Abstract
Atrial fibrillation is the most common postoperative arrhythmia in patients who undergo cardiac surgery. We sought to determine whether the administration of statins reduces the incidence of postoperative atrial fibrillation in cardiac surgery patients. We performed a meta-analysis on all studies published between 2004 and 2008 that reported comparisons between statin treatment or nontreatment in these patients. Our primary focus was the incidence of postoperative atrial fibrillation. Random-effects modeling and sensitivity analysis were used to evaluate the consistency of the calculated treatment effect. Ten qualifying studies generated a total of 4,459 patients. The incidence of postoperative atrial fibrillation was 22.6% (622/2,758) in the statin-treated group and 29.8% (507/1,701) in the untreated group. Using the random-effects model, we calculated an odds ratio (OR) of 0.60 (95% confidence interval [CI], 0.48–0.76). When we considered only the 4 randomized studies (919 patients) in order to reduce the effects of heterogeneity, this significant reduction in the incidence of postoperative atrial fibrillation in the statin group was maintained (OR, 0.55; 95% CI, 0.41–0.73) with no heterogeneity (χ2 of heterogeneity, 2.96; P = 0.4). In studies wherein only coronary artery bypass grafting was performed, statin treatment decreased postoperative atrial fibrillation (OR, 0.64; 95% CI, 0.43–0.95). We conclude that statin administration results in a reduction in the incidence of atrial fibrillation in patients who undergo cardiac surgery. Further research into the underlying mechanism can elucidate possible relationships between the dosage and type of statin used.
Key words: Atrial fibrillation/drug therapy/physiopathology/prevention & control; cardiac surgical procedures/complications; data interpretation, statistical; hydroxymethylglutaryl-CoA reductase inhibitors/therapeutic use; meta-analysis as topic; models, statistical; postoperative complications/drug therapy/epidemiology/prevention & control; randomized controlled trials as topic/methods; risk factors
Atrial fibrillation (AF) is an important and common complication after cardiac surgery. Long-term AF (>5 yr) is associated with a 3-to 4-fold increase in stroke risk and a mortality rate double that of the general population.1 Similarly, postoperative AF is associated with increased death and morbidity, often as a result of perioperative stroke.2,3 Furthermore, in this group, hospital length of stay is prolonged to the stage of excessive consumption of hospital resources and therefore increased costs.4,5 Patient morbidity, including anxiety,4,6 is increased because of an augmentation in the incidence of AF-associated complications. These include transient ischemic attacks, renal dysfunction, stroke, congestive heart failure, and neurocognitive impairment.7,8 Morbidity may also result from adverse effects of additional pharmacotherapy that is required to treat the dysrhythmia.
Atrial fibrillation most commonly occurs on the 2nd or 3rd postoperative day, and 70% of arrhythmias are diagnosed within the first 4 days.9 Of the patients who experience postoperative AF, those in whom pharmacologic or electrical cardioversion fails face the prospect of oral anticoagulation and its associated risks, which may be substantial.10
The cause of AF is complex and multifactorial. Nonsurgical AF has been associated with established risk factors11,12 and, more recently, with genetic factors and inflammation.13 Several theories relate to the pathophysiology of AF. It is understood that AF leads to electrical, contractile, or structural remodeling of the atria.14–16 Multiple wavelets of excitation propagate around the atrium,17 and both single-focus and multiple-source causes have been described.18 Once the rhythm is established, changes in the atrial substrate facilitate perpetuation of the dysrhythmia.17 Multiple influences have been connected with the cause and pathophysiology of the condition, including genetic factors (perhaps as a consequence of a channelopathy19 and metabolic changes).14
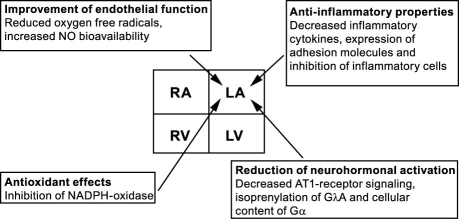
Statins (3-hydroxy-3-methylglutaryl-coenzyme A [HMG-CoA] reductase inhibitors) have been shown to suppress cholesterol biosynthesis and to reduce cardiovascular pathology significantly in patients who are at risk of developing atherosclerotic disease. Recent clinical and experimental data suggest that the benefit of statins extends beyond this ability to lower lipid levels. Mechanisms besides remodeling that are mediated by statins are oxidative stress, inflammation, endothelial pathology, and neurohormonal influence (Fig. 1).20
Fig. 1. Molecular effects of statins on the left atrium (adapted from Adam and colleagues,20 with permission).
AT-1 = angiotensin-1; Gα = G protein, α subunit; GΓA = G protein, γ subunit; LA = left atrium; LV = left ventricle; NADPH = reduced form of nicotinamide-adenine dinucleotide phosphate; NO = nitrous oxide; RA = right atrium; RV = right ventricle
We sought to evaluate whether statin treatment reduces the incidence of postoperative AF. To date, studies in human beings have analyzed the impact of statins in new-onset or recurrent AF, postcardioversion AF, and postoperative AF. We studied the postoperative-AF group only. We searched the world medical literature for relevant comparative studies of patients who had or had not been treated with statins, with our focus on randomized clinical trials (RCTs) and the best available high-quality studies.
We chose to perform a meta-analysis, which provides meaningful data when it would otherwise be difficult to construct an adequately powered, large-scale, high-quality RCT.10 It is crucial to evaluate the best evidence in the available literature,8 because a meta-analysis can be judged only on the basis of the studies that it includes and the factors that it takes into account. Accordingly, the accuracy of estimates of treatment effects can be quantitatively evaluated.
Materials and Methods
Literature Search
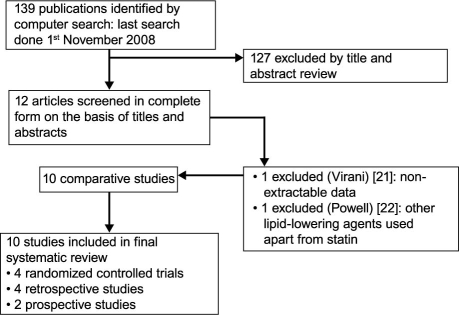
A literature search was performed on the EMBASE, MEDLINE®, Cochrane Library, and Google™ Scholar databases for comparative studies through 2008 that investigated a clinical significance of statin treatment on the incidence of postoperative AF. The following MeSH search headings were used: cardiac surgery, atrial fibrillation, and statins. Searches were also performed under the headings of coronary artery bypass graft, cardiothoracic surgery, and HMG-CoA reductase inhibitors. The “related articles” function was used to broaden the search, and all identified citations were reviewed. No studies comparing AF incidence and statin treatment were found from before 2004; therefore, all relevant papers were published from 2004 through 2008. Studies that compared statin-treated and untreated groups of patients were identified, and data were extracted regarding the outcome of interest (postoperative AF). Figure 2 shows the search approach and the qualified studies.
Fig. 2. Search approach and selection of the studies for the meta-analysis.
Data Extraction
Two reviewers (SS and CR) independently extracted from each study the lead author, year of publication, study design, types of statins used, number of statin-treated and untreated patients who were operated upon, type of cardiac surgery, and incidence of AF in each study group.
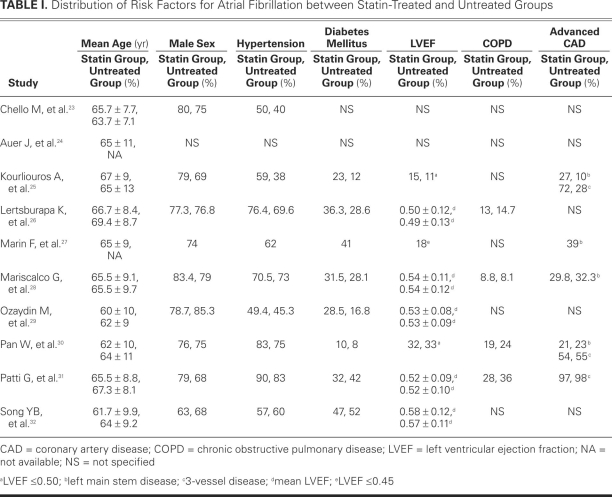
Variables that generally predispose surgical patients to AF10—advanced age, male sex, hypertension, diabetes mellitus, low left ventricular ejection fraction, chronic obstructive pulmonary disease, and advanced 3-vessel and left-main-stem coronary artery disease—were focused upon in order to compare the 2 groups. Table I shows the distribution of these variables between groups.23–32
TABLE I. Distribution of Risk Factors for Atrial Fibrillation between Statin-Treated and Untreated Groups
Inclusion and Exclusion Criteria
We included all comparative studies of statin treatment and nontreatment when the incidence of AF was reported as an outcome in patient groups that underwent cardiac surgery. When 2 studies by the same institution reported the same outcomes, we included either the better-quality or the more informative publication in the analysis. We excluded studies in which the primary interventional approach could not be defined, lipid-lowering agents other than statins were administered, and outcomes of interest were not reported, and in which it was impossible to calculate AF incidence from the published results.
The outcome of interest was identified in the selected studies wherever the term atrial arrhythmia or atrial fibrillation was used. Ventricular and other arrhythmias were excluded from our analysis. The selected studies reported new-onset postoperative AF and, by definition, excluded patients with existing AF. It was not our interest to consider intermittent or persistent AF, protocols for prophylaxis, or methods of monitoring patients.
Statistical Analysis
Meta-analysis was performed in accordance with recommendations from The Cochrane Collaboration and the Quality of Reporting of Meta-Analyses (QUOROM) guidelines.33,34 For categorical variables, we used the odds ratio (OR) as the summary statistic. This ratio represented the odds of an adverse event's occurrence in the statin-treated group compared with the untreated group. An OR of less than 1 favored the statin group. The point estimate of the OR was considered statistically significant at P <0.05 if the 95% confidence interval (CI) did not include the value 1. To translate these results into a quantitatively beneficial clinical outcome, we calculated the risk difference and number needed to treat (NNT). Risk difference (or absolute risk reduction) was the difference in the incidence of postoperative complications between treated and untreated groups. The NNT was the number of patients in the treatment group who needed to be treated in order to prevent 1 complication event (NNT = 1/risk difference).
Aggregation of the overall rates of the outcome of interest was performed with use of the Mantel-Haenszel method. The Yates correction was used for those studies that contained a zero in 1 cell for the number of events of interest in 1 of the 2 groups.35,36 Because “zero cells” create problems in the computation of ratio measure and its standard error of the treatment effect, we added the value 0.5 in each cell of the 2 × 2 table for the study in question. When there were no events for the statin-treated and untreated groups, the study was discarded from the meta-analysis.
We used both fixed-effects and random-effects models. In a fixed-effects model, it is assumed that the treatment effect in each study is the same—whereas in a random-effects model, variation is assumed between studies, and the calculated OR consequently has a more conservative value.37,38 For surgical research, meta-analysis using the random-effects model was preferable, particularly because patients who have undergone operations in different centers have varying risk profiles and are selected by differing criteria for each surgical procedure. In order to evaluate the highest-quality evidence that was available, we focused on RCTs in our subgroup analysis.
We used 3 approaches in order to evaluate heterogeneity quantitatively:
Statistical tests—reanalyzing data via 2 different statistical approaches, using random- and fixed-effects models.
Graphic exploration—using funnel plots to evaluate publication bias.39,40
Sensitivity analysis by subgroup analysis. Five subgroups were selected: RCTs, studies of patients who underwent only coronary artery bypass grafting (CABG), studies in which atorvastatin was the only lipid-lowering agent used, studies with ≥300 patients in each group (sample-size effect), and studies that had ≥6 matching criteria (evaluation of study quality).
Analysis was conducted by using Review Manager version 4.2 (The Cochrane Collaboration, Software Update, Oxford, U.K.) and Sample Power 2.0 (SPSS Inc.; Chicago, Ill) for power-analysis calculations. All data conformed to each test that was used to analyze them.
Results
Selected Studies
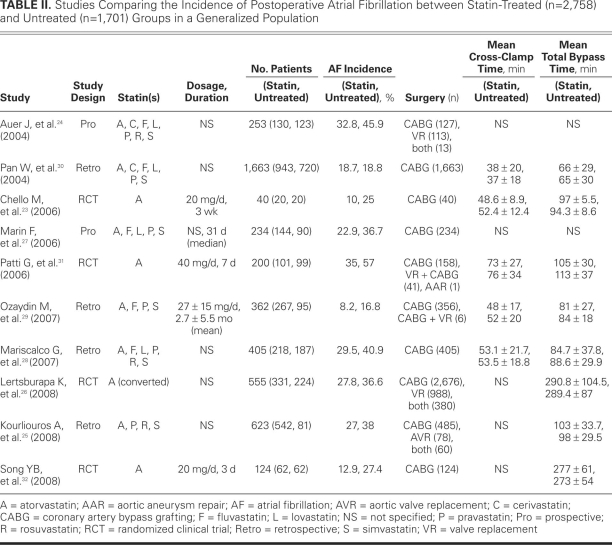
We initially identified 139 studies, 12 of which21–32 were selected for the meta-analysis. One study21 was then excluded, because the incidence of postoperative AF could not be calculated from the published results. Another study22 was excluded because lipid-lowering agents other than statins had been administered. Of the remaining studies, 4 were RCTs, 4 were retrospective, and 2 were prospective (Fig. 2). We included these 10 studies in our final analysis, which comprised 4,459 patients. Of these, 2,758 had undergone statin treatment and 1,701 had not (Table II).23–32
TABLE II. Studies Comparing the Incidence of Postoperative Atrial Fibrillation between Statin-Treated (n = 2,758)and Untreated (n = 1,701) Groups in a Generalized Population
There was 100% agreement between the 2 reviewers regarding the data extraction. All the studies contained groups that were comparable in age. However, 2 studies24,27 gave a mean age of the entire patient group without distinguishing between the treated and untreated groups. One study24 provided no values for any of the other risk factors for postoperative AF. The incidences of preoperative chronic obstructive pulmonary disease26,28,30,31 and advanced coronary artery disease were comparable in both cases in 4 studies.25,28,30,31 Comparable data for all 7 risk factors were available in 3 studies.28,30,31
Meta-Analysis
Eight studies24–29,31,32 showed a statistically significant difference between the 2 groups in the incidence of postoperative AF. Taking all 10 studies into account, the incidence was 22.6% in the statin-treated cohort (622 of 2,758) and 29.8% in the untreated cohort (507 of 1,701). In order to rule out a 7% relative risk reduction with a 5% significance level and 80% power, we calculated that a traditional RCT would require 636 patients in each arm and 842 patients for 90% power.
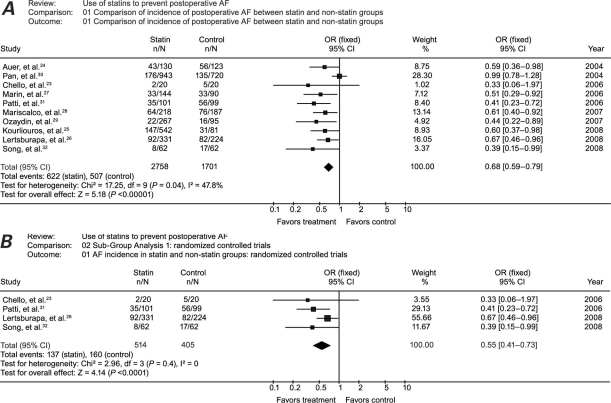
Using the random-effects model (Fig. 3A), we calculated an OR of 0.60 (95% CI, 0.48–0.76) and a χ2 of heterogeneity of 17.25 (P = 0.04). With use of a fixed-effects model, the OR was 0.68 (95% CI, 0.59–0.79) with an identical 95% CI when compared with the random-effects model, and with statistically significant heterogeneity.
Fig. 3. Meta-analysis of the A) studies and B) randomized controlled trials that compared the incidence of postoperative atrial fibrillation in statin-treated patients versus patients who received no statin treatment. Squares indicate point estimates of treatment effect (odds ratio), the size of the squares represents the weight attributed to each study, and horizontal bars indicate the 95% confidence interval (CI). The diamond represents the summary OR from the pooled studies with 95% CI.
P < 0.05 was considered statsitically significant.
AF = atrial fibrillation; OR = odds ratio
When we focused only on the 4 RCTs23,26,31,32 (919 patients) in order to calculate more precise estimates for the treatment effect, meta-analysis using the random-effects model revealed an OR of 0.55 (95% CI, 0.41–0.73), and χ2 of heterogeneity of 2.96 (P = 0.4) (Fig. 3B). With use of a fixed-effects model for all of the RCTs, the OR was the same value as that of the random-effects model with identical heterogeneity.
Subgroup Analysis
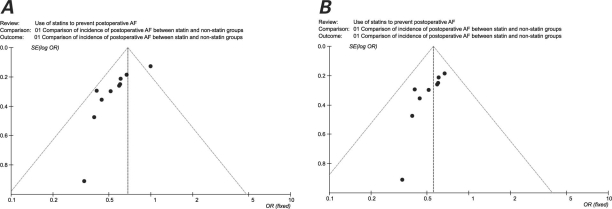
Graphic exploration was used to investigate heterogeneity further. Significant differences in the OR and heterogeneity for the chief outcome of interest were identified. Figure 4A is a scatter plot of the treatment effects estimated from all the individual studies included in this meta-analysis on the horizontal axis (OR), against a measure of study size on the vertical axis (SE[log OR]). This plot resembles a symmetric, inverted funnel (the 95% CI), within which lie most of the studies that were included in our analysis. The name “funnel plot” arises from the fact that precision in the estimation of the underlying treatment effect will increase as the sample size of the component studies increases. Figure 4A shows asymmetry, with 1 study30 outside the 95% CI. When the analysis was repeated after exclusion of that study, the OR for the incidence of AF was 0.56 (95% CI, 0.47–0.67), with a nonsignificant heterogeneity of 3.77 (P = 0.88). This was identical with use of random- and fixed-effects models. Figure 4B is a funnel plot of the meta-analysis with the outlying study excluded.
Fig. 4. Funnel plot A) that identifies sources of heterogeneity, and B) after exclusion of an outlying study, resulting in heterogeneity.
AF = atrial fibrillation; OR = odds ratio; SE = standard error
The RCTs were already analyzed as described above; neither the random-nor the fixed-effects models showed significant heterogeneity. Further random-effects subanalysis showed that including only large studies (≥300 patients)24,26,28–30 resulted in a calculated OR of 0.69 (95% CI, 0.52–0.91), with a nonsignificant heterogeneity of 9.37 (P = 0.05). Six studies24,25,27–30 used multiple types of statins. Upon exclusion of these studies from the analysis, the OR in the studies wherein atorvastatin was the only drug administered23,26,31,32 was 0.55 (95% CI, 0.41–0.73) with a nonsignificant heterogeneity of 2.96 (P = 0.4). Different types of cardiac surgery were used in 5 studies.24–26,29,31 Excluding these studies enabled us to analyze the data from 5 studies in which CABG was the only surgery performed23,27,28,30,32: the OR was 0.64 (95% CI, 0.43–0.95), with significant heterogeneity of 10.07 (P = 0.04). Finally, 5 studies24,26–29 underwent analysis of at least 6 out of 7 matching criteria. The OR was 0.58 (95% CI, 0.47–0.71) with a heterogeneity of 2.28 (P = 0.69).
Discussion
On the basis of this study, we can conclude that statin treatment likely reduces the incidence of postoperative AF in a generalized population (mean age, <70 yr) when compared with an untreated group. The data from the RCTs revealed a significant reduction in AF incidence: OR = 0.55 (95% CI, 0.41–0.73) without significant heterogeneity (2.96; P = 0.4) in the random- and fixed-effects models. The meta-analysis also showed a similarly significant reduction in postoperative-AF incidence in studies wherein CABG was the sole cardiac surgical procedure performed and atorvastatin was the specific statin administered.
In practice, it is common that clinicians select the treatment for a particular patient on the basis of individual characteristics (such as age, severity of disease, or type of anatomy). It follows that the type of treatment that patients receive can be influenced by many factors in a nonrandomized observational study. We have specifically analyzed RCTs, because these represent the highest level of evidence for comparing a treatment with a control. A key factor is that RCTs contain a random mechanism that controls treatment assignment, which limits bias in patient selection.10
One aim of a well-conducted meta-analysis is to identify causes of heterogeneity. We found that outlying values, size of the study, study design, and different statin protocols are important factors to consider. The effect that the study by Pan and colleagues30 caused on our meta-analysis is illustrated by the asymmetry of the funnel plot in Figure 4A. Of note, exclusion of that study from the analysis resulted in a much more symmetric funnel plot with a nonsignificant heterogeneity. The significant heterogeneity between all 10 studies is likely to be a result of the underlying clinical heterogeneity or, less likely, due to chance. Despite the degree of heterogeneity, it would be wrong to consider the conclusions to be automatically misleading. Even were our meta-analysis without significant heterogeneity, it can never be interpreted as direct evidence in favor of statin use, because tests that look for heterogeneity have low power in detecting even a moderate degree of genuine heterogeneity that is statistically significant.10,41
The most important contributing effects of statins are their anti-inflammatory properties and their role in the promotion of improved endothelial function. Several investigators have examined anti-inflammatory treatment in AF. Favorable effects have been achieved by use of angiotensin-converting enzyme inhibitors, b-blocking agents, oral glucocorticoids, and nonsteroidal anti-inflammatory medications.8
With respect to statin treatment, data emerging from animal and human studies apply to the protective effects of statins against AF risk. Studies have correlated C-reactive protein (CRP) with AF. C-Reactive protein is an acute-phase reactant that reflects low-grade systemic inflammation and acts as a clinical marker. Kumagai and colleagues42 reported a canine sterile-pericarditis model, and Shiroshita-Takeshita and associates43,44 reported atrial rapid-pacing and ventricular tachycardic canine models that showed a reduced inducibility and sustainability of the arrhythmia and reduced CRP levels when compared with the control group (which experienced shortened intra-atrial conduction time and AF duration). Furthermore, large trials20,45,46 have shown that statins effectively and rapidly lower CRP levels in hyper- and normocholesterolemic patients alike, and reduce the production of pro-inflammatory cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6.20,47 This indicates that statins are effective in decreasing systemic inflammation.
Impaired endothelium-dependent vasodilation and ischemia–reperfusion injury are associated with AF20,48 and lead to impaired left ventricular function.20,47 Because statin treatment has improved endothelial function in experimental models of heart failure, it follows that this effect may influence the incidence of AF.20,47,49
A dosage-dependent effect of statins has been suggested. Lertsburapa and co-authors26 achieved the greatest effect on postoperative AF with the administration of 40 mg of atorvastatin (30 min–14 hr) when compared with no statin treatment. Atorvastatin dosages of 20 to 40 mg and 20-mg equivalents also conferred a statistically nonsignificant reduction. According to Kourliouros and colleagues,50 statins exhibit pleiotropic properties, whereas the division of statin dosages to high, intermediate, and low26 in clinical practice is on the basis of their lipid-lowering efficacy. This random division may have influenced the accuracy of some results.50 Statins have a dosage-related effect on postoperative AF.25 However, by propensity-score analysis, it was shown that the antifibrillatory effect of statins did not match their lipid-lowering capacity.50 For example, simvastatin 20 mg (which is equivalent to atorvastatin 10 mg in lipid-lowering efficacy), had a statistically significant effect on postoperative AF (30 min–3 hr) when compared with no statins, whereas atorvastatin 10 mg had no impact on AF.25,50 Unfortunately, when we considered all 10 of our selected studies, the different statins and dosages ensured heterogeneous results, and no definitive conclusions could be reached.
Study Limitations
The chief limitation of our meta-analysis is that clinical and statistical heterogeneity are introduced because of AF's pathophysiologic complexity. To overcome this degree of heterogeneity, it would be necessary to devise a blocked RCT design, which would be expensive, time-consuming, and impractical. Instead, in order to minimize heterogeneity, we conducted subgroup analyses of the 4 RCTs and of the studies that contained 6 or more matched criteria. Furthermore, in using the 7 specified AF risk factors to evaluate the incidence of postoperative AF, we believe that we effectively reduced the statistical heterogeneity of our comparative analysis.
Different statins and dosages were used in the studies. The meta-analysis included only 4 RCTs from which to draw the main conclusions. We were unable to determine the influence of the duration and dosage of preoperative statin therapy and its postoperative effects on the incidence of AF. The incidence of new postoperative AF depended upon the type and duration of postoperative AF-monitoring techniques, which may have varied between studies. Definitions of postoperative AF were inconsistent from study to study. Operations were performed by different surgeons who used a variety of equipment, conduits, and surgical and anesthetic techniques, which increased clinical heterogeneity in the groups of patients. Finally, we did not evaluate postoperative pulmonary complications, bleeding, and pericardial tamponade, all of which can cause hypoxemia and an increased inotropic requirement, and therefore an increase in AF incidence.
Conclusions
Considerable evidence, including our meta-analysis, points to an effect of statins in decreasing the incidence of postoperative AF. This effect is independent of their main lipid-lowering function, and we have outlined certain mechanisms that could explain this. The results of our analysis suggest the desirability of a large-scale, multicenter, prospective RCT of statin treatment versus nontreatment, with the development of postoperative AF the primary endpoint. Secondary aims should include identifying the type and dosage of statin and duration of use that will reduce postoperative AF most effectively. This would be necessary before definitive conclusions could be drawn regarding whether specific statins and dosages reduce the incidence of postoperative AF in patients who undergo cardiac surgery and any implementation of these results in surgical practice.
Acknowledgment
We thank Francisco Marin, MD, PhD, for access to data from his publication.27
Footnotes
Address for reprints: Srdjan Saso, MBBS, BSc, Department of Biosurgery & Surgical Technology, Imperial College Healthcare Trust, 10th fl., QEQM Wing, St. Mary's Campus, Praed St., London W2 1NY, UK
E-mail: srdjan.saso@imperial.ac.uk
Source of support: NIHR Biomedical Research Centre Funding Scheme
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82 (8A):2N–9N. [DOI] [PubMed]
- 2.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56(3):539–49. [DOI] [PubMed]
- 3.Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis 1989;31(5):367–78. [DOI] [PubMed]
- 4.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004;291(14):1720–9. [DOI] [PubMed]
- 5.Ferguson TB Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL; STS National Database Committee. A decade of change–risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg 2002;73(2):480–90. [DOI] [PubMed]
- 6.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997;226(4):501–13. [DOI] [PMC free article] [PubMed]
- 7.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery [published erratum appears in N Engl J Med 1997;337(3):209]. N Engl J Med 1997;336(20):1429–34. [DOI] [PubMed]
- 8.Sanchez-Quinones J, Marin F, Roldan V, Lip GY. The impact of statin use on atrial fibrillation. QJM 2008;101(11):845–61. [DOI] [PubMed]
- 9.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94(3):390–7. [DOI] [PubMed]
- 10.Athanasiou T, Aziz O, Mangoush O, Al-Ruzzeh S, Nair S, Malinovski V, et al. Does off-pump coronary artery bypass reduce the incidence of post-operative atrial fibrillation? A question revisited. Eur J Cardiothorac Surg 2004;26(4):701–10. [DOI] [PubMed]
- 11.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110(9): 1042–6. [DOI] [PubMed]
- 12.Tsang TS, Miyasaka Y, Barnes ME, Gersh BJ. Epidemiological profile of atrial fibrillation: a contemporary perspective. Prog Cardiovasc Dis 2005;48(1):1–8. [DOI] [PubMed]
- 13.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of ‘not-so-lone atrial fibrillation’. Europace 2008;10(6):668–73. [DOI] [PubMed]
- 14.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol 2008;51(5):585–94. [DOI] [PubMed]
- 15.Jahangiri M, Weir G, Mandal K, Savelieva I, Camm J. Current strategies in the management of atrial fibrillation. Ann Thorac Surg 2006;82(1):357–64. [DOI] [PubMed]
- 16.Schoonderwoerd BA, Van Gelder IC, Van Veldhuisen DJ, Van den Berg MP, Crijns HJ. Electrical and structural remodeling: role in the genesis and maintenance of atrial fibrillation. Prog Cardiovasc Dis 2005;48(3):153–68. [DOI] [PubMed]
- 17.Peters NS, Schilling RJ, Kanagaratnam P, Markides V. Atrial fibrillation: strategies to control, combat, and cure. Lancet 2002;359(9306):593–603. [DOI] [PubMed]
- 18.Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol 2008;31(2):55–62. [DOI] [PMC free article] [PubMed]
- 19.Otway R, Vandenberg JI, Fatkin D. Atrial fibrillation–a new cardiac channelopathy. Heart Lung Circ 2007;16(5):356–60. [DOI] [PubMed]
- 20.Adam O, Neuberger HR, Bohm M, Laufs U. Prevention of atrial fibrillation with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Circulation 2008;118(12):1285–93. [DOI] [PubMed]
- 21.Virani SS, Nambi V, Razavi M, Lee VV, Elayda M, Wilson JM, Ballantyne CM. Preoperative statin therapy is not associated with a decrease in the incidence of postoperative atrial fibrillation in patients undergoing cardiac surgery. Am Heart J 2008;155(3):541–6. [DOI] [PubMed]
- 22.Powell BD, Bybee KA, Valeti U, Thomas RJ, Kopecky SL, Mullany CJ, Wright RS. Influence of preoperative lipid-lowering therapy on postoperative outcome in patients undergoing coronary artery bypass grafting. Am J Cardiol 2007;99(6): 785–9. [DOI] [PubMed]
- 23.Chello M, Patti G, Candura D, Mastrobuoni S, Di Sciascio G, Agro F, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med 2006;34(3):660–7. [DOI] [PubMed]
- 24.Auer J, Weber T, Berent R, Lamm G, Ng CK, Hartl P, et al. Use of HMG-coenzyme a-reductase inhibitors (statins) and risk reduction of atrial fibrillation after cardiac surgery: results of the SPPAF study, a randomised placebo-controlled trial [abstract]. Eur Heart J 2004;25(Suppl):353.
- 25.Kourliouros A, De Souza A, Roberts N, Marciniak A, Tsiouris A, Valencia O, et al. Dose-related effect of statins on atrial fibrillation after cardiac surgery. Ann Thorac Surg 2008;85(5): 1515–20. [DOI] [PubMed]
- 26.Lertsburapa K, White CM, Kluger J, Faheem O, Hammond J, Coleman CI. Preoperative statins for the prevention of atrial fibrillation after cardiothoracic surgery. J Thorac Cardiovasc Surg 2008;135(2):405–11. [DOI] [PubMed]
- 27.Marin F, Pascual DA, Roldan V, Arribas JM, Ahumada M, Tornel PL, et al. Statins and postoperative risk of atrial fibrillation following coronary artery bypass grafting. Am J Cardiol 2006;97(1):55–60. [DOI] [PubMed]
- 28.Mariscalco G, Lorusso R, Klersy C, Ferrarese S, Tozzi M, Vanoli D, et al. Observational study on the beneficial effect of preoperative statins in reducing atrial fibrillation after coronary surgery. Ann Thorac Surg 2007;84(4):1158–64. [DOI] [PubMed]
- 29.Ozaydin M, Dogan A, Varol E, Kapan S, Tuzun N, Peker O, et al. Statin use before by-pass surgery decreases the incidence and shortens the duration of postoperative atrial fibrillation. Cardiology 2007;107(2):117–21. [DOI] [PubMed]
- 30.Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004;110(11 Suppl 1):II45-9. [DOI] [PubMed]
- 31.Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation 2006;114(14):1455–61. [DOI] [PubMed]
- 32.Song YB, On YK, Kim JH, Shin DH, Kim JS, Sung J, et al. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off-pump coronary artery bypass grafting surgery. Am Heart J. 2008 Aug;156(2):373.e9-16. [DOI] [PubMed]
- 33.Cochrane Reviewers' Handbook. The Cochrane Collaboration; Issue 3. Oxford (UK): Update Software; 2001.
- 34.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354(9193):1896–900. [DOI] [PubMed]
- 35.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27(5):335–71. [DOI] [PubMed]
- 36.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22(4):719–48. [PubMed]
- 37.Systematic reviews in health care: meta-analysis in context. Egger M, Smith GD, Altman DG, editors. 2nd ed. London: BMJ Publishing Group; 1995.
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–88. [DOI] [PubMed]
- 39.Stamou SC, Jablonski KA, Pfister AJ, Hill PC, Dullum MK, Bafi AS, et al. Stroke after conventional versus minimally invasive coronary artery bypass. Ann Thorac Surg 2002;74(2): 394–9. [DOI] [PubMed]
- 40.England MR, Gordon G, Salem M, Chernow B. Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA 1992;268(17):2395–402. [PubMed]
- 41.Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet 1991;338(8775):1127–30. [DOI] [PubMed]
- 42.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res 2004;62(1):105–11. [DOI] [PubMed]
- 43.Shiroshita-Takeshita A, Brundel BJ, Burstein B, Leung TK, Mitamura H, Ogawa S, Nattel S. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res 2007;74(1):75–84. [DOI] [PubMed]
- 44.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004;110(16):2313–9. [DOI] [PubMed]
- 45.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352(1): 29–38. [DOI] [PubMed]
- 46.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352(1):20–8. [DOI] [PubMed]
- 47.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005;45:89–118. [DOI] [PMC free article] [PubMed]
- 48.Takahashi N, Ishibashi Y, Shimada T, Sakane T, Ohata S, Sugamori T, et al. Impaired exercise-induced vasodilatation in chronic atrial fibrillation–role of endothelium-derived nitric oxide. Circ J 2002;66(6):583–8. [DOI] [PubMed]
- 49.Di Napoli P, Taccardi AA, Grilli A, De Lutiis MA, Barsotti A, Felaco M, De Caterina R. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc Res 2005;66(3):462–71. [DOI] [PubMed]
- 50.Kourliouros A, Roberts N, Jahangiri M. Statins with equivalent lipid-lowering capacity exhibit differential effects on atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg 2008;136(4):1100–1. [DOI] [PubMed]