Abstract
Metabolic syndrome is associated with intravascular inflammation, as determined by increased levels of inflammatory biomarkers and an increased risk of ischemic atherothrombotic events. Evidence suggests that atherothrombosis and intravascular inflammation share predictive biomarkers, including high-sensitivity C-reactive protein, CD40 ligand, P-selectin, and N-terminal pro-brain natriuretic peptide.
Patients who had metabolic syndrome were randomized to receive clopidogrel 75 mg/day plus aspirin 81 mg/day (n = 89) or placebo plus aspirin 81 mg/day (n = 92) for 9 weeks to assess the efficacy of each treatment in suppression of inflammatory markers. Change from baseline in the levels of high-sensitivity C-reactive protein, CD40 ligand, P-selectin, and N-terminal pro-brain natriuretic peptide at 6 weeks was assessed to evaluate each treatment.
There was a significant difference at Week 6 in model-adjusted CD40-ligand levels in favor of clopidogrel plus aspirin compared with placebo plus aspirin in both the intent-to-treat population (difference between least-squares means = −186.5; 95% confidence interval, −342.3 to −30.8; P = 0.02) and the per-protocol population (P = 0.05). No significant differences were observed between the treatment arms for high-sensitivity C-reactive protein, P-selectin, and N-terminal pro-brain natriuretic peptide. There were no deaths or serious adverse events in either treatment arm.
Data from this study suggest that clopidogrel can decrease the expression of the CD40-ligand biomarker.
Key words: Aspirin/therapeutic use; biological markers/blood; CD40 ligand/drug effects; clopidogrel/therapeutic use; C-reactive protein/drug effects; inflammation; metabolic syndrome X; platelet aggregation inhibitors/therapeutic use; probrain natriuretic peptide, N-terminal/drug effects; P-selectin/drug effects
Cumulative evidence concerning predictive biomarkers for atherothrombosis, including high-sensitivity C-reactive protein (hs-CRP), CD40 ligand, soluble P-selectin, and N-terminal pro-brain natriuretic peptide (NT-proBNP), suggests that these are also markers of intravascular inflammation.1–6 It is not completely clear whether lowering the levels of these marker proteins also lowers cardiovascular risk.
Currently available therapies for patients at risk of, or with clinically manifest, atherothrombotic disease are effective in reducing levels of these biomarkers. Statins lower levels of hs-CRP by approximately 15% to 37%, regardless of baseline hs-CRP levels and duration of treatment.7–10 Indeed, results of the JUPITER trial (Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin)7 showed that lowering hs-CRP levels with use of rosuvastatin significantly reduced the risk of adverse cardiovascular events in persons with elevated hs-CRP levels who were otherwise healthy.
Use of the antiplatelet agent clopidogrel prior to percutaneous coronary intervention (PCI) has also been shown to attenuate periprocedural increases in hs-CRP levels.11 In 1 study, patients with the highest pre-PCI quartile of hs-CRP (>5 mg/L) had a significant reduction in death or reinfarction at 30 days.12 In addition to lowering hs-CRP levels, clopidogrel has also been shown to decrease levels of CD40 ligand and P-selectin expressed by platelets.13–15
Patients who have metabolic syndrome (that is, ≥3 of the following: large waist circumference, hypertriglyceridemia, hypertension, or abnormally high fasting plasma glucose levels) are at risk for diabetes mellitus and ischemic events.16–21 Furthermore, elevated CRP levels are significantly associated with metabolic syndrome22 and are also significant predictors of diabetes and metabolic syndrome.23 Given the treatment benefits of clopidogrel toward reducing the risk of ischemic events in patients with diabetes24 and reducing levels of inflammatory markers,11–15 patients with metabolic syndrome are an ideal population in which to evaluate the effects of clopidogrel therapy on CRP levels and other inflammatory markers.
PROCLAIM (A Pilot Study to Examine the Effects of Clopidogrel Compared to Placebo on Markers of Inflammation in Subjects with Metabolic Syndrome Who Are Receiving Background Therapy, including Low-Dose Aspirin) was a Phase IV multicenter, double-blind, randomized clinical trial that compared the effect of clopidogrel plus aspirin versus placebo plus aspirin on levels of hs-CRP, CD40 ligand, soluble P-selectin, and NT-proBNP in patients with metabolic syndrome and elevated levels of hs-CRP (≥2 mg/L and ≤10 mg/L) at baseline. A 2nd goal of PROCLAIM was to evaluate adjusted mean changes from baseline in these biomarkers between treatment arms.
Patients and Methods
A total of 181 consenting and eligible patients were enrolled in the PROCLAIM study at 55 sites in the United States between 2 November 2005 and 14 June 2006. The study duration for each enrolled subject was approximately 9 weeks.
Patients
Subjects were eligible for enrollment if they were ≥18 years of age with metabolic syndrome, had been on stable medications for 6 weeks prior to entry, had experienced an atherothrombotic vascular event or cardiovascular intervention ≥6 months earlier, and had an hs-CRP level between 2 mg/L and 10 mg/L at screening. Diagnostic criteria for metabolic syndrome included having ≥3 of the 5 National Cholesterol Education Program criteria for metabolic syndrome: triglycerides ≥150 mg/dL, systolic blood pressure ≥130 mmHg and diastolic blood pressure >85 mmHg, fasting blood glucose ≥110 mg/dL, waist circumference >101.6 cm (40 in) for men and >88.9 cm (35 in) for women, and high-density lipoprotein cholesterol <40 mg/dL for men and <50 mg/dL for women. All women were required to use a medically accepted, effective method of contraception for the duration of the study.
The main exclusion criteria were intolerance or contraindication to the use of clopidogrel or aspirin; platelet count <100,000/mm3; use of any of the following medications within the last 3 months: oral anticoagulants, dipyridamole, ticlopidine, clopidogrel, oral glucocorticoids, daily aspirin >81 mg/day or chronic nonsteroidal anti-inflammatory agents, use of oral hormone-replacement therapy, oral contraceptives, or transdermal contraceptive patch; history of pathologic bleeding (e.g., peptic ulcer or intracranial hemorrhage); history of chronic inflammatory disease or any recent medical event resulting in tissue injury, infection, or inflammation; myocardial infarction, coronary artery bypass graft, or angioplasty within the 6 months before screening; uncontrolled hypertension (systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥100 mmHg); history of cancer in the past 2 years (except for successfully resected basal-cell carcinoma of the skin); alcohol or substance abuse within 12 months before screening; and current or recent (within 30 days before screening) use of other investigational study medication. Informed, written consent was obtained from all subjects before any study-related procedures were performed. The informed consent form was in compliance with U.S. Food and Drug Administration regulations and was reviewed and approved by each participating institution's Institutional Review Board or Ethics Committee. Consenting patients who met all of the inclusion and none of the exclusion criteria were enrolled in the study.
Treatment
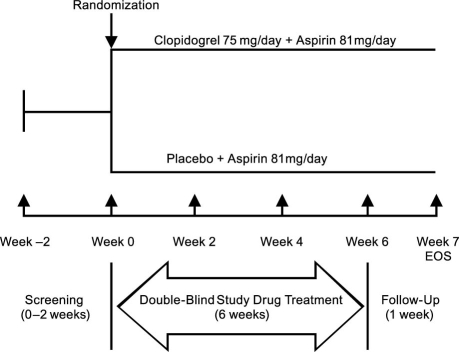
The total study duration for each subject was approximately 9 weeks. Figure 1 outlines the overall study design. From Weeks −2 to 0, patients were screened for study eligibility. At Week 0, consenting, eligible patients were randomly assigned to receive blinded treatment with clo<?__anchored_object__ “ro_u5accins1da2”?><?__anchored_object__ “ro_u5accins1da3”?><?__anchored_object__ “ro_u5accins1da3”?><?__anchored_object__ “ro_u5accins1da4”?>pidogrel 75 mg/day plus aspirin 81 mg/day or placebo plus aspirin 81 mg/day. Randomization to the 2 study arms occurred in a 1:1 ratio using permuted blocks of size 4, and subjects were assigned unique identification numbers. Vital signs were measured again, study medication was dispensed according to the regimen assigned, and blood samples were obtained for baseline measurement of markers of inflammation and platelet activation. All subjects were instructed to take their medications at the same time each day (±1 hour) and to return all unused medication for compliance assessment. At Weeks 2, 4, and 6, unused medication was collected; vital signs were measured; subjects were surveyed for adverse events; and blood samples were obtained for hematology, serum chemistries, and markers of inflammation and platelet activation. At Week 7, subjects returned for their final study visit, at which time a brief physical examination was performed, and adverse events were assessed.
Fig. 1. Schematic representation of the overall study design.
EOS = end of study
Evaluation
For both treatment arms, efficacy variables included point estimates of the median and mean change from baseline to Week 6 in hs-CRP, CD40-ligand, soluble P-selectin, and NT-proBNP levels, and estimates of the treatment differences in model-adjusted mean changes from baseline for each biomarker. In addition to these biomarkers, laboratory variables included hematology (hemoglobin, hematocrit, red blood count, white blood count, platelet count, hemoglobin A1c, fasting glucose, and lipids), chemistry (blood urea nitrogen, creatinine, sodium, potassium, chloride, bicarbonate, aspartate aminotransferase, alanine aminotransferase, and total bilirubin), and urinalysis.
At each visit, subjects were carefully evaluated and questioned for signs and symptoms of adverse events, and any clinically significant changes in the physical examination or laboratory findings were recorded as an adverse event. The investigator described the nature, severity, and duration of each event, its relationship to the study medication (no relation, unlikely, unknown, or likely), and the outcome.
Analyses
The planned sample size was driven primarily by the desired precision of the parameter estimates for endpoints and associated variances. Using an expected standard deviation of 1.5, it was estimated that to achieve a 100(1–α)% interval of size 0.8 around the difference between mean change from baseline, where α=0.05, it would be necessary for 108 patients from each treatment arm to complete the study. Assuming a post-randomization attrition rate of 40%, it was estimated that an enrollment of 180 patients per treatment arm, or 360 patients total, would be needed.
The intent-to-treat population included all randomized subjects. The per-protocol population included all subjects in the intent-to-treat population who did not violate any major entry condition that was likely to confound the assessment of efficacy and who did not deviate substantially from the protocol between randomization and study completion. Bias-corrected bootstrap point estimates for the efficacy variables were calculated. The associated bias-corrected bootstrap confidence intervals (CIs) were also calculated, using the 2.5% and 97.5% percentiles constructed from 1,000 bootstrap samples.25
In evaluating and comparing the effect of the treatment arms on each biomarker, analysis of covariance (ANCOVA) models were constructed to obtain least-squares mean estimates for each treatment (adjusted for the average value of the specified covariates in the model), and differences between least-squares mean estimates were calculated, along with associated 95% CIs and P values. In addition to a treatment variable, prespecified model covariates included baseline patient characteristics and clinical variables. Patients' characteristics included age, sex, and race/ethnicity (African American/not African American; Asian/not Asian; and Hispanic-Latino/not Hispanic-Latino). Age was entered as a continuous variable. Clinical variables included baseline biomarker levels, triglycerides, blood pressure (systolic and diastolic), fasting glucose, waist circumference, high-density lipoprotein cholesterol, and body mass index. In addition, 2 dummy variables were included to capture qualitative changes in the inclusion criteria. The 1st of these captured the effect of a change in the inclusion criterion that required subjects to have a stable medication regimen from 6 weeks to 3 months prior to enrollment. The 2nd was included to capture the effect of a protocol amendment to the inclusion criterion that prohibited the enrollment of asymptomatic patients without a previous atherothrombotic event (a consequence of findings from the CHARISMA trial [Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance],26 which were released during the PROCLAIM study period). A dummy variable capturing the class of qualifying symptomatic events was also included in the model, as was a dummy variable to reflect the achievement of 80% to 120% compliance in study medication use by subjects. All interval and ratio level baseline measures were defined using the arithmetic average of visits 1 and 2. Subjects with missing biomarker data at baseline were not included in the estimation of efficacy variables. A last-observation-carried-forward approach was used for the analysis of primary and secondary endpoints. SAS version 9.0 (SAS Institute, Inc.; Cary, NC) was used for all calculations.
Results
Patients
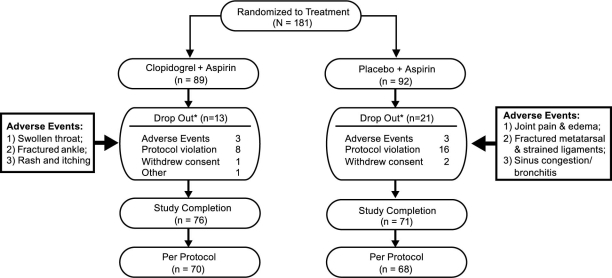
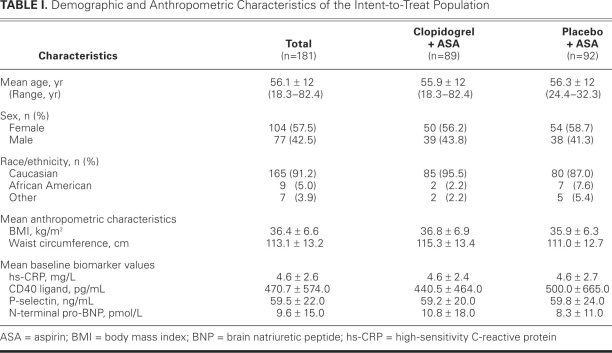
A total of 181 patients were enrolled in the study, 89 ofwhom were assigned to the clopidogrel plus aspirin groupand 92, to the placebo plus aspirin group. Among these, 34 (19%) discontinued the study before taking a singledose of the study agent: 13 (38%) in the clopidogrel plus aspirin treatment arm and 21 (62%) in the placebo plus aspirin arm. Figure 2 depicts the flow of patients from enrollment to final disposition, including the number and reasons for discontinuation in each group. Overall, baseline characteristics were similar between the 2 treatment groups (Table I). The entire study population was 56.1 years old on average and predominantly Caucasian (91.2%); a little over half were women (57.5%).
Fig. 2. Study population flow through the study.
*Discontinued on Day 1 before taking a single dose of study agent
TABLE I. Demographic and Anthropometric Characteristics of the Intent-to-Treat Population
Biomarkers: Individual Treatment Point Estimates of Change
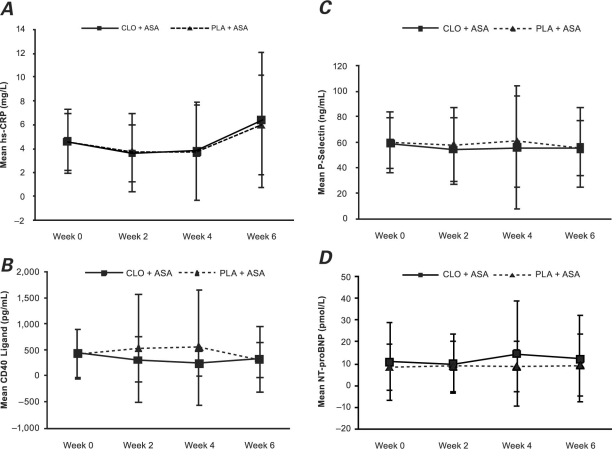
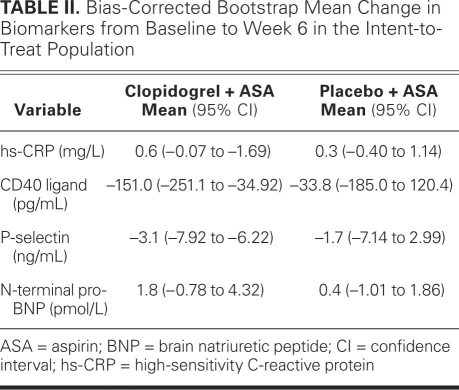
High-Sensitivity C-Reactive Protein. Evidence shown in Figure 3A suggests that, in both treatment arms, hs-CRP levels were above the threshold value of ≥2 mg/L for study eligibility at each time point, with a trend toward increasing levels at Week 6 in both the intent-to-treat and per-protocol populations (not presented). Bias-corrected bootstrap estimates of mean changes in hs-CRP from baseline to Week 6 in the intent-to-treat population were similar in the 2 treatment groups at 0.6 mg/L (95% CI, −0.07 to 1.69 mg/L) for the clopidogrel plus aspirin group and 0.3 mg/L (95% CI, −0.4 to 1.14 mg/L) for the placebo plus aspirin group (Table II).
Fig. 3. Mean values and standard deviations at baseline (Week 0) and Weeks 2, 4, and 6 for A) hs-CRP, B) CD40 ligand, C) P-selectin, and D) NT-proBNP in the intent-to-treat population.
ASA = aspirin; CLO = clopidogrel; hs-CRP = high-sensitivity C-reactive protein; NT-proBNP = N-terminal pro-brain natriuretic peptide; PLA = placebo
TABLE II. Bias-Corrected Bootstrap Mean Change in Biomarkers from Baseline to Week 6 in the Intent-to-Treat Population
CD40 Ligand. Estimates of mean changes in CD40 ligand from baseline to Week 6 in the intent-to-treat population were −151 pg/mL (95% CI, −251.1 to −34.92 pg/mL) for the clopidogrel plus aspirin group and −33.8 pg/mL (95% CI, 185 to 120.4 pg/mL) for the placebo plus aspirin group (Table II, Fig. 3B). These changes were similar in the per-protocol population (not presented).
P-Selectin. Estimates of mean changes in P-selectin from baseline to Week 6 in the intent-to-treat population were somewhat larger in the clopidogrel plus aspirin group (–3.1 ng/mL; 95% CI, −7.92 to 6.22 ng/mL) than in the placebo plus aspirin group (–1.7 ng/mL; 95% CI, −7.14 to 2.99 ng/mL) (Table II). Figure 3C, which depicts means at baseline and at Weeks 2, 4, and 6, suggests that the profile of P-selectin was similar in the 2 treatment arms.
N-Terminal Pro-Brain Natriuretic Peptide. For the NT-proBNP biomarker, the estimates of mean changes from baseline to Week 6 in the clopidogrel plus aspirin treatment group were 1.8 pmol/L (95% CI, −0.78 to 4.32) and in the placebo plus aspirin treatment group, 0.4 pmol/L (95% CI, −1.01 to 1.86) (Table II, Fig. 3D).
Treatment Comparison
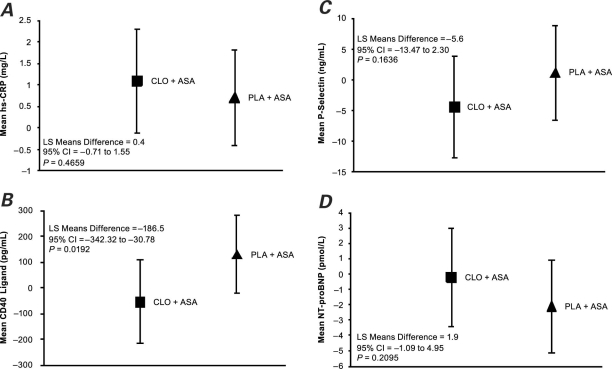
The comparison of treatment effects indicated a significant difference at Week 6 in model-adjusted CD40-ligand levels in favor of clopidogrel plus aspirin (Fig. 4B) compared with placebo plus aspirin in both the intent-to-treat population (difference between least-squares means = −186.5; 95% CI, −342.3 to −30.8; P = 0.02) and the per-protocol population (P = 0.05) (not presented). No significant differences between the study arms were found for hs-CRP, P-selectin, and NT-proBNP(Figs. 4A, 4C, and 4D).
Fig. 4. Change in biomarkers based on a multivariate model analysis from baseline (Week 0) to Week 6 in the levels of A) hs-CRP, B) CD40 ligand, C) P-selectin, and D) NT-proBNP in the intent-to-treat population. Values represent least-squares means (LS means) with SE.
ASA = aspirin; CLO = clopidogrel; hs-CRP = high-sensitivity C-reactive protein; NT-proBNP = N-terminal pro-brain natriuretic peptide; PLA = placebo
Safety
No subjects died during this study, there were no serious adverse events, and all treatment-related adverse events were of mild-to-moderate severity. Six adverse events (3 in each group) led to treatment discontinuation (Fig. 2). Approximately half of the patient population in each study arm experienced an adverse event: gastrointestinal disorders, along with infections and infestations, were the most commonly reported (17% in the clopidogrel group and 9% in the placebo group). No patients experienced internal bleeding. Minor adverse events that were observed only in the clopidogrel plus aspirin group included immune hypersensitivity, seasonal allergy, hematuria, and renal failure. The adverse events that were observed only in the placebo plus aspirin group included peripheral edema, cardiac palpitations, dyslipidemia, and spinal stenosis. All adverse events lasted only for the duration of the study.
Results of laboratory tests at Week 6 were similar in both treatment groups with respect to hematocrit, hemoglobin, platelet count, and red and white blood cell counts. Basophils were negative in both study groups at baseline and at the end of treatment, and there were minimal changes in eosinophils, lymphocytes, monocytes, and neutrophils. Physical examination, blood chemistry, and urinalysis evaluations were similarly unremarkable, with the exception of a slight change in urine pH in both treatment groups observed at Week 6 (pH, 5.2), compared with baseline (pH, 5.4).
Discussion
It has been well established that higher levels of inflammatory markers, such as hs-CRP, are significant predictors of cardiovascular events.23 PROCLAIM was a pilot study designed to examine the effects of clopidogrel 75 mg/day plus aspirin 81 mg/day compared with placebo plus aspirin 81 mg/day on 4 inflammatory biomarkers—hs-CRP, CD40 ligand, P-selectin, and NT-proBNP—in patients who had metabolic syndrome and elevated hs-CRP levels at study baseline. Enrollment criteria, however, were amended after publication of the CHARISMA study,26 which showed that asymptomatic patients with multiple atherothrombotic risk factors did not benefit from the addition of clopidogrel to aspirin. The amended enrollment criteria allowed only symptomatic subjects with a history of an atherothrombotic vascular event or a cardiovascular intervention more than 6 months earlier to be included. Because enrollment proceeded at an extremely slow pace, a decision was made to terminate enrollment early in the study, at 181 patients instead of the initially estimated 360 patients.
In both the intent-to-treat and per-protocol populations, subjects in the clopidogrel plus aspirin arm, compared with those in the placebo plus aspirin arm, had significantly greater reductions in model-adjusted mean changes in CD40-ligand levels from baseline to Week 6.CD40 is a ligand expressed on the membranes of activated platelets, immune cells, and the vasculature. It is cleaved to generate the soluble hydrolytic fragment sCD401, nearly all of which is derived from platelets.27 The interaction of CD40 with its ligand initiates inflammatory responses that are crucial to the pathogenesis of acute or subacute thrombogenic complications in some acute coronary syndrome patients.28–30 Because sCD40 ligand is a prothrombic and proinflammatory molecule, it is often elevated, along with CRP, in patients with acute coronary syndrome or cardiovascular risk factors.27,31–33 In addition, high sCD40-ligand levels have been associated with late restenosis after PCI, and some data have shown that this biomarker is markedly increased 24 hours after PCI.34,35 In patients with unstable angina who had undergone coronary stenting, the use of clopidogrel (300-mg loading dose followed by 75-mg daily maintenance) significantly decreased sCD40-ligand levels 24 hours after stenting (P <0.01), and levels were further decreased at 7 days (P <0.0001).36 Decreases were sustained up to 90 days,36 with a rebound increase after clopidogrel discontinuation.37 Patients in the active group had high circulating levels of sCD40 ligand. In a randomized study of 73 patients with stable coronary artery disease, use of clopidogrel for 8 weeks significantly decreased sCD40-ligand levels from baseline (P = 0.03), but levels remained unchanged in the placebo group. The greatest decreases were seen in patients who had higher circulating levels of sCD40 ligand; decreases were less in patients who were receiving statins and aspirin. Levels of hs-CRP, however, were not affected by clopidogrel.38 These data suggest that the effects of clopidogrel are more pronounced in patients who are not receiving concomitant statins or aspirin and in patients with higher baseline levels of sCD40 ligand. The results of PROCLAIM support this suggestion, since patients in this study were already receiving extensive therapy at baseline, including aspirin and statins, which are known to reduce CD40-ligand levels.39 Thus, it is possible that the effects of clopidogrel on CD40 ligand and other biomarkers were attenuated by the use of aspirin, statins, or both. It is also possible that the large variability observed in the treatment effects (Fig. 4) masked any significant changes.
In contrast, a recent study found no effect of clopidogrel plus aspirin on CD40-ligand or P-selectin levels in patients with chronic angina,40 whereas another study found a reduction in elevated P-selectin levels with the administration of clopidogrel plus aspirin.41 Activated platelets express platelet selectin, and a derivative of this molecule is secreted into the plasma in a soluble form, P-selectin, which has been used in clinical trials as an in vivo measurement of platelet activity in patients with acute coronary syndrome.42,43 The results of our study suggest an influence of clopidogrel on P-selectin levels; however, the change did not reach statistical significance. This lack of significance may be explained in part by baseline levels of inflammatory biomarkers in the study population. Previous evidence indicates that the effects of clopidogrel on some inflammatory markers are more pronounced in patients who have elevated baseline levels of these biomarkers.44,45 In a study of patients with non–ST-segment-elevation acute coronary syndrome, no differences were found in P-selectin levels between patients who received clopidogrel plus aspirin and those who received aspirin monotherapy, with the exception of a significant elevation at 8 hours in the aspirin monotherapy group compared with the dual antiplatelet therapy group. In contrast, patients with evidence of greater inflammation (indicated by higher levels of hs-CRP and sCD40 ligand) had significant reductions in P-selectin and outcomes that were significantly less adverse with clopidogrel plus aspirin therapy compared with aspirin therapy alone.45
We also examined brain natriuretic peptide (BNP). An increase in plasma levels of this biomarker is related to an increase in left ventricular filling pressure, which is seen mostly in patients with chronic heart failure46 and is associated with increased mortality rates.47,48 Aspirin has been shown to increase BNP levels, whereas the addition of clopidogrel attenuates this increase.44 Since the effects of aspirin on BNP levels are observed primarily in patients with more advanced disease (that is, higher baseline BNP levels),44 the lack of change in this biomarker in the current study may not be surprising.
Data from the 2 PROCLAIM study groups indicate that both treatments—clopidogrel plus aspirin, and placebo plus aspirin—are safe in this patient population. There were no deaths or serious adverse events during this study, and all treatment-related adverse events were of mild-to-moderate severity. Extensive laboratory testing and physical examination variables were consistent and unremarkable in both groups.
This pilot study confirms previous reports that treatment with clopidogrel reduces levels of CD40 ligand and suggests an influence on the expression of P-selectin in patients with atherothrombosis. A larger trial conducted over a longer time interval might have shown more changes in the inflammatory markers of interest and might have reduced the large degree of variability noted in the model-adjusted treatment comparisons. Nonetheless, these effects provide further insight into the mechanisms by which clopidogrel confers protection in patients with atherothrombosis. To fully assess whether some of the benefit of clopidogrel plus aspirin therapy arises from lowering the expression of inflammatory markers such as CD40 ligand, a larger trial with clinical endpoints conducted over a longer time period is necessary.
Acknowledgments
This manuscript was written and edited by the authors, who take full responsibility for its content. Editorial assistance (coordinating revisions and creating figures and tables) was provided by Susan Abulhawa and was funded by the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership. The authors did not receive any compensation for this work.
References
APPENDIX
Investigators and other important participants in the PROCLAIM (A Pilot Study to Examine the Effects of Clopidogrel Compared to Placebo on Markers of Inflammation in Subjects with Metabolic Syndrome who are Receiving Background Therapy, including Low-Dose Aspirin) study.
Allen Adolphe, MD
Cesar Albarracin, MD
Georges M. Argoud, MD
Anthony J. Bartkowiak, Jr., MD
Andrew Behnke, MD
James Borders, MD
Donald Brandon, MD
Robert Broker, MD
Patricia Buchanan, MD
Dennis K. Buth, MD
Christopher Chappel, MD
Louis Chaykin, MD
Steven Chrysant, MD, PhD
James Compton, MD
Jimmy Durden, MD
Saeed Etezadi, MD
James Fidelholtz, MD
Neil Joseph Fraser, MD
Vicki Greiff, MD
Boyde Harrison, MD
Mark Henderson, MD
Charles Herring, MD
Kenneth Hershon, MD
John Arthur Hoekstra, MD
Alan F. Jacobson, PhD
Mark Kipnes, MD
Sam Lerman, MDCM
Kevin Lutz, MD
Rodney Magargle, MD
James Mersey, MD
Rodney Michaels, MD
John Moloney, MD
Joseph Moran, MD
Jeffrey Morton, MD
Mim Ilene Mulford, MD
Joel Neutel, MD
Andrea Phillips, MD
Julie Poludniak, MD
Michael Reeves, MD
Dennis Riff, MD
Michael Rosemore, DO
Julio Rosenstock, MD
Eli Meyer Roth, MD
Gary Ruoff, MD
Richard Sachson, MD
Jaime Sandoval, MD
Sherwyn Schwartz, MD
Michael Seidner, MD
Malcolm Sperling, MD
Ronald Stegemoller, MD
Charles Stoner, MD
Danny Sugimoto, MD
Louise A. Taber, MD
Raymond Tidman, MD
Melvin Jerome Tonkon, MD
Minou Phuong-Lan Tran, MD
Robert Jay Weiss, MD
Peter Weissman, MD
John Wilker, MD
Footnotes
Address for reprints: James T. Willerson, MD, Texas Heart Institute at St. Luke's Episcopal Hospital, MC 3–116, 6770 Bertner Ave., Houston, TX 77030
E-mail: jwillerson@heart.thi.tmc.edu
*The list of investigators is found in the Appendix.
The PROCLAIM study was sponsored by the Bristol-Myers Squibb and sanofi-aventis Pharmaceuticals Partnership.
Disclosures/Conflict of Interest: Dr. Willerson was the primary investigator of the PROCLAIM study; Dr. Cable is an employee of sanofi-aventis.
References
- 1.Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol 1999;34(5):1512–21. [DOI] [PubMed]
- 2.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001;103(13):1813–8. [DOI] [PubMed]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men [published erratum appears in N Engl J Med 1997;337(5):356]. N Engl J Med 1997;336 (14):973–9. [DOI] [PubMed]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342 (12):836–43. [DOI] [PubMed]
- 5.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347(20):1557–65. [DOI] [PubMed]
- 6.Yeh ET. CRP as a mediator of disease. Circulation 2004;109 (21 Suppl 1):II11-4. [DOI] [PubMed]
- 7.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359(21):2195–207. [DOI] [PubMed]
- 8.Albert MA, Danielson E, Rifai N, Ridker PM; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286(1):64–70. [DOI] [PubMed]
- 9.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001;344(26):1959–65. [DOI] [PubMed]
- 10.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1998;98(9):839–44. [DOI] [PubMed]
- 11.Vivekananthan DP, Bhatt DL, Chew DP, Zidar FJ, Chan AW, Moliterno DJ, et al. Effect of clopidogrel pretreatment on periprocedural rise in C-reactive protein after percutaneous coronary intervention. Am J Cardiol 2004;94(3):358–60. [DOI] [PubMed]
- 12.Chew DP, Bhatt DL, Robbins MA, Mukherjee D, Roffi M, Schneider JP, et al. Effect of clopidogrel added to aspirin before percutaneous coronary intervention on the risk associated with C-reactive protein. Am J Cardiol 2001;88(6):672–4. [DOI] [PubMed]
- 13.Cha JK, Jeong MH, Lee KM, Bae HR, Lim YJ, Park KW, Cheon SM. Changes in platelet P-selectin and in plasma C-reactive protein in acute atherosclerotic ischemic stroke treated with a loading dose of clopidogrel. J Thromb Thrombolysis 2002;14(2):145–50. [DOI] [PubMed]
- 14.Klinkhardt U, Graff J, Harder S. Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and P-selectin expression in a human ex vivo in vitro model. Clin Pharmacol Ther 2002;71(3):176–85. [DOI] [PubMed]
- 15.Quinn MJ, Bhatt DL, Zidar F, Vivekananthan D, Chew DP, Ellis SG, et al. Effect of clopidogrel pretreatment on inflammatory marker expression in patients undergoing percutaneous coronary intervention. Am J Cardiol 2004;93(6):679–84. [DOI] [PubMed]
- 16.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M; Bruneck study. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care 2003; 26(4):1251–7. [DOI] [PubMed]
- 17.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24(4):683–9. [DOI] [PubMed]
- 18.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288(21):2709–16. [DOI] [PubMed]
- 19.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285(19):2486–97. [DOI] [PubMed]
- 20.Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes 2004;53(4):1068–73. [DOI] [PubMed]
- 21.Kawamoto R, Tomita H, Oka Y, Kodama A. Metabolic syndrome as a predictor of ischemic stroke in elderly persons. Intern Med 2005;44(9):922–7. [DOI] [PubMed]
- 22.Bo S, Gentile L, Ciccone G, Baldi C, Benini L, Dusio F, et al. The metabolic syndrome and high C-reactive protein: prevalence and differences by sex in a southern-European population-based cohort. Diabetes Metab Res Rev 2005;21(6):515–24. [DOI] [PubMed]
- 23.Bo S, Gambino R, Uberti B, Mangiameli MP, Colosso G, Repetti E, et al. Does C-reactive protein identify a subclinical metabolic disease in healthy subjects? Eur J Clin Invest 2005; 35(4):265–70. [DOI] [PubMed]
- 24.Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002;90(6):625–8. [DOI] [PubMed]
- 25.An introduction to the bootstrap (monographs on statistics and applied probability). Efron B, Tibshirani RJ, editors. London: Chapman & Hall/CRC; 1993.
- 26.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354(16):1706–17. [DOI] [PubMed]
- 27.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on ac-tivated human platelets is temporally limited by coexpressed CD40. Blood 2001;98(4):1047–54. [DOI] [PubMed]
- 28.Azar RR, McKay RG, Kiernan FJ, Seecharran B, Feng YJ, Fram DB, et al. Coronary angioplasty induces a systemic inflammatory response. Am J Cardiol 1997;80(11):1476–8. [DOI] [PubMed]
- 29.Dibra A, Mehilli J, Braun S, Hadamitzky M, Baum H, Dirschinger J, et al. Association between C-reactive protein levels and subsequent cardiac events among patients with stable angina treated with coronary artery stenting. Am J Med 2003; 114(9):715–22. [DOI] [PubMed]
- 30.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A 1997;94(5):1931–6. [DOI] [PMC free article] [PubMed]
- 31.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med 2002;8(3):247–52. [DOI] [PubMed]
- 32.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 2003;348(12): 1104–11. [DOI] [PubMed]
- 33.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994;331(7):417–24. [DOI] [PubMed]
- 34.Azar RR, Badaoui G, Sarkis A, Kassab R, Salame E, Klayme S, et al. Effects of tirofiban and statins on high-sensitivity C-reactive protein, interleukin-6, and soluble CD40 ligand following percutaneous coronary interventions in patients with stable coronary artery disease. Am J Cardiol 2005;95(2):236–40. [DOI] [PubMed]
- 35.Cipollone F, Ferri C, Desideri G, Paloscia L, Materazzo G, Mascellanti M, et al. Preprocedural level of soluble CD40L is predictive of enhanced inflammatory response and restenosis after coronary angioplasty. Circulation 2003;108(22):2776–82. [DOI] [PubMed]
- 36.Yip HK, Chang LT, Sun CK, Yang CH, Hung WC, Cheng CI, et al. Impact of clopidogrel on suppression of circulating levels of soluble CD40 ligand in patients with unstable angina undergoing coronary stenting. Am J Cardiol 2006;97(2):192–4. [DOI] [PubMed]
- 37.Yip HK, Wu CJ, Yang CH, Chang HW, Fang CY, Hung WC, Hang CL. Serial changes in circulating concentrations of soluble CD40 ligand and C-reactive protein in patients with unstable angina undergoing coronary stenting. Circ J 2005;69 (8):890–5. [DOI] [PubMed]
- 38.Azar RR, Kassab R, Zoghbi A, Aboujaoude S, El-Osta H, Ghorra P, et al. Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J 2006;151(2):521.e1-4. [DOI] [PubMed]
- 39.Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation 2002;106(4):399–402. [DOI] [PubMed]
- 40.Walter T, Szabo S, Kazmaier S, Swoboda S, Suselbeck T, Brueckmann M, et al. Effect of clopidogrel on adhesion molecules, hemostasis, and fibrinolysis in coronary heart disease. J Cardiovasc Pharmacol 2008;51(6):616–20. [DOI] [PubMed]
- 41.Perneby C, Wallen NH, Hofman-Bang C, Tornvall P, Ivert T, Li N, Hjemdahl P. Effect of clopidogrel treatment on stress-induced platelet activation and myocardial ischemia in aspirin-treated patients with stable coronary artery disease. Thromb Haemost 2007;98(6):1316–22. [DOI] [PubMed]
- 42.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease [published erratum appears in Eur Heart J 2006;27(4):501]. Eur Heart J 2003;24(24):2166–79. [DOI] [PubMed]
- 43.Mulvihill NT, Foley JB, Murphy R, Crean P, Walsh M. Evidence of prolonged inflammation in unstable angina and non-Q wave myocardial infarction. J Am Coll Cardiol 2000; 36(4):1210–6. [DOI] [PubMed]
- 44.Meune C, Wahbi K, Fulla Y, Cohen-Solal A, Duboc D, Mahe I, et al. Effects of aspirin and clopidogrel on plasma brain natriuretic peptide in patients with heart failure receiving ACE inhibitors. Eur J Heart Fail 2007;9(2):197–201. [DOI] [PubMed]
- 45.Vavuranakis M, Latsios G, Aggelis D, Bosinakou I, Karambelas I, Tousoulis D, et al. Randomized comparison of the effects of ASA plus clopidogrel versus ASA alone on early platelet activation in acute coronary syndromes with elevated high-sensitivity C-reactive protein and soluble CD40 ligand levels. Clin Ther 2006;28(6):860–71. [DOI] [PubMed]
- 46.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000; 36(5):1587–93. [DOI] [PubMed]
- 47.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2003; 107(9):1278–83. [DOI] [PubMed]
- 48.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, et al. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2002;106(19):2454–8. [DOI] [PubMed]