreover, a BMI ≥30 kg/m2 may not be the most sensitive definition of obesity, and uniform BMI cutoffs may not apply across all races and ethnic groups. Other measures, such as waist circumference and waist-to-hip ratios, might have provided a more accurate reading of the increased risk associated with obesity.28 Although this was a single-institution study, the high volume of cases and the large, multiyear cohort likely enable the results to be applied, in general, to other settings; and the paucity of studies on this important topic lends interest to our findings, as well. Despite these limitations, the strengths of our study include carefully applied regression and propensity analyses.
Conclusions
Our results have important clinical implications. If the results of our retrospective review are confirmed in the setting of a randomized clinical trial, obesity as a risk factor for the development of postoperative renal insufficiency could be viewed as modifiable. Indeed, in our multivariate analysis, obesity was one of the few modifiable risk factors for the development of postoperative renal failure. Carefully executed randomized clinical trials are needed to confirm these preliminary findings and to determine whether preventive measures—such as preoperative weight loss in selected patients not undergoing emergent or urgent procedures and the use of perioperative statin therapy (shown to be associated with a decreased incidence of postoperative renal insufficiency29)—can ameliorate some of the detrimental renal outcomes that are associated with obesity in cardiac surgery patients.
Acknowledgment
The authors acknowledge Joanna A. Brooks, BA, for editorial assistance.
Footnotes
Address for reprints: Christie M. Ballantyne, MD, Baylor College of Medicine, 6565 Fannin, MS A-601, Suite A656, Houston, TX 77030
E-mail: cmb@bcm.tmc.edu
Source of support: This work was supported by the Texas Heart Institute Muller Fund for Cardiovascular Research and the Houston VA Health Services Research & Development Center of Excellence (grant number HFP90-020).
References
- 1.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 2008;19(3):593–9. [DOI] [PMC free article] [PubMed]
- 2.Grassi G, Seravalle G, Scopelliti F, Dell'oro R, Fattori L, Quarti-Trevano F, et al. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2009; doi:10.1038/oby.2009.195. Available from: http://www.nature.com/oby/journal/vaop/ncurrent/abs/oby2009195a.html [DOI] [PubMed]
- 3.Karpoff L, Vinet A, Schuster I, Oudot C, Goret L, Dauzat M, et al. Abnormal vascular reactivity at rest and exercise in obese boys. Eur J Clin Invest 2009;39(2):94–102. [DOI] [PubMed]
- 4.Sarafidis PA, Whaley-Connell A, Sowers JR, Bakris GL. Cardiometabolic syndrome and chronic kidney disease: what is the link? J Cardiometab Syndr 2006;1(1):58–65. [DOI] [PubMed]
- 5.Chandie Shaw PK, Berger SP, Mallat M, Frolich M, Dekker FW, Rabelink TJ. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care 2007;30(7):1840–4. [DOI] [PubMed]
- 6.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Effects of obesity and small body size on operative and long-term outcomes of coronary artery bypass surgery: a propensity-matched analysis. Ann Thorac Surg 2005; 79(6):1976–86. [DOI] [PubMed]
- 7.Rockx MA, Fox SA, Stitt LW, Lehnhardt KR, McKenzie FN, Quantz MA, et al. Is obesity a predictor of mortality, morbidity and readmission after cardiac surgery? Can J Surg 2004; 47(1):34–8. [PMC free article] [PubMed]
- 8.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004;291(7):844–50. [DOI] [PubMed]
- 9.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 2005; 46(4):587–94. [DOI] [PubMed]
- 10.Cignarelli M, Lamacchia O. Obesity and kidney disease. Nutr Metab Cardiovasc Dis 2007;17(10):757–62. [DOI] [PubMed]
- 11.Wigfield CH, Lindsey JD, Munoz A, Chopra PS, Edwards NM, Love RB. Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg 2006;29(4):434–40. [DOI] [PubMed]
- 12.Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons' database. Ann Thorac Surg 2002;74(4):1125–31. [DOI] [PubMed]
- 13.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation 1996;94(9 Suppl):II87-92. [PubMed]
- 14.Reeves BC, Ascione R, Chamberlain MH, Angelini GD. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2003;42(4):668–76. [DOI] [PubMed]
- 15.Kuduvalli M, Grayson AD, Oo AY, Fabri BM, Rashid A. Risk of morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 2002;22(5):787–93. [DOI] [PubMed]
- 16.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26(4):734–53. [DOI] [PubMed]
- 17.Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People's Republic of China Study and the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2008;167(11):1365–74. [DOI] [PMC free article] [PubMed]
- 18.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345(11):790–7. [DOI] [PubMed]
- 19.Elahi MM, Khan JS, Matata BM. Deleterious effects of cardiopulmonary bypass in coronary artery surgery and scientific interpretation of off-pump's logic. Acute Card Care 2006;8 (4):196–209. [DOI] [PubMed]
- 20.Gravlee GP. Update on cardiopulmonary bypass. Curr Opin Anaesthesiol 2001;14(1):11–6. [DOI] [PubMed]
- 21.D'Agati VD, Markowitz GS. Supersized kidneys: lessons from the preclinical obese kidney. Kidney Int 2008;73(8):909–10. [DOI] [PubMed]
- 22.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 2005;46 (5):871–80. [DOI] [PubMed]
- 23.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006;17(6):1695–702. [DOI] [PubMed]
- 24.Tyson GH 3rd, Rodriguez E, Elci OC, Koutlas TC, Chitwood WR Jr, Ferguson TB, Kypson AP. Cardiac procedures in patients with a body mass index exceeding 45: outcomes and long-term results. Ann Thorac Surg 2007;84(1):3–9. [DOI] [PubMed]
- 25.Yap CH, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust 2007;186(7):350–4. [DOI] [PubMed]
- 26.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104 (4):343–8. [DOI] [PubMed]
- 27.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 1998;128 (3):194–203. [DOI] [PubMed]
- 28.Yang L, Kuper H, Weiderpass E. Anthropometric characteristics as predictors of coronary heart disease in women. J Intern Med 2008;264(1):39–49. [DOI] [PubMed]
- 29.Virani SS, Polsani V, Lee VV, Elayda M, Wilson JM, Ballantyne CM. Is preoperative statin therapy associated with a decrease in the incidence of postoperative renal insufficiency [abstract]? J Am Coll Cardiol 2008;51:A381.